Abstract
Objective:
To determine predictors of symptomatic uretero-enteric anastomotic strictures (UAS) formation following radical cystectomy (RC) and urinary diversion (UD).
Materials and Methods:
2,888 consecutive patients who underwent open RC at our institution from 1995–2014 were included for analysis. Data was collected from institutional databases and individual medical records. Symptomatic benign UAS was defined as percutaneous nephrostomy tube insertion for rising creatinine or unilateral hydronephrosis comparing pre- and post-operative imaging. Univariate and multivariable Cox proportional hazards models were utilized to identify features associated with UAS formation.
Results:
UAS developed in 123/2,888 patients following RC. There were 94 symptomatic and 29 asymptomatic strictures. Median follow-up was 32 months (IQR 12, 72) for patients without stricture. Higher BMI (p=0.002), ASA score >2 (p<0.0001), lymph node positive disease (p=0.027) and 30-day post-operative complications grade 3+ (p=0.017) on univariate analysis and male gender on multivariable analysis were significantly associated with time to stricture development. However, history of prior abdominal surgery (PAS) had the strongest association with time to stricture formation (HR 3.25, 95% CI 1.78, 5.94, p=0.0001). Risk of developing a stricture within 10 years was 1.9% for patients without PAS vs. 9.3% with PAS.
Conclusions:
Associated factors with increased risk of benign UAS include higher BMI, ASA score >2, lymph node involvement, grade III/IV complications within 30 days, male sex, and a history of PAS. We conclude that while surveillance is important for patients who undergo cystectomy for malignancy, it may be beneficial for patients with history of PAS undergo more intensive follow-up compared to those patients without history of PAS.
Keywords: urinary diversion, anastomotic stricture, radical cystectomy
INTRODUCTION
Radical cystectomy is a well-established operation for advanced stages of bladder cancer and sometimes even indicated for other aggressive pelvic organ disease states. [1] Although uncommon, development of a benign uretero-enteric anastomotic stricture is a known complication of radical cystectomy and urinary diversion. Published data demonstrates some variation in reported rates with a prevalence of 3% to 10% during long-term, post-operative follow-up. Much of the published data reports stricture rates as a finding of post-operative complications, however our goal is to illustrate the factors that make patients more vulnerable to stricture formation. [2–6]
It is thought that ureteral strictures develop from scar formation secondary to vascular compromise of the ureter or bowel segment, resulting in tissue ischemia and inflammation or from periureteral scar tissue that may form as a result of urine leak, surgery or infection. [2] Ureteral strictures often lead to symptomatic obstruction, infection, stones or loss of renal function requiring intervention. [7] Anastomotic strictures may take years to develop, but most occur within 1 to 2 years following surgery. [3, 4, 7]
Patient factors that have been previously suggested to be associated with anastomotic strictures include pelvic radiation [8] and type of urinary diversion [12]. Specifically, continent urinary diversion has been associated with increased readmission rates and other long-term complications including anastomotic stricture formation and renal function decline. [9] Intra-operative techniques, such as tissue handling, the use of interrupted or running anastomotic sutures, or post-operative ureteral stents have not been proven to affect stricture formation. [10–14]
Although formation of uretero-enteric anastomotic strictures is an infrequent sequela following radical cystectomy, they are associated with significant morbidity. Management of such strictures often is extremely challenging and open surgical revision remains to be the gold standard for treatment. However, the patient undergoing radical cystectomy often has multiple comorbidities and post-operatively is often debilitated, leading to the growing popularity of endoscopic treatment of strictures, at the cost of higher rates of recurrence. [15]
In order to better understand the formation of uretero-enteric anastomotic strictures, we identify the patient characteristics in our cohort that may be predisposing factors of stricture formation. Following radical cystectomy and urinary diversion, we define the incidence of symptomatic uretero-enteric anastomotic strictures and determine the predictors related to this long-term complication.
MATERIALS AND METHODS
After obtaining institutional review board approval, we identified 3,025 patients who underwent radical cystectomy at our center between 1995 and 2014 among five surgeons. We aimed to assess which patient or disease characteristics were associated with time to developing a stricture in an attempt to identify patients who are at highest risk of stricture post-operatively. By definition, symptomatic strictures could not occur within 30 days of surgery. We also planned to include complications within the first 30 days post-operatively as a covariate in our analyses. Therefore, we used a 30 day landmark in our survival analysis. Patients were excluded from the cohort if they were missing follow-up data or had fewer than 30 days of follow-up (n=133).
Patients were diagnosed with symptomatic uretero-enteric anastomotic stricture if they showed hydronephrosis with obstruction to the level of the uretero-enteric anastomosis which required percutaneous nephrostomy tube placement or ureteral stent placement. We also included patients with asymptomatic stricture in the analysis. Typically, patients are followed up with upper tract imaging studies from 3 to 12 months after cystectomy based on initial tumor stage and time from surgery. Pre-operative and post-operative reports of imaging studies were reviewed for each patient with documented hydronephrosis in this cohort who did not undergo percutaneous nephrostomy tube placement. If a patient was found to have a report of post-operative unilateral hydronephrosis on imaging and did not have pre-operative hydronephrosis in the same kidney, this was considered as an asymptomatic stricture. The date of first imaging with post-operative hydronephrosis was considered to be the date of stricture in these patients. Some patients had pre-operative hydronephrosis that did not resolve after surgery. While an asymptomatic stricture in these patients would not have been identified via imaging, these patients represent a small portion of the cohort. We do not believe there is a latency period between the development of an asymptomatic stricture that can be found on imaging, and a symptomatic stricture requiring nephrostomy tube placement. Therefore, time to stricture is not underestimated among patients with symptomatic stricture. Furthermore, asymptomatic stricture based on our method likely overestimates the true rate because we cannot account for new-onset hydronephrosis due to urine reflux. Given that reflux occurs at uretero-enteric anastomotic sites, we believe that comparing pre-operative and post-operative imaging in conjunction with unilateral hydronephrosis appropriately reflected asymptomatic strictures.
We excluded those patients found to have a symptomatic or asymptomatic stricture within the first 30 days postoperatively (n=4), leaving 2,888 patients available for analysis. These patients were excluded in order to include first 30-day complications as a covariate in statistical model and survival analysis. Patients with hydronephrosis secondary to extrinsic compression or hydronephrosis secondary to malignant ureteral recurrence were excluded to ensure that analysis included benign stricture formation only.
The primary outcome of the study was to determine clinicopathologic associations in the group of patients who developed uretero-enteric anastomotic strictures. Pathological (T) and nodal (N) stage were based on the 2010 American Joint Committee on Cancer respective cancer staging system based on patient’s primary diagnosis (i.e. bladder, prostate, colon, etc). Patient comorbidity was estimated using ASA class.
Because our institution is a tertiary referral center, some patients chose follow up with their local urologist. All medical records and imaging from outside facilities were reviewed by professionals at our institution. When collecting data on patients with symptomatic strictures, it was found that information on a patient’s history of prior abdominal surgery was not captured in the prospectively-collected institutional database for all patients. To ensure that a complete prior abdominal history was captured, we matched each of the 94 patients who developed a symptomatic stricture with up to 4 controls, or patients who did not develop a stricture, and performed a subset complete chart review for these patients to gather more data on patients who were missing data on history of prior abdominal surgery. Matching was performed only for further clarification of history of previous abdominal surgery.
Each patient with a symptomatic stricture was matched with 4 patients who were at risk of stricture at the time that the case developed a stricture. Patients who had strictures could be matched as a control if they were free of symptomatic stricture at the time that their matched case developed a stricture. Matching was based on sex, age (+/− 1 year), and date of surgery (+/− 1 year). There were 94 patients with symptomatic strictures, with 77 matched to 4 controls. Chart reviews were performed for a total of 413 patients, including the 94 patients with symptomatic strictures. The outcome of asymptomatic stricture was added after the matching and chart review of matched controls was performed. However, chart reviews were also performed for all 29 patients identified as having asymptomatic strictures. Although patients with asymptomatic strictures were not matched to controls, multiple imputation was used to account for missing data in all patients, including those patients who would have been matched as controls to patients with asymptomatic strictures.
Multiple imputation using predictive mean matching based on age and sex were used to impute BMI (n=543) and estimated blood loss (n=133) for non-selected patients. Multiple imputation using logit models based on age and sex were used to impute a history of prior abdominal surgery (n=1716), ASA score III/IV (n=162), pathologic stage T3 or higher (n=52) and continent or non-continent urinary diversion (n=344) for non-selected patients. Statistical analyses were performed utilizing the measured and imputed values combined across 30 imputations using Rubin’s rules.
Univariate and multivariable Cox proportional hazards models were created to assess the association between time from 30 days post-cystectomy to stricture using the following covariates: age, sex, BMI, ASA score, neo-adjuvant chemotherapy, type of urinary diversion (continent or non-continent), history of prior abdominal surgery, estimated blood loss, pathologic T stage (T3+ or less than T3), presence of positive nodes, receipt of radiation therapy, any grade 3+ complications within 30 days and grade 3+ abdominal infection or fluid collection complications within 30 days. Given the number of stricture events, a limited number of covariates could be included in the multivariable model. As the incidence of grade 3+ abdominal infection or fluid collection was low (1.9% of our patient cohort), we chose to exclude this covariate from the multivariable model. However, this covariate was assessed on univariate analysis. The cumulative incidence function was presented for time to developing a stricture for patients with and without prior abdominal surgery. All analyses were performed using Stata 13 (StataCorp, College Station, TX).
RESULTS
Patient and disease characteristics for patients in our cohort are described in Table 1. There were 94 symptomatic and 29 asymptomatic strictures. Strictures were left-sided in 66 patients, right sided in 50 and bilateral in 7. Median follow-up was 32 months (IQR 12, 72) for patients without a stricture. On univariate analysis, higher BMI (HR 1.05, 95% CI 1.02, 1.08, p=0.002), an ASA score of 3 or 4 (HR 2.40, 95% CI 1.60, 3.61, p<0.0001), lymph node positive disease (HR 1.64, 95% CI 1.06, 2.53, p=0.027), neoadjuvant chemotherapy (HR 1.56, 95% CI 1.03, 2.36, p=0.036) and ≥ grade 3 Clavien-Dindo complications within 30 days of cystectomy (HR 1.90, 95% CI 1.12, 3.22, p=0.017) were all significantly associated with time to developing a stricture (Table 2). There was also evidence that male gender (HR 1.54, 95% CI 0.98, 2.43, p=0.061) and age (HR 1.18, 95% CI 0.99, 1.40, p=0.059) increased risk of stricture, although these associations did not reach conventional levels of statistical significance. A history of prior abdominal surgery had the strongest association with time to stricture (HR 3.25, 95% CI 1.78, 5.94, p=0.0001).
Table 1.
Patient characteristics among all patients, N=2888. Data are reported as frequency (%) or median (interquartile range).
| Male | 2141 (74%) |
| BMI* | 28 (25, 30) |
| Age | 68 (60, 75) |
| ASA III/IV* | 1784 (62%) |
| EBL (per 100 mL)* | 9 (6, 14) |
| Continent urinary diversion* | 970 (34%) |
| Grade 3+ complications within 30 days | 243 (8.4%) |
| Grade 3+ abdominal infection or fluid collection within 30 days | 55 (1.9%) |
| Pathologic T3 or higher* | 1037 (36%) |
| Neoadjuvant chemotherapy | 580 (20%) |
| Prior abdominal surgery* | 2175 (75%) |
| Positive lymph nodes | 540 (19%) |
| Positive ureteral margins | 274 (9.5%) |
| Pre-operative radiation therapy | 173 (6.0%) |
| Post-operative radiation therapy | 225 |
Proportion of values imputed: BMI (19%), ASA (5.6%), EBL (4.6%), urinary diversion (12%), pathologic T stage (1.8%), and prior abdominal surgery (59%).
Table 2.
Univariate and multivariable Cox proportional hazards models for time from 30 days after surgery to stricture, N=2888.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Male | 1.54 | 0.98, 2.43 |
0.061 | 1.81 | 1.13, 2.91 |
0.014 |
| Age | 1.18 | 0.99, 1.40 |
0.059 | 1.13 | 0.92, 1.40 |
0.3 |
| BMI | 1.05 | 1.02, 1.08 |
0.002 | 1.04 | 1.01, 1.07 |
0.013 |
| EBL (per 100 mL) | 1.01 | 1.00, 1.02 |
0.059 | 1.01 | 1.00, 1.02 |
0.084 |
| Prior abdominal surgery | 3.25 | 1.78, 5.94 |
0.0001 | 3.40 | 1.84, 6.28 |
<0.0001 |
| ASA III/IV | 2.40 | 1.60, 3.61 |
<0.0001 | 1.88 | 1.21, 2.91 |
0.005 |
| Continent urinary diversion | 0.90 | 0.62, 1.30 |
0.6 | 1.17 | 0.75, 1.82 |
0.5 |
| Pathologic stage T3/T4 |
1.22 | 0.84, 1.78 |
0.3 | 1.13 | 0.75, 1.70 |
0.6 |
| Positive lymph nodes | 1.64 | 1.06, 2.53 |
0.027 | 1.62 | 1.01, 2.60 |
0.044 |
| Grade 3+ complications within 30 days | 1.90 | 1.12, 3.22 |
0.017 | 1.59 | 0.92, 2.74 |
0.094 |
| Neoadjuvant chemotherapy | 1.56 | 1.03, 2.36 |
0.036 | 1.37 | 0.89, 2.11 |
0.15 |
| Positive ureteral margin | 1.33 | 0.76, 2.32 |
0.3 | 1.26 | 0.72, 2.22 |
0.4 |
| Radiation therapy | 1.35 | 0.94, 1.95 |
0.10 | 1.31 | 0.90, 1.91 |
0.2 |
| Grade 3+ abdominal infection or fluid collection within 30 days |
0.48 | 0.07, 3.47 |
0.5 | - | - | - |
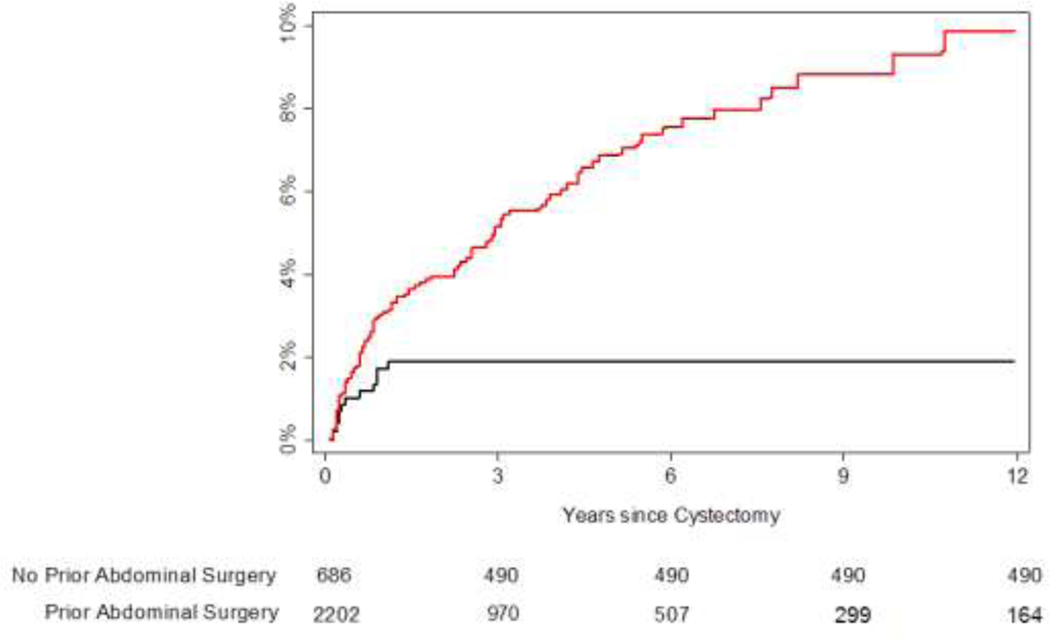
Hazard ratios did not change appreciably between univariate and multivariable analysis. All covariates that were significant on univariate analysis remained significant on multivariable analysis, with the exception of grade 3 or higher complications within 30 days, which did not reach conventional levels of statistical significance (p=0.094, Table 2), and neoadjuvant chemotherapy (p=0.15), while male gender did show statistical significance (p= 0.014). On multivariable analysis, prior abdominal surgery was the only covariate with a hazard ratio greater than 2 (HR 3.40, 95% CI 1.84, 6.28, p<0.0001). For patients without a history of prior abdominal surgery, the risk of developing a stricture within 10 years was 1.9%. For patients with a history of prior abdominal surgery, the risk of stricture within 5 years was 6.9% and the risk of stricture by 10 years was 9.3% (Figure 1).
Figure 1.
Cumulative incidence estimates for time to developing a stricture. Black line represents patients with no history of prior abdominal surgery; red line represents patients with a history of prior abdominal surgery. The number at risk is approximate due to the use of imputation.
COMMENT
In our large cohort of patients who underwent radical cystectomy and urinary diversion, 123 patients developed uretero-enteric anastomotic strictures. 98 patients (3.4%) developed a stricture within 3 years post-operatively, which is consistent with published data. [4–7, 16] However, the true stricture rate following radical cystectomy is difficult to quantify given variability in follow up and the non-standardized approach to postoperative upper tract imaging among patients.
In a study showing the long term complications of open radical cystectomy and ileal conduit, a 10% rate of stricture formation was identified at a median time of 1.1 years postoperatively (0.1 to 25 years). [4] Comparatively, Hautmann et al. published data assessing long term complications of radical cystectomy and neobladder diversions, reporting an 11% rate of strictures which developed 3 to 160 months following surgery. [3] Tal et al. reported a cohort of 221 patients who underwent open radical cystectomy with a median follow up of 5 years and a rate of 12.7% who developed benign uretero-enteric anastomotic strictures, with 80% involving the left side and median time to stricture formation was 7 months postoperatively. They found no significant different in stricture rates between continent and incontinent diversion types. [16] It has been hypothesized that tissue handling or ureteral stretching may influence stricture development. Previously, Hautmann et al. have suggested concerns for intraoperative technique and creation of the anastomosis to play a role in development of stricture formation, however more recent studies have shown no difference between the technical aspects of anastomotic suturing. [3, 11, 17] Albeit in our study, the same end-to-side Bricker anastomosis was used for all diversion types in our cohort of patients. We did not compare running and interrupted suturing techniques. [5] Historically, prior pelvic radiation has been a major associated risk factor for stricture development, however we did not detect a difference in stricture formation in those patients who had a history of radiotherapy compared to those who did not. This was likely due to the small subset of patients who had a history of radiation therapy in our cohort (6%) and a subsequent lack of a statistical difference.
A history of prior abdominal surgery is the strongest predictor of uretero-enteric anastomotic stricture formation in our patient population. This was an unexpected finding, but because the potential predictive variables were defined prior to initiating this analysis, it is unlikely that this is a spurious finding. In fact, these patients continued to have an incremental risk of UAS for at least a decade beyond radical cystectomy (see Figure 1). Previous abdominal surgery is associated with formation of intra-abdominal adhesions [18, 19], which may lead to higher morbidity such as a higher number of immediate post-operative grade 3 complications, intra-abdominal infections, or anastomotic leak. Parsons et al. has previously described the effects of previous abdominal surgery in urologic laparoscopy, and demonstrated a rate of 48% of patients who underwent laparoscopic urologic surgery had a history of prior abdominal surgery. They reported that a history of previous abdominal surgery was associated with female gender, higher ASA classification, an increased age, and an increased rate of perioperative transfusion requirement (p<0.0001). [20] Autopsy studies show that intra-abdominal adhesions occur in 75% to 90% of patients with a history of abdominal surgery, while adhesions develop in only 10% of patients without prior surgery. [18] We hypothesize that inflammation from previous abdominal surgery and the subsequent formation of adhesions may distort tissue planes and may lead to variable blood supply. Therefore, if vascularity has been compromised, patients with previous abdominal surgery may be more susceptible to uretero-enteric anastomotic stricture formation. Given that ureters are retroperitoneal structures, this data may reflect the consequences of change in bowel vascularity from previous abdominal surgery or adhesions. Our hypothesis of the consequences related to bowel vascularity may be an addition to the well-known importance of ureteral dissection and distal ureteral blood supply which can lead to stricture formation.
Conversely, in patients without a history of prior abdominal surgery, while there is a risk of UAS formation up to 2 years post-operatively, it is negligible beyond this time point (see Figure 1). This calls into question the role of follow-up imaging beyond standard oncologic follow-up in patients without a history of prior abdominal surgery.
Our data supports that higher BMI led to a higher rate of stricture formation, which may reflect the influence of surgical technical feasibility of the operation. In addition to this, the higher ASA score and grade III/IV complications within 30 days seen in patients that developed uretero-enteric anastomotic strictures portrays the influence of comorbidity leading to stricture formation. The association of lymph node disease and stricture formation may further support the causality of neovascularization and macro-metastatic disease leading to changes in blood supply and scar tissue formation.
There is growing support for feasibility and equivalent long-term complications of robotic-assisted radical cystectomy (RARC), however, there is shorter follow up and higher stricture rates in studies that compare open radical cystectomy to robotic-assisted radical cystectomy [7, 12] Given the small number of patients who underwent robotic assisted radical cystectomy and sheer surgeon preference up until recent years at our institution, we did not include this component in our analysis. However, to our knowledge, this is the largest cohort of consecutive patients from a single-institutional tertiary center who underwent open radical cystectomy and urinary diversion.
Despite this limitation, we experienced a similar proportion of patients diagnosed with uretero-enteric strictures after open radical cystectomy and urinary diversion. While many potential confounders and clinicopathologic characteristics were identified and adjusted for, there are a number of immeasurable factors that may lead to stricture formation. Of note, our patient selection criteria excluded all stricture formation caused by disease recurrence or malignant etiology.
CONCLUSION
Higher BMI, male sex, increased co-morbidities as noted by ASA score >2, positive lymph nodes on final pathology, grade III/IV complications within 30 days, and a history of previous abdominal surgery increase the risk of developing uretero-enteric strictures. While imaging is important for ongoing surveillance in patients who undergo cystectomy for urothelial cancer and studies to validate the importance of previous abdominal surgery’s impact on uretero-enteric stricture formation are eagerly awaited, our report suggests that patients with a history of prior abdominal surgery will benefit from more intensive follow-up (e.g. renal sonogram) for at least a decade post-cystectomy. Conversely, consideration should be given to limiting imaging during follow-up beyond 2 years in patients without a history of prior abdominal surgery outside of oncologic surveillance guidelines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Campbell MF, Wein AJ, and Kavoussi LR. Campbell-Walsh urology. 2007; 9th:[4 v. (xlii, 3945, cxv p.) ill. + 1 CD-ROM(s) (4 3/4 in.).].
- 2.Madersbacher S, et al. , The long-term outcome of medical therapy for BPH. Eur Urol, 2007. 51(6): p. 1522–33. [DOI] [PubMed] [Google Scholar]
- 3.Hautmann RE, de Petriconi RC, and Volkmer BG, 25 years of experience with 1,000 neobladders: long-term complications. J Urol, 2011. 185(6): p. 2207–12. [DOI] [PubMed] [Google Scholar]
- 4.Shimko MS, et al. , Long-term complications of conduit urinary diversion. J Urol, 2011. 185(2): p. 562–7. [DOI] [PubMed] [Google Scholar]
- 5.Katkoori D, et al. , Is the incidence of uretero-intestinal anastomotic stricture increased in patients undergoing radical cystectomy with previous pelvic radiation? BJU Int, 2010. 105(6): p. 795–8. [DOI] [PubMed] [Google Scholar]
- 6.Studer UE, et al. , Twenty years experience with an ileal orthotopic low pressure bladder substitute--lessons to be learned. J Urol, 2006. 176(1): p. 161–6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CB, et al. , Ureteroenteric anastomotic strictures after radical cystectomy-does operative approach matter? J Urol, 2013. 189(2): p. 541–7. [DOI] [PubMed] [Google Scholar]
- 8.Kim HL and Steinberg GD, Complications of cystectomy in patients with a history of pelvic radiation. Urology, 2001. 58(4): p. 557–60. [DOI] [PubMed] [Google Scholar]
- 9.Gandaglia G, et al. , Short-term perioperative outcomes of patients treated with radical cystectomy for bladder cancer included in the National Surgical Quality Improvement Program (NSQIP) database. Can Urol Assoc J, 2014. 8(9–10): p. E681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg MS, et al. , Long-term renal function outcomes after radical cystectomy. J Urol, 2014. 191(3): p. 619–25. [DOI] [PubMed] [Google Scholar]
- 11.Large MC, et al. , The impact of running versus interrupted anastomosis on ureterointestinal stricture rate after radical cystectomy. J Urol, 2013. 190(3): p. 923–7. [DOI] [PubMed] [Google Scholar]
- 12.Skinner DG, Re: Ureteroenteric anastomotic strictures after radical cystectomy--does operative approach matter?:Anderson CB, Morgan TM, Kappa S, Moore D, Clark PE, Davis R, Penson DF, Barocas DA, Smith JA Jr., Cookson MS and Chang SS. J Urol 2013; 189: 541–547. J Urol, 2013. 190(1): p. 362–3. [DOI] [PubMed] [Google Scholar]
- 13.Mullins JK, et al. , Ureteral stents placed at the time of urinary diversion decreases postoperative morbidity. Urol Int, 2012. 88(1): p. 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards KA, et al. , The effect of length of ureteral resection on benign ureterointestinal stricture rate in ileal conduit or ileal neobladder urinary diversion following radical cystectomy. Urol Oncol, 2015. 33(2): p. 65 e1–8. [DOI] [PubMed] [Google Scholar]
- 15.Nassar OA and Alsafa ME, Experience with ureteroenteric strictures after radical cystectomy and diversion: open surgical revision. Urology, 2011. 78(2): p. 459–65. [DOI] [PubMed] [Google Scholar]
- 16.Tal R, et al. , Management of benign ureteral strictures following radical cystectomy and urinary diversion for bladder cancer. J Urol, 2007. 178(2): p. 538–42. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, et al. , Technique selection of bricker or wallace ureteroileal anastomosis in ileal conduit urinary diversion: a strategy based on patient characteristics. Ann Surg Oncol, 2014. 21(8): p. 2808–12. [DOI] [PubMed] [Google Scholar]
- 18.Weibel MA and Majno G, Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg, 1973. 126(3): p. 345–53. [DOI] [PubMed] [Google Scholar]
- 19.Liakakos T, et al. , Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg, 2001. 18(4): p. 260–73. [DOI] [PubMed] [Google Scholar]
- 20.Parsons JK, et al. , The effect of previous abdominal surgery on urological laparoscopy. J Urol, 2002. 168(6): p. 2387–90. [DOI] [PubMed] [Google Scholar]