Abstract
Background/Purpose:
Relapsing polychondritis (RP) is a systemic disease. Failure to recognize RP can lead to diagnostic delay and further complications including death. The study objective was to identify clinical patterns in a prospective cohort of patients with RP.
Methods:
Patient subgroups were identified using latent class analysis by eight clinical variables: saddle nose deformity, subglottic stenosis (SGS), tracheomalacia (TM), bronchomalacia (BM), ear chondritis, tenosynovitis/synovitis, inflammatory eye disease, and audiovestibular disease. Model selection was based on Akaike Information Criterion.
Results:
Seventy-three patients were included. Three subgroups were identified: Type 1 RP (14%); Type 2 RP (29%); and Type 3 RP (57%). Type 1 RP was characterized by ear chondritis (100%), TM (100%), saddle nose deformity (90%), and SGS (80%). These patients had the shortest time to diagnosis (median = 1 year), highest disease activity, and greatest frequency of ICU admissions and tracheostomy. Type 2 RP was characterized by TM (100%) and BM (52%), but without saddle nose deformity or SGS. These patients had the longest delay to diagnosis (median = 10 years) and highest percentage of work disability. Type 3 RP was characterized by tenosynovitis/synovitis (60%) and ear chondritis (55%). There were no significant differences in sex, ethnicity, and treatment strategies between the three subgroups.
Conclusions:
Three subgroups of patients with RP were identified that differ in time to diagnosis, clinical and radiological characteristics, and disease-related complications. Recognizing a broader spectrum of clinical patterns in RP, beyond just cartilaginous involvement of the ear and upper airway, may facilitate more timely diagnosis.
Relapsing polychondritis (RP) is a rare, systemic and in some cases fatal, inflammatory disease with a predilection for cartilaginous structures [1, 2]. Although, auricular chondritis is often considered the hallmark feature of the disease, RP can affect multiple organs including the tracheobronchial tree, upper airway, nose, joints, central nervous system, eyes, inner ear, blood vessels, heart valves, and skin. Due to the potential widespread organ involvement, clinical manifestations may vary, and recognizing the disease across a range of presentations can be challenging.
Early identification of RP, with prompt initiation of treatment, may help decrease uncontrolled inflammation and ultimate organ damage. RP carries significant morbidity including tracheomalacia, subglottic stenosis (SGS), intensive care unit (ICU) admissions, hearing loss, and disability [3, 4]. RP is also associated with significant mortality, with an incidence ranging from 55 to 91% at 10 years after diagnosis [3, 5]. Diagnostic delay is common, with reported time to diagnosis ranging anywhere from 1 to 20 years [4, 6]. Lack of auricular chondritis has been associated with diagnostic delay, indicating that broader understanding of the spectrum of possible clinical manifestations and patterns of organ involvement could facilitate more timely diagnosis [4].
Defining disease subgroups in RP based on clinical and radiographic features may enable earlier diagnoses, while also leading to the development of diagnostic and treatment algorithms. Therefore, the objective of the current study was to employ unbiased analytic approaches to identify subgroups of patients with RP based on clinical patterns of disease.
METHODS
Study population
Patients 18 years and older were recruited into a prospective observational cohort of RP at the National Institutes of Health (NIH). All patients met McAdams or Damiani’s diagnostic criteria for RP [7, 8]. Patients could be enrolled at any point in their disease course. All patients provided written informed consent, and the study protocol was approved by local ethics review (14-AR-0200).
Clinical assessments and disease definitions
All patients were seen in consultation at the NIH Clinical Center. Detailed standardized clinical assessment was performed by the investigative study team. Findings attributed to RP were recorded using standardized case report forms. All patients underwent audiology testing and standardized assessment of the airways, including dynamic computed tomography (CT) scan of the chest, direct laryngoscopy by an otolaryngologist, and pulmonary function tests (PFT’s). Outside clinical records were directly reviewed by the study team. Bronchoscopy was not performed as part of standardized assessment; however, data from prior bronchoscopies was recorded. Clinical laboratory testing was performed in every patient including erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), complete blood cell count, comprehensive metabolic panel, lipid panel, antineutrophil cytoplasmic antibodies (ANCA), lupus anticoagulant, anti-cardiolipin antibodies, rheumatoid factor, ANA, anti-dsDNA, ENA, and urinalysis.
Standardized definitions were applied to disease-relevant features. Ear chondritis was defined as physician-observed tender swelling of the pinna with associated redness; and/or cauliflower ear; and/or other evidence of cartilage damage including floppy ears and/or thickened cartilage. Sinonasal disease was defined as tenderness over the bridge of the nose with redness and/or swelling; tip of the nose tenderness with associated swelling and/or redness; saddle nose deformity; nasal crusting; or nasal septal perforation. Airway chondritis was defined as tracheomalacia, bronchomalacia, tracheal thickening, or SGS. Tracheomalacia (TM) was defined as anterior and/or lateral flattening of the tracheal wall of ≥50% visualized during bronchoscopy or dynamic CT scan [9]. Bronchomalacia (BM) was defined as bronchial collapse visualized during bronchoscopy or dynamic CT scan. Tracheal thickening was defined as >3mm wall thickness as measured by chest CT [10]. Subglottic stenosis (SGS) was defined as pathological narrowing of the subglottis visualized by direct laryngoscopy. Arthritis was defined as physician observed synovitis/tenosynovitis. Vestibular/cochlear damage was defined as documented sensorineural hearing loss by audiometry and/or documented vestibular dysfunction by vestibular testing. Ocular inflammation was defined as physician-observed scleritis, episcleritis, iritis, or uveitis. Skin involvement was defined as biopsy-proven skin pathology clinically attributed to RP. Disease activity was defined by the physician global assessment (PGA) score, rated 0 to 10 with higher scores indicating more disease activity. A PGA score of >0 indicated active disease.
Statistical analysis
Latent class analysis (LCA) was performed to identify subsets of patients with RP based on pre-defined input variables. LCA is an unbiased partitioning method that can be used to classify individuals into subgroups that share similar characteristics based on a set of categorical input variables. Input variables for LCA modeling were chosen a priori based on criterion items from McAdams diagnostic criteria for RP. Variables that represented objective cartilaginous involvement were selected, including ear chondritis, saddle nose deformity as a surrogate for nose chondritis, airway chondritis (SGS, TM, BM), arthritis/tenosynovitis, sensorineural hearing loss or vestibular dysfunction, and inflammatory eye disease. Airway chondritis was divided into three different anatomic locations to differentiate upper airway involvement from lower airway involvement since patients with RP can have isolated upper or lower airway disease.
Model fit was assessed by the Akaike Information Criteria (AIC). Models were fit with two to six subgroups, and the model with the lowest AIC was ultimately selected. Each resultant subgroup was labelled with a descriptive term based on the common manifestations defining the group. Categorical and continuous variables were compared using the chi square or Kruskal-Wallis test. Median values with interquartile range (IQR) are reported. All analyses were performed using JMP version 14. A p<0.05 defined statistical significance, and adjustment for repeated measures was not performed given the exploratory nature of the study and modest sample size.
RESULTS
Clinical characteristics of study participants
A total of 73 patients were included in analyses. Most patients were female (n=62, 85%) and Caucasian (n=63, 86%). The median age at symptom onset was 36 years (IQR 28–43) and at diagnosis was 43 years (IQR 33–59). The median time to diagnosis was 5 years (IQR 2–10). The median disease duration was 8 years (IQR 4–15).
Severe organ damage was not uncommon, including saddle nose deformity (n= 10, 14%), subglottic stenosis (n= 11, 15%), tracheomalacia (n=31, 42%), bronchomalacia (n=16, 22%), and hearing loss (n=19, 26%). Four patients had nasal septal perforation (%), two patients had scleromalacia (%), one patient had vestibular dysfunction (%), and one patient had severe aortic insufficiency (%). Other significant disease-related complications included work disability (n=25, 34%) and ICU admission due to RP (n=12, 16%). Most patients were taking disease modifying anti-rheumatic drugs (DMARD’s) at the time of initial evaluation (n=49, 67%). Most patients received prednisone ≥ 60 mg per day (n=36, 50%) at some point in the disease course as part of their treatment. Every patient had active disease at the baseline visit. No patient had a positive anti-PR3 or anti-MPO ANCA.
Analytic identification of subgroups
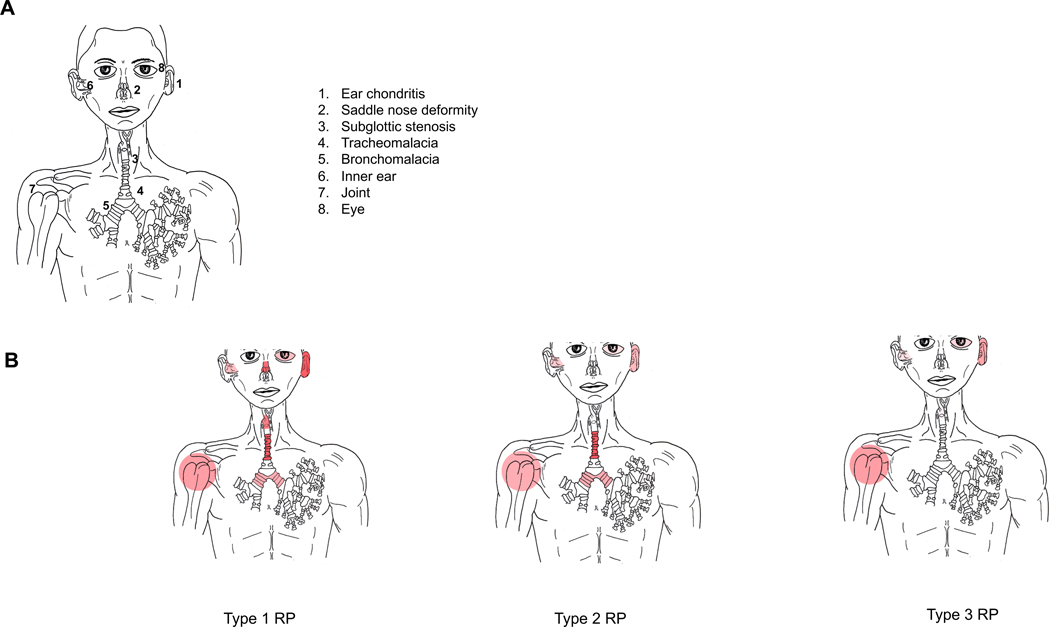
LCA identified three subgroups of patients with RP based on clinical manifestations. The first subgroup (n=10, 14%) was characterized by ear chondritis (100%), TM (100%), saddle nose deformity (90%), SGS (80%), tenosynovitis/synovitis (60%), BM (50%), hearing loss (50%), and inflammatory eye disease (40%). This group, defined by extensive cartilage damage and ear involvement, was labeled Type 1 RP. The second subgroup (n=21, 29%) was characterized by TM (100%), BM (52%), tenosynovitis/synovitis (52%), ear chondritis (43%), hearing loss (33%), and inflammatory eye disease (19%), in absence of saddle nose deformity and SGS. (Supplement 1). This group, characterized by prominent lower airway disease with less overt involvement of cranial cartilaginous structures, was labeled Type 2 RP. The final subgroup (n=42, 58%) was characterized by tenosynovitis/synovitis (60%), ear chondritis (55%), inflammatory eye disease (26%), and hearing loss (19%). This group, defined by minimal overt cartilage damage including SGS (7%), saddle nose deformity (2%) without evidence of TM or BM, was labeled Type 3 RP. (Figure 1).
Figure 1.
Figure 1A Represent input variables used for latent class analysis modeling which were chosen based on criterion items from McAdams diagnostic criteria for RP.
Figure 1B Reprepresent clinical subtypes identified by latent class analysis. The color demonstrate the percentage of organ involvement. The darker the color the higher the percentage of involvement.
When comparing the three subgroups, there were no differences in demographic characteristics including race, sex, age at symptom onset, age at diagnosis, and disease duration. Two patients in the cohort died (Type 1 RP=1; Type 2 RP=1), and the cause of death was not directly related to RP. Compared to the other subgroups, patients with Type 1 RP experienced the shortest time to diagnosis (median = 1 year, IQR 0.6–5, p=0.007), were the youngest at the time of diagnosis (39 years, IQR 23–49, p=0.08) and had the highest disease activity (median PGA =5, IQR 3.75–6). Patients with Type 2 RP experienced the longest time to diagnosis (median = 10 years, IQR 3.5–20, p=0.007) and were the oldest subgroup at diagnosis (48 years, IQR 43–56, p=0.08). (Table 1)
Table 1.
Clinical Characteristics of Three Subgroups of Patients with Relapsing Polychondritis
| All Patients n=73 | Type 1 RP n=10 | Type 2 RP n=21 | Type 3 RP n=42 | P value | |
|---|---|---|---|---|---|
|
| |||||
| Variables Included in Latent Class Analysis | |||||
|
| |||||
| Saddle nose n (%) | 10 (13) | 9 (90) | 0 | 1 (2) | <0.001 |
| Subglottic stenosis n (%) | 11 (15) | 8 (80) | 0 | 3 (7) | <0.001 |
| Tracheomalacia n (%) | 31 (42) | 10 (100) | 21 (100) | 0 | <0.001 |
| Bronchomalacia n (%) | 16 (22) | 5 (50) | 11 (52) | 0 | <0.001 |
| Ear chondritis n (%) | 42 (57) | 10 (100) | 9 (43) | 23 (55) | 0.001 |
| Tenosynovitis/Synovitis n (%) | 42 (57) | 6 (60) | 11 (52) | 25 (60) | 0.85 |
| Eye inflammatory disease n(%) | 20 (27) | 4(40%) | 4 (19) | 11(26) | 0.47 |
| Sensorineural hearing loss n(%) | 19 (26) | 4 (40%) | 7 (33) | 8 (19) | 0.26 |
|
| |||||
| Demographic Characteristics | |||||
|
| |||||
| Race, White n (%) | 63 (86) | 7 (70%) | 18 (86%) | 38 (90) | 0.29 |
| Sex, Female n (%) | 62 (84) | 9 (90%) | 17 (81%) | 36 (86%) | 0.78 |
| Age, symptom onset (years, IQR) | 36 (27–43) | 37 (22–40) | 37 (31–46) | 35 (26–43) | 0.36 |
| Age at diagnosis (years, IQR) | 43 (33–52) | 39 (23–49) | 48 (43–56) | 42 (31–50) | 0.08 |
| Diagnostic Delay (years, IQR) | 5 (2–10) | 1 (0.6–5) | 10 (3.5–20) | 4.5 (2–8.5) | 0.007 |
|
| |||||
| Clinical Symptoms | |||||
|
| |||||
| Fever n (%) | 18 (24) | 2 (20) | 7 (33) | 9 (21) | 0.55 |
| Weight loss n (%) | 8 (11) | 4 (40) | 0(0) | 4 (9) | 0.02 |
| Oral ulcers n (%) | 22 (30) | 2 (20) | 10 (48) | 10 (24) | 0.12 |
| Genital ulcers n (%) | 12 (16) | 0 (0) | 7 (33) | 5 (12) | 0.02 |
| Audiovestibular n (%) | 57 (78) | 7 (70) | 16 (76) | 34 (80) | 0.73 |
| Sinonasal disease n (%) | 68 (94) | 9 (90) | 19 (95) | 40 (95) | 0.82 |
| Costochondritis n (%) | 63 (86) | 9 (90) | 17 (80) | 37 (88) | 0.70 |
| Dry cough n (%) | 62 (85) | 10 (100) | 20 (95) | 32(76) | 0.09 |
| Skin involvement n (%) | 19 (26) | 0 (0) | 0(0) | 6 (14) | 0.03 |
| Sicca symptoms n (%) | 30 (41) | 5 (50) | 8 (28) | 17 (40) | 0.82 |
| Wheezing n (%) | 30 (41) | 5 (50) | 13 (62) | 12 (28) | 0.03 |
| Stridor n (%) | 10 (13) | 7 (70) | 0 () | 3 (7) | <0.0001 |
|
| |||||
| Other Diagnosis prior to RP Diagnosis | |||||
|
| |||||
| Asthma n (%) | 28 (38) | 5 (50) | 13 (62) | 12 (28) | 0.009 |
| Ear infections n (%) | 9 (12) | 2 (20) | 1 (5) | 6 (15) | 0.36 |
| Sinusitis n (%) | 18 (24) | 4 (40) | 4 (19) | 10 (24) | 0.46 |
N = number; IQR = interquartile range
The prevalence of additional symptoms not included as input variables in the LCA models was compared across the subgroups. Patients with Type 1 R reported a significantly greater prevalence of weight loss [40% vs 0% (Type 2 RP) vs 9% (Type 3 RP); p=0.02] and stridor [70% vs 0% (Type 2 RP) vs 7% (Type 3 RP); p<0.0001]. Patients with Type 2 RP had more genital ulcers [33% vs 0% (Type1 RP) vs 12% (Type 3 RP); p=0.02] and wheezing [62% vs 50% (Type 1 RP) and 28% (Type 2 RP); p=0.03]. While patients with Type 3 RP had significantly more skin involvement [14% vs 0% (Type 2 RP) vs 0% (Type 1 RP); p=0.03]. A prior diagnosis of asthma was significantly more common in the Type 2 RP subgroup [62% vs 50% (Type 1 RP) vs 24% (Type 3 RP); p=0.009]. There were no other significant differences in clinical symptoms between the subgroups (Table 1).
The three subgroups differed based on a few of the dynamic CT and PFT results. Patients with Type 1 RP had a significantly greater prevalence of tracheal wall thickening (90% vs 14% (Type 2 RP) vs 10% (Type 3 RP); p<0.0001) and air trapping (89% vs 67% (Type 2 RP) vs 34% (Type 3 RP); p=0.002) on dynamic CT. Compared to the other subgroups, the Type 1 RP subgroup had significantly decreased median FEF 25–75% predicted (38 vs 88 vs 82; p=0.01) and FEV1/FVC % of predicted (60 vs 76 vs 79; p<0.001).
There were no statistically significant differences between the subgroups at study entry in use of synthetic or biologic DMARD’s, daily prednisone requirement, or inflammatory markers. Microcytic anemia was significantly more common in patients with Type 1 RP [20% vs 0% (Type 2 RP) vs 2% (Type 3 RP); p=0.02] (Table 2).
Table 2.
Differences in Imaging, Pulmonary Function Test, Treatment, and Outcomes Among Three Subgroups of Patients with Relapsing Polychondritis (RP)
| All Patients n=73 | Type 1 RP n=10 | Type 2 RP n=21 | Type 3 RP n=42 | p value | |
|---|---|---|---|---|---|
|
| |||||
| Chest Dynamic CT Scan | |||||
|
| |||||
| Airtrapping n (%) | 35 (51) | 8 (89%) | 14 (67%) | 13 (34%) | 0.002 |
| Tracheal wall >3 mm n (%) | 16 (22) | 9 (90%) | 3 (14%) | 4 (10%) | <0.0001 |
|
| |||||
| Pulmonary Function Test | |||||
|
| |||||
| FVC Pre % (median, IQR) | 91 (83–101) | 94 (58–102) | 90 (81–103) | 91 (85–100.5) | 0.92 |
| FVC L/sec (median, IQR) | 3.3 (2.8–3.8) | 2.9 (1.9–3.2) | 3.3 (2.4–3.8) | 3.4 (3–3.9) | 0.12 |
| FEV1 Pre % (median, IQR) | 85 (75–96) | 76 (26–84) | 83 (67–97) | 89 (81–89) | 0.06 |
| FEV1 L/sec (median, IQR) | 2.4 (2–3) | 1.34 (0.7–2.3) | 2.3 (1.9–2.9) | 2.75 (2.3–3.1) | 0.01 |
| FEF 25–75 %Ref (median, IQR) | 80 (51–94) | 38 (15–66) | 88 (47–92) | 82 (65–97) | 0.01 |
| FEF 25–75 L/sec (median, IQR) | 2.32 (1.49–2.85) | 0.9 (0.4–1.7) | 1.98 (1.4–2.5) | 2.58 (2–3) | 0.01 |
| FEV1/FVC % (median, IQR) | 76 (72–80) | 60 (43–71) | 76 (66–80) | 79 (75–81) | 0.0006 |
| VC% Ref (median, IQR) | 95 (86–107) | 91 (44–102) | 94 (86–111) | 96 (87–107) | 0.55 |
| VC L/sec (median, IQR) | 3.34 (2.86–3.99) | 3 (1.42–3.2) | 3.1 (2.7–3.8) | 3.46 (3.1–1.4) | 0.04 |
| TLC % Ref (median, IQR) | 93 (87–101) | 92 (66–97) | 94 (87–105) | 93 (89–99) | 0.78 |
| TLC L/sec (median, IQR) | 4.8 (4.2–5.4) | 4.7 (4.5–5.3) | 4.7 (4–5.7) | 4.9 (4.2–5.4) | 0.7 |
| RV % Ref (median, IQR) | 74 (61–86) | 75 (51–101) | 82 (66–97) | 74 (61–83) | 0.36 |
| RV L/sec (median, IQR) | 1.36 (1.1–1.5) | 1.5 (1.4–2.7) | 1.4 (1.1–1.6) | 1.2 (0.9–1.5) | 0.06 |
| RV/TLC Ref (median, IQR) | 77 (68–88) | 80 (65–104) | 87 (71–95) | 72 (64–85) | 0.12 |
| DLCO Adj %(median, IQR) | 18 (17–22) | 18 (15–19) | 18 (17–22) | 19 (17–21) | 0.25 |
| DLCO Adj (median, IQR) | 68 (64–74) | 66 (61–70) | 67 (64–80) | 69 (61–74) | 0.61 |
|
| |||||
| Physician Global Assessment | 3 (2–4) | 3 (2–4) | 4 (3–5) | 5 (3.75–6) | 0.0003 |
|
| |||||
| Treatment | |||||
|
| |||||
| Current prednisone dose | 7 (0–20) | 10 (5–20) | 7.5 (0–25) | 5 (0–20) | 0.69 |
| Sythentic DMARD’s n (%) | 49 (67) | 8 (80) | 16 (76) | 25 (59) | 0.25 |
| Biologics n (%) | 32 (43) | 7 (70) | 8 (38) | 17 (40) | 0.19 |
|
| |||||
| Complications | |||||
|
| |||||
| Tracheostomy n (%) | 7 (1) | 5 (50) | 0 (0) | 2 (5) | 0.0003 |
| Disability n (%) | 25 34) | 2 (20) | 12 (57) | 11 (26) | 0.10 |
| ICU admission n (%) | 12 (16) | 5 (50) | 2 (9) | 5 (12) | 0.02 |
|
| |||||
| Inflammartoy Markers | |||||
|
| |||||
| CRP (mg/dL) | 2.4 (0.8–8.6) | 4.7 (0.37–12.5) | 3.9 (1.25–8.1) | 2 (0.77–0.52) | 0.568 |
| ESR (mm/hr) | 11 (6–20) | 13.5 (3.5–54.5) | 9 (6.5–20) | 11.5 (6–19.5) | 0.974 |
N = number; IQR = interquartile range; DMARDs = disease modifying antirheumatic drugs
DISCUSSION
Relapsing polychondritis is a potentially severe, debilitating, and in some cases fatal disease that can be difficult to diagnose due to heterogenous disease manifestations. In this study, three clinical subgroups of RP were identified based on overall patterns of disease using data-driven approaches. Although all of the patients met diagnostic criteria for RP, clinical patterns of disease varied considerably. Only a minority of patients (14%) had a pattern of disease considered classic for RP (Type 1 RP), with ear chondritis and extensive damage to the cartilage of the nose and upper airway. These patients, perhaps not surprisingly, had the fastest time to diagnosis, yet still experienced a diagnostic delay of a median of one year. The other two subgroups of patients were defined by either lower airway-predominant disease (Type 2 RP) or disease in absence of overt cartilage damage (Type 3 RP). Interestingly, patients with predominant lower airway involvement had the lowest prevalence of ear involvement and experienced diagnostic delays of ten years on average. RP can be a devastating condition particularly when patients develop cartilage damage due to untreated inflammation [11–14]. Recognition of a wider range of disease patterns in RP and development of disease-specific assessment tools may facilitate diagnosis and earlier medical intervention which may, in turn, prevent the development of permanent damage. [15]
Findings from this study emphasize that patients with RP have a high burden of serious disease-related complications. Damage to critical cartilaginous structures was common, including tracheomalacia, bronchomalacia, SGS, ear cartilage damage, and saddle nose deformity. Many patients required admission to the ICU or developed disabilities. Despite aggressive treatment with DMARD’s, biologics and glucocorticoids, all the patients had persistently active disease at initial evaluation. Even patients with Type 3 RP without overt cartilage damage reported a high rate of disability and had active disease despite treatment. Both timely intervention and more effective treatments are needed for this condition.
Other investigators have defined subgroups in RP based on clinical patterns of disease. Shimizu et al compared clinical features in 239 patients with RP based on auricular involvement, respiratory involvement, and overlap of auricular and airway involvement [16]. Accordingly, the present study confirms, using unbiased analytic approaches, the presence of a group of patients with RP who have predominant airway disease. Dion et al, retrospectively analyzed 142 patients over a 12-year period. Cluster analysis was used to identify three subsets of patients defined by associated hematologic disease (n=12); respiratory predominant disease (n=37); or mild disease (n=93) [3]. Patients with associated hematologic disease, defined mostly by myelodysplastic syndromes, were older, typically male, and had the most severe phenotype, with a mortality rate of 58% and an ICU admission rate of 50%. In this study, there were a few patients with hematologic abnormalities including anemia and elevated MCV; however, none of these patients met diagnostic criteria for myelodysplastic syndrome. In these patients, cartilaginous involvement was limited to the ear, nose and joints without evidence of damage such as saddle nose deformity or tracheobronchomalacia; therefore they were classified in the Type 3 RP group. Patients with respiratory predominant disease identified in the Dion et al study were younger, had tracheobronchial and laryngeal involvement with abnormal functional respiratory tests, were most likely to receive biologic therapy, and had the highest rate of ICU admissions. Although a group with predominantly lower airway disease (Type 2 RP) was also identified in the current study, these patients were the oldest, with the longest time to diagnosis, suggesting that this subgroup perhaps is the most difficult to recognize. In this study, a higher prevalence of tracheomalacia (43%) was observed compared with Dion et al (22%), potentially because every patient underwent dynamic CT scan of the chest to evaluate for airway involvement per study protocol.
The last subgroup reported by Dion et al was termed as having a “mild phenotype” defined by an absence of severe tracheobronchial involvement or hematologic disease, low mortality, infrequent ICU admissions, and increased likelihood of achieving sustained clinical remission. In the present study a similar group was identified, termed here as Type 3 RP. Although patients in this group lacked evidence of significant cartilage damage, this group did not have “mild disease” as seen by Dion et al. Patients with Type 3 RP had a high frequency of disability (26%), hearing loss (19%), and active disease despite the use of DMARDs in 60% and a maximum daily prednisone of ≥60mg in 45% of these patients. Differences in study design could explain some of the differences between the current results and previous studies. Unlike the study by Dion et al, which was retrospective and subject to assessment bias, patients in the current study were evaluated in a prospective cohort and underwent comprehensive standardized clinical assessment including direct laryngoscopy, dynamic CT imaging of the airways, PFTs, and audiometry testing. Additionally, the present study employed standardized definitions of disease features and a different statistical analytic method than prior studies.
Some potential study limitations should be considered. This study cohort was smaller than prior studies which have attempted to define subgroups within RP; however, RP is a rare disease and this study represents the first prospective cohort study where every patient underwent standardized clinical assessment under a research protocol. No differences in mortality or treatment response was identified between the subgroups, possibly due to the fact that the analysis was done using only data from the initial study visit. Future longitudinal studies from the cohort may be required to identify differences in clinical outcomes among patients with RP. This study was not an inception cohort. It is possible that partitioning strategies using data collected at the time of diagnosis could identify different subgroups of patients and that a patient could change subgroup status over time. Further research is needed to evaluate whether biologic differences underlie this clinical classification scheme.
In conclusion, this study employed latent class analysis to identify three subgroups of patients with RP which differed in clinical and radiographical characteristics of disease, time to diagnosis, and disease-related complications. Future studies should investigate whether subgroups of patients with RP also differ in terms of causal factors such as genetic risk or environmental exposures, immunologic mechanisms of disease, and responses to treatment. Despite differences in the clinical spectrum of disease, patients with RP commonly experience long diagnostic delays, inadequate clinical response to therapy, and substantial morbidity. Findings from this study underscore a tremendous unmet need for clinical advancement in this potentially devastating and under-studied disease.
Supplementary Material
Acknowledgment:
We would like to thank Cade J. Motta and Kelly T. Byrne for creating figure 1.
Funding and Conflict of Interest Disclosure: This research was supported by the Division of Intramural Research of the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Sane DC, Vidaillet HJ Jr, Burton CS Iii: Saddle nose, red ears, and fatal airway collapse.Chest 1987, 91(2):268–270. [DOI] [PubMed] [Google Scholar]
- 2.Dion J, Costedoat-Chalumeau N, Sene D, Cohen-Bittan J, Leroux G, Francés C, Piette JC. Description of 142 cases of relapsing polychondritis followed in a single center since 2000. Arthritis and rheumatism 2013, 65:S868. [Google Scholar]
- 3.Dion J, Costedoat-Chalumeau N, Sene D, Cohen-Bittan J, Leroux G, Dion C, et al. Relapsing Polychondritis Can Be Characterized by Three Different Clinical Phenotypes: Analysis of a Recent Series of 142 Patients. Arthritis Rheumatol 2016, 68(12):2992–3001. [DOI] [PubMed] [Google Scholar]
- 4.Ferrada MA, Grayson PC, Banerjee S, Sikora K, Colbert R, Sinaii N, et al. Patient Perception of Disease-Related Symptoms and Complications in Relapsing Polychondritis. Arthritis Care Res (Hoboken) 2018, 70(8):1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michet CJ Jr, McKenna CH, Luthra HS, O’Fallon WM. Relapsing polychondritis: Survival and predictive role of early disease manifestations. Annals of internal medicine 1986, 104(1):74–78. [DOI] [PubMed] [Google Scholar]
- 6.Winstanley S, Boyde A, Attanoos R. Fatal relapsing tracheobronchial polychondritis diagnosed at autopsy. BMJ Case Rep 2015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damiani JM, Levine HL. Relapsing polychondritis--report of ten cases. Laryngoscope 1979, 89(6 Pt 1):929–946. [PubMed] [Google Scholar]
- 8.McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 1976, 55(3):193–215. [PubMed] [Google Scholar]
- 9.Quinn KA, Gelbard A, Sibley C, Sirajuddin A, Ferrada MA, et al. Subglottic stenosis and endobronchial disease in granulomatosis with polyangiitis. Rheumatology (Oxford) 2019, 48(12):2203–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence DA, Branson B, Oliva I, Rubinowitz A. The wonderful world of the windpipe: a review of central airway anatomy and pathology. Can Assoc Radiol J 2015, 66(1):30–43. [DOI] [PubMed] [Google Scholar]
- 11.Bollet AJ, Smith JG, Mushet GR, Austin F. Arthritis, deafness, and saddle nose(relapsing polychondritis). Medical Grand Rounds Medical College of Georgia. J Med Assoc Ga 1969, 58(7):315–319. [PubMed] [Google Scholar]
- 12.Cansiz H, Yilmaz S, Duman C. Relapsing polychondritis: a case with subglottic stenosis and laryngotracheal reconstruction. J Otolaryngol 2007, 36(6):E82–84. [PubMed] [Google Scholar]
- 13.Lee CC, Singer AJ. Respiratory failure due to subglottic stenosis from relapsing polychondritis. Am J Emerg Med 2006, 24(6):750–752. [DOI] [PubMed] [Google Scholar]
- 14.Mezghani Ben Salah S, Harzallah MF, Marouen F, Aissa S, Hayouni A, Khlifa M. et al. Tracheomalacia bronchomalacia: a severe complication of relapsing polychondritis. Tunis Med 2013, 91(1):84–85. [PubMed] [Google Scholar]
- 15.Sharma A, Kumar R, Mb A, Naidu G, Sharma V, Sood A, Dhir V, Verma R. et al. Fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis, assessment of disease activity and therapeutic response in relapsing polychondritis. Rheumatology (Oxford) 2020, 59(1):99–106. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu J, Yamano Y, Kawahata K, Suzuki N. Relapsing polychondritis patients were divided into three subgroups: patients with respiratory involvement (R subgroup), patients with auricular involvement (A subgroup), and overlapping patients with both involvements (O subgroup), and each group had distinctive clinical characteristics. Medicine (Baltimore) 2018, 97(42):e12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.