To the Editor:
Good syndrome (GS), originally described in 1954 by Dr Robert Good in a series of three patients, classically comprises hypogammaglobulinemia (HG) and thymoma [1]. We encountered a challenging 85-year-old patient with 5 months duration of normocytic, normochromic, reticulo-cytopenic anemia, a thymoma, and HG, compatible with a provisional diagnosis of pure red cell aplasia (PRCA). A blood smear revealed reactive large granular lymphocytic (LGL) leukemia with an absolute LGL count of 2020/µL. A bone marrow aspiration/biopsy showed a paucity of erythroid precursors, increased myeloid/erythroid ratio, no dysplastic changes, nor cytogenetic abnormalities suggestive for a concomitant myelodysplastic syndrome and CD8+ lymphocytic infiltrates negative for somatic STAT3/STAT5B mutations. Flow cytometry demonstrated increased CD56+CD16+CD8+ cells with a positive TCR-gamma rearrangement. This presentation resembled GS, with the additional presence of PRCA and LGL. We subsequently identified two additional cases, both presenting with the combination of GS, PRCA, and LGL, TCR-gamma rearrangement but no bone marrow dysplasia, cytogenetic abnormalities, STAT3/STAT5B, or other myeloid mutations. Of note, all these three patients were male and two of them presented with lichen planus, a chronic autoinflammatory disorder that most often affects middle-aged adults, involving both the skin and mucous membranes. While the association between LGL and PRCA has been previously studied [2, 3], to the best of our knowledge, only one patient with coexisting GS and LGL has been reported [4]. This suggests that our series did not represent a random combination of rare clinical features, pointing instead to common pathogenetic links. They may include regulatory circuits involving precursor B- and T-cell maturation and thymic epithelium function explaining coexistence of HG, T-cell autoimmunity, and propensity for clonal expansion [5]. This report investigates whether GS associations constitute a novel continuum of clinical features with unifying pathogenesis.
We analyzed three separate cohorts of patients with thymoma (N = 327), PRCA (N = 74), and LGL (N = 257) referred to The Cleveland Clinic Foundation between the years of 2000 and 2019 (Fig. 1A, B). Thymoma was classified according to the Suster–Moran classification based on morphologic features of differentiation (thymoma, atypical thymoma, and thymic carcinoma) and on whether the cellular nuclei had a spindle/oval shape (type A), an epithelioid appearance (type B), or mixed (AB) [6]. GS was defined as “classic” in case of concomitant presence of thymoma and HG or “probable” with any unclassified immunodeficiency [7]. T-LGL was diagnosed if more than three of the following criteria were present: (i) LGL count >500/µL, (ii) marrow LGL aggregates, (iii) phenotypically aberrant cytotoxic T-lymphocytes expressing CD2, CD56, and CD57 and lacking CD28, (iv) preferential usage of a TCR Vb-family by flow cytometry, and (v) positive TCR-gamma rearrangement [8]. PRCA was defined as reticulocyte count <10,000/μL, <5% marrow erythroblasts [2, 9]. Clinical and laboratory data were abstracted in accordance to approval of the Cleveland Clinic IRB.
Fig. 1. Overlapping relationships between Good syndrome, large granular lymphocytic leukemia, and pure red cell aplasia.
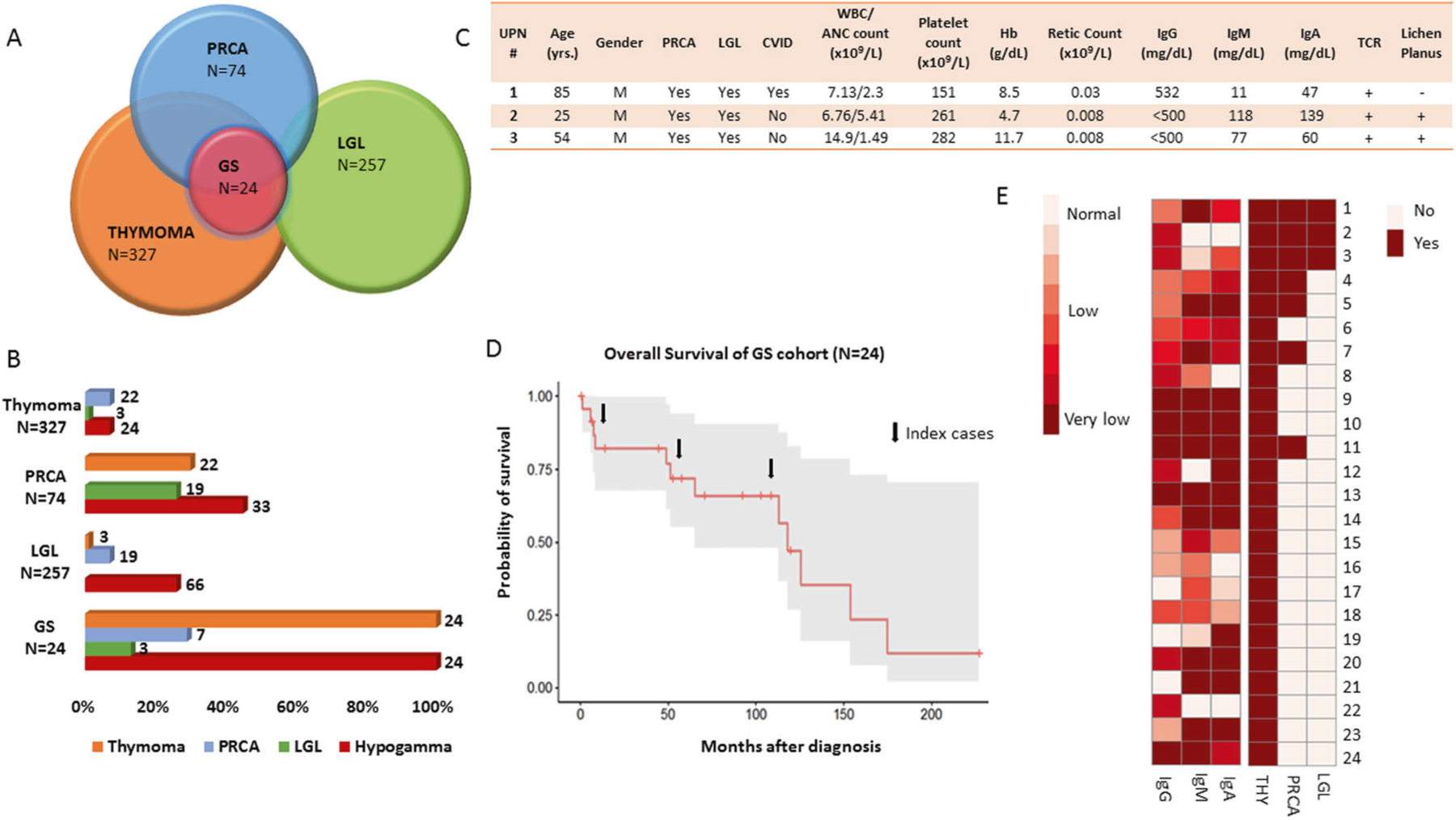
A Venn diagram representation and circle plot of the overlapping relationship of Good syndrome with LGL and PRCA. The Venn diagram circles indicate overlap (not proportional in size to the prevalence of these diseases). B Bar chart illustrates the frequencies of each clinical condition across the different cohorts (numbers above each of the bars are the total numbers of patients in each category). C Demographic and hematological characteristics of our case series of patients. UPN 1 is the original patient who was identified to have the tetrad of diseases. T-cell receptor gamma rearrangement was noted in all patients. UPN 1, 2, and 3 noted to have very low levels of absolute reticulocyte counts consistent with the diagnosis of pure red cell aplasia. D Five-year overall survival of patients with Good syndrome (66%, 47.9–90.5; median follow-up time of 64 months (range, 0.4–227). E Idealized heat map generated to demonstrate combinatorial configuration of clinical features, e.g, immunoglobulin levels, PRCA, and LGL. Normal ranges were considered according to the followings: IgG 717–1411 mg/dL, IgA 78–391 mg/dL, IgM 53–334 mg/dL. ANC absolute neutrophils count, GS good syndrome, Hb hemoglobin, Hypogamma hypogammaglobulinemia, Ig immunoglobulin, LGL Large granular lymphocytic leukemia, M male, retic reticulocytes, PRCA pure red cell aplasia, TCR T-cell receptor rearrangement, WBC white blood cell.
Our initial series of three index patients with the fully blown tetrad of thymoma, HG, PRCA, and LGL (Fig. 1C) inspired a systematic review of similar cases seen by our service among the aforementioned three cohorts of patients (Fig. 1A). In addition to the initial cases, 21 patients with GS were identified for a total of 24 patients (median age at diagnosis of GS was 60 years, see also Table 1). Among patients with GS, PRCA had a total frequency of 30%, while within the cohorts of thymoma and LGL, isolated PRCA was present in 7% of cases. (Fig. 1B, Table 1). Overall, the prevalence of GS was 7.3% in thymoma, 9.4% in PRCA, and 1% in LGL cohorts. An additional case of lichen planus was also noted for a total of 3/24 within the cohort of all GS cases (Fig. 1C, Table 1). With a median follow-up of 64 months (range, 0.4–227), the 5-year overall survival for these patients was 66% (95% confidence interval: 47.9–90.5; see Fig. 1D).
Table 1.
Demographic and hematological characteristics of patients with “Good syndrome”.
| UPN # | AGE (years) | Gender (M/F) | Good syndrome | PRCA | LGL | WBC/neutrophil count (×109/L) | Platelet count (×109/L) | Hb (g/dL) | Retic count (×109/L) | IgG (mg/dL) | IgM (mg/dL) | IgA (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | M | Classic | Yes | Yes | 7.13/2.3 | 151 | 8.5 | 0.03 | 532 | 11 | 47 |
| 2 | 25 | M | Classic | Yes | Yes | 6.76/5.41 | 261 | 4.7 | 0.008 | <500 | 118 | 139 |
| 3 | 54 | M | Classic | Yes | Yes | 14.9/1.49 | 282 | 11.7 | 0.008 | <500 | 77 | 60 |
| 4 | 54 | M | Classic | Yes | No | 0.15/0 | 12 | 8.5 | <0.4 % a | 590 | 39 | 38 |
| 5 | 63 | F | Classic | Yes | No | 4.96/3.76 | 82 | 7.6 | 0.019 | 541 | 7 | 5 |
| 6 | 60 | M | Classic | No | No | 1.78/0.62 | 240 | 8.5 | NA | 487 | 35 | 30 |
| 7 | 62 | F | Classic | Yes | No | 18.5/13.96 | 409 | 15.5 | <0.4% a | 285 | <4 | 25 |
| 8 | 58 | F | Classic | No | No | 17.70/16.67 | 213 | 12.6 | NA | 240 | 50 | 133 |
| 9 | 51 | F | Classic | No | No | 6.06/3.3 | 218 | 14.03 | NA | 51 | <5 | <8 |
| 10 | 71 | F | Classic | No | No | 11.58/4.17 | 312 | 12.6 | NA | 69 | 4 | 7 |
| 11 | 83 | F | Classic | Yes | No | 2.92/1.87 | 165 | 7.5 | 0.029 | 50 | <4 | 10 |
| 12 | 65 | M | Classic | No | No | 7.16/4.18 | 260 | 15.2 | 0.126 | 247 | 184 | <7 |
| 13 | 40 | F | Classic | No | No | 9.32/6.79 | 265 | 15 | NA | NA | NA | NA |
| 14 | 47 | M | Classic | No | No | 4.1/3.1 | 178 | 16.9 | NA | 470 | NA | NA |
| 15 | 87 | F | Classic | No | No | 11.1/9.1 b | 555 | 12.8 | NA | 648 | 23 | 83 |
| 16 | 60 | F | Classic | No | No | 3.1 /1.0 | 287 | 14.9 | 0.039 | 704 | 51 | 141 |
| 17 | 46 | F | Probable | NA | NA | NA | NA | NA | NA | 907 | 42 | 108 |
| 18 | 65 | M | Classic | No | No | 11.38/9.41 | 155 | 11 | NA | 420 | 48 | 105 |
| 19 | 72 | M | Probable | No | No | 8.72/7.05 | 337 | 13.1 | NA | 860 | 78 | <7 |
| 20 | 42 | F | Classic | No | No | 5.23/4.41 | 191 | 11.9 | 0.135 | <500 | NA | NA |
| 21 | 56 | M | Probable | No | No | 3.42/2.2 | 391 | 8.8 | 0.045 | 1800 c | 6 | 17 |
| 22 | 52 | F | Classic | No | No | 3.98/2.33 | 271 | 12 | 0.036 | <500 | 161 | 409 |
| 23 | 84 | F | Classic | No | No | 10.1/7.3 | 208 | 14.4 | NA | 694 | NA | NA |
| 24 | 31 | F | Probable | No | No | 5.72/3.06 | 248 | 14 | NA | NA | NA | 26 |
All findings were registered at the time of Good syndrome onset.
Abnormal values are highlighted in bold. Normal ranges were considered according to the followings: WBC, 3.70–11.00 ×109/L; neutrophil count, 1.45–7.50 ×109/L; platelet count, 150–400 ×109/L; Hb, 11.5–15.5 g/dL; reticulocytes count, 0.0180–0.1000 M/µL; IgG, 717–1411 mg/dL; IgA, 78–391 mg/dL; IgM, 53–334 mg/dL.
M male, F female, Hb hemoglobin, Ig immunoglobulin, LGL large granular lymphocytic leukemia, retic reticulocytes, PRCA pure red cell aplasia, TCR T-cell receptor rearrangement, WBC white blood cell, MGUS monoclononal gammopathy of undertermined significance.
Patients with absolute reticulocytes counts not reported due to very low counts below analyzer linearity.
Patient with essential thrombocythemia (ET).
Patient with monoclonal gammopathy of undetermined significance (MGUS IgGk).
Notably, one patient with GS developed a monoclonal gammopathy of undetermined significance (MGUS), whereas three patients had normal IgG but lower IgM and/ or IgA levels. As matter of fact, these patients were considered as “probable GS,” according to the GS Study Group proposed criteria [7]. In regard to other associations, HG was present in 45% (33/74) of patients with PRCA, 26% (66/257) of patients with LGL, and 6% (24/327) of thymomas (Fig. 1B).
Autoimmune disorders were identified in the majority (70%) of the 24 patients with GS with myasthenia gravis, hypothyroidism, and lichen planus being the most frequent findings (13% for each of the three disorders; Table 2). All patients underwent thymectomy, and AB thymoma was the most common histological subtype (67%).
Table 2.
Autoimmune disorders among patients with Good syndrome (N = 24).
| Autoimmune disorders | Frequency, N (%) |
|---|---|
| Hypothyroidism | 3 (13) |
| Myasthenia gravis | 3 (13) |
| Lichen planus | 3 (13) |
| Asthma | 1 (4) |
| Urticaria | 1 (4) |
| CIDP | 1 (4) |
| Sjogren’s syndrome | 1 (4) |
| Celiac disease | 1 (4) |
| Auto-immune enteropathy | 1 (4) |
| Chronic otitis externa | 1 (4) |
| Fisher–Evans syndrome | 1 (4) |
| Pernicious anemia | 1 (4) |
| Arthritis | 1 (4) |
| Myopathy | 1 (4) |
CIDP chronic inflammatory demyelinating polyneuropathy (rare and progressive autoimmune neurological disorder characterized by progressive weakness and impaired sensory function in the legs and arms).
Fisher–Evans Syndrome is characterized by the concomitant occurrence of autoimmune hemolytic anemia and immune thrombocytopenic purpura with a positive direct anti-human globulin test.
Lichen planus, chronic autoinflammatory disorder that most often affects middle-aged adults, involving both the skin and mucous membranes.
Infections were registered in 62% (15/24) of patients with GS and were most commonly of bacterial origin (Supplementary Fig. 1), in some cases requiring hospitalization (meningitis, JC virus infection, osteomyelitis, zoster encephalitis).
The molecular characterization (Supplementary Table 1), available for the three index cases and other two patients with the combination of GS and PRCA, showed in one case (UPN11) the presence of a concomitant heterozygous germ line Tier-1 FANCE frameshift variant (variant allele frequency (VAF) 54%) and a BCORL1 somatic mutation (VAF 8%). Thus, no mutations were found in the three index cases presenting the peculiar combination of features GS/LGL/PRCA. Of note, in our internal cohort of patients with PRCA and LGL, respectively, 17% and 26% of patients harbored at least one somatic mutation in myeloid/ cancer predisposition genes.
Here we report, for the first time, a clinical tetrad of thymoma, HG, PRCA, and LGL. Incomplete presentation with the triad thymoma, HG, and PRCA was also found, indicating that this peculiar clinical combination is not coincidental, but rather represents an overlapping continuum of features with possibly shared pathogenic mechanisms.
Our monocentric GS cohort, examined specifically for hematologic manifestations, is one of the largest to date. Only one previously described case showed the association of GS and LGL [4], while PRCA was reported in 8 out of 33 cases (24%) of patients with GS presenting with auto-immune disorders studied in another multicenter GS cohort [7]. As the concept of immunodeficiency associated with GS is not clearly delineated, Jansen et al. suggested the inclusion of patients with “unclassified immunodeficiency not fulfilling the criteria for classic GS” into the definition of “probable” [7]. Thus, following the recommendations of the Good Syndrome Study Group [7] we also included four patients with “probable GS” in our cohort (UPN 17, 19, 21, and 24): one also had MGUS, and the other three with hypo-IgA and/or -IgM classifications. Remarkably one of these patients presented with GS associated with myasthenia gravis and Isaac’s syndrome (neuromyotonia or continuous muscle fiber activity syndrome, a rare disorder caused by continuous firing of the peripheral nerve axons characterized by progressive muscle stiffness), described only in one case report outlining this syndrome in combination with thymoma and myasthenia gravis, but without clear evidence of immunodeficiency [10].
The classic combination of thymoma and PRCA is well documented [11] and GS was also found in cases of idiopathic PRCA [12]. Moreover, our cohort of T-LGL also demonstrated the associations with HG and PRCA [8]. Of note, this latter combination has been previously described [3] and recently STAT3 mutations, typical of LGL, have been found in clonal CD8+ cells of a cohort of patients with PRCA with and without a concomitant LGL diagnosis [13]. Overall, our separate analysis of thymoma, T-LGL, and PRCA cohorts further strengthen the conclusions drawn from the GS cohort indicating the presence of partial overlap of these features, as in the three original index cases.
The variety of immunological abnormalities affecting adaptive immunity in either B- or T-cell compartments may be responsible for the plethora of autoimmune manifestations accompanying GS, reported in about half of the patients [5, 7]. The high frequency of autoimmune disorders is another factor that may indicate the original common insult to be of immune origin, instead of a more classical molecular pathway activation. In a series of 50 patients with lichen planus, 6% of cases (3/50) have been reported to have thymoma [14]. Interestingly, one of these cases had pan-hypoglobulinemia and received IVIG, although there is no speculation of a possible diagnosis of GS, emphasizing how underdiagnosed this condition truly remains. As a matter of fact, GS still represents a conundrum to many clinicians, underlying the need of clinical acumen and awareness of the accompanying clinical features, aiming at avoiding delays in diagnosis and treatment. Moreover, in our study one of the most common autoimmune manifestations was myasthenia gravis, representing a classic association with thymoma [11].
LGL has often been associated with B-cell dyscrasias, myelodysplastic syndromes, and various other neoplastic conditions [8, 9]. Classical LGL occurs in older patients and encompasses semi-reactive oligoclonal to clonal CD8+ cells expansions [8]. The presence of isolated, mostly canonical STAT3 mutations [15] points toward neoplastic transformation of the initially reactive process driving CD8+ cells clonality. The recent report of somatic STAT3 mutations in thymoma-associated PRCA with or without LGL diagnosis and the peculiar pattern of responsiveness to immunosuppressive treatment (e.g., cyclosporine) acquired by STAT3-mutated cases may suggest a common pathogenetic T-cell–mediated mechanism by linking all the clinical characteristics presented also in our index cases. However, when we reviewed the mutational status of STAT3 in our patients with LGL and/ or PRCA we found that no STAT3 mutations were detected (5/7 patients with available mutational screening), while in one GS/PRCA case we found a somatic BCORL1 and a germ line heterozygous FANCE variants. The absence of any somatic mutation in the three index cases suggested more probably an immunologic rather than a molecular mechanism as a primum movens. For instance, considerable overlap of pathologic features in GS with PRCA and LGL treatments indicates a potentially similar pathogenesis irrespective of etiology, the latter perhaps involving an inability to eliminate a cryptic viral infection triggering an exuberant CD8+ cells response resembling LGL. Deficient clearance of viral antigens may drive LGL clonal expansion, either via a molecular mimicry or breach of thymic tolerance. Alternatively, thymic dysfunction may excite T-cell autoimmunity, e.g., directed against antigens located on erythroid precursors, causing PRCA. (Supplementary Fig. 2).
Treatment of GS is aimed at resolving the two major features characterizing the syndrome: thymoma and HG. The first involves surgical resection of the tumor, while regular immunoglobulin replacement is used to maintain adequate IgG levels preventing infections [16]. Various schedules of IVIG are recommended depending on the purpose of preventing infections in case of immunodeficiencies (e.g., the case of patients with GS) or for anti-inflammatory or immunomodulatory properties. Most commonly, IVIG should be used either as weekly subcutaneous or monthly intravenous replacement after a loading dose (2 g/kg), to maintain appropriate IgG levels.
In sum, due to the rarity of its presentation, GS studies have only been possible in a retrospective fashion. Our large clinical series demonstrates that there is a high degree of overlap between GS, LGL, and PRCA, serving as clues to the overlapping pathogenesis and in turn to diagnostic schemes and therapeutic strategies.
Supplementary Material
Acknowledgements
Henry and Marilyn Taub Foundation research grant (to JPM), R01HL118281, R01HL123904, R01HL132071, R35HL135795 (to JPM); and Vera and Joseph Dresner Foundation MDS Research Fund (to VV) and American Italian Cancer Foundation (to CG).
Footnotes
Supplementary information The online version of this article (https://doi.org/10.1038/s41375–020-01114-z) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Good RA. A provocative experiment of nature. Bull Uni Minn Hosp Minn Med Found 1954;26:1–19. [Google Scholar]
- 2.Balasubramanian SK, Sadaps M, Thota S, Aly M, Przychodzen BP, Hirsch CM, et al. Rational management approach to pure red cell aplasia. Haematologica 2018;103:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go RS, Li C-Y, Tefferi A, Phyliky RL. Acquired pure red cell aplasia associated with lymphoproliferative disease of granular T lymphocytes. Blood 2001;98:483–85. [DOI] [PubMed] [Google Scholar]
- 4.Caperton C, Agrawal S, Gupta S. Good syndrome presenting with CD8(+) T-Cell large granular lymphocyte leukemia. Oncotarget 2015;6:36577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelleher P, Misbah SA. What is Good’s syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol 2003;56:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suster S, Moran CAThymoma. atypical thymoma, and thymic carcinoma. A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol 1999;111:826–33. [DOI] [PubMed] [Google Scholar]
- 7.Jansen A, van Deuren M, Miller J, Litzman J, de Gracia J, Saenz-Cuesta M, et al. Prognosis of Good syndrome: mortality and morbidity of thymoma associated immunodeficiency in perspective. Clin Immunol 2016;171:12–7. [DOI] [PubMed] [Google Scholar]
- 8.Sanikommu SR, Clemente MJ, Chomczynski P, Afable MG, Jerez A, Thota S, et al. Clinical features and treatment outcomes in large granular lymphocytic leukemia (LGLL). Leuk Lymphoma 2018;59:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durrani J, Awada H, Kishtagari A, Visconte V, Kerr C, Adema V, et al. Large granular lymphocytic leukemia coexists with myeloid clones and myelodysplastic syndrome. Leukemia 2019;34: 957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul BS, Singh G, Bansal RK, Singla M. Isaac’s syndrome associated with myasthenia gravis and thymoma. Indian J Med Sci 2010;64:320–24. [PubMed] [Google Scholar]
- 11.Bailey RO, Dunn HG, Rubin AM, Ritaccio AL. Myasthenia gravis with thymoma and pure red blood cell aplasia. Am J Clin Pathol 1988;89:687–93. [DOI] [PubMed] [Google Scholar]
- 12.Okui M, Yamamichi T, Asakawa A, Harada M, Horio H. Pure red cell aplasia associated with Good syndrome. Korean J Thorac Cardiovasc Surg 2017;50:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami T, Sekiguchi N, Kobayashi J, Imi T, Matsuda K, Yamane T, et al. Frequent STAT3 mutations in CD8+ T cells from patients with pure red cell aplasia. Blood Adv 2018;2:2704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motegi S, Uchiyama A, Yamada K, Toki S, Amano H, Ishikawa O. Lichen planus complicated with thymoma: report of three Japanese cases and review of the published work. J Dermatol 2015;42:1072–77. [DOI] [PubMed] [Google Scholar]
- 15.Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmaki H, Andersson EI, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med 2012;366:1905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarr PE, Sneller MC, Mechanic LJ, Economides A, Eger CM, Strober W, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Med (Baltim) 2001;80:123–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


