Abstract
Background
Although social distancing is a key public health response during viral pandemics, psychosocial stressors, such as social isolation, have been implicated in adverse health outcomes in general [1] and in the context of infectious disease, such as human immunodeficiency virus (HIV) [2, 3]. A comprehensive understanding of the direct pathophysiologic effects of psychosocial stress on viral pathogenesis is needed to provide strategic and comprehensive care to patients with viral infection.
Methods
To determine the effect of psychosocial stress on HIV pathogenesis during acute viral infection without sociobehavioral confounders inherent in human cohorts, we compared commonly measured parameters of HIV progression between singly (n = 35) and socially (n = 41) housed simian immunodeficiency virus (SIV)-infected pigtailed macaques (Macaca nemestrina).
Results
Singly housed macaques had a higher viral load in the plasma and cerebrospinal fluid and demonstrated greater CD4 T-cell declines and more CD4 and CD8 T-cell activation compared with socially housed macaques throughout acute SIV infection.
Conclusions
These data demonstrate that psychosocial stress directly impacts the pathogenesis of acute SIV infection and imply that it may act as an integral variable in the progression of HIV infection and potentially of other viral infections.
Keywords: animal models, HIV, macaques, psychosocial stress, SIV
This study demonstrates that psychosocial stress directly impacts the pathogenesis of acute SIV infection and implies that psychosocial stress may act as an integral variable in the progression of HIV infection and potentially of other viral infections.
Stress is broadly defined as an individual’s physical and psychological responses to changes in the physical and social environments known as “stressors.” The idea of psychosocial stress comes from the understanding that the effect of social stressors can be mediated by the psychological understanding and processing of the social stressor [4–6]. Loneliness, social disruption, and social integration are examples of psychosocial stressors. Observational studies have demonstrated that psychosocial stress has a measurable impact on general health outcomes [1]. Today, psychosocial stress, particularly that caused by reduced social contact, has taken on greater societal significance, with social distancing practices vital to slow the severe acute respiratory syndrome coronavirus 2 pandemic [7], but how psychosocial stress impacts the body’s responses to pathogens, including viral infections, remains incompletely understood. Although data are currently unavailable for analysis in the SARS CoV-2 2019 pandemic, other viral pandemics, such as human immunodeficiency virus (HIV), may provide insight. Human immunodeficiency virus infection remains widespread and outcomes for people with HIV (PWH) vary greatly and depend upon a number of factors, including psychosocial stressors. People with HIV are at higher risk for psychosocial stress due to stigma associated with infection and marginalization of people from demographics that are considered at higher infection risk [8]. In particular, PWH experience societal stigma more often compared with the general population [9], and this isolation has been associated with poor health outcomes and more rapid disease progression in PWH [10–12].
Determination of the direct physiologic effects of psychosocial stressors on viral pathogenesis is complicated by its multifaceted nature and by sociobehavioral confounders that potentially limit access to effective care or otherwise compromise health. The present study aims to determine whether single housing, as a psychosocial stressor, directly affects simian immunodeficiency virus (SIV) pathogenesis by comparing how single housing affects the progression of acute SIV infection in pigtailed macaques (Macaca nemestrina) in a retrospective analysis. Simian immunodeficiency virus infection in macaques recapitulates many aspects of HIV pathogenesis, including decline in circulating CD4 T cells after infection and persistence of virus in latent reservoirs despite antiretroviral therapy (ART) [13]. We hypothesized that acute SIV infection would have a greater impact on macaques housed individually before and after viral inoculation compared to macaques socially housed. We demonstrate that housing macaques individually affects SIV viral load, CD4 T-cell decline, and T-cell activation during acute infection. Psychosocial stress thus directly influences disease progression in a SIV-infected macaque model of HIV, and psychosocial stressors are likely to have direct pathophysiologic impact on health outcomes of PWH and on the pathogenesis of other viral infections.
METHODS
Animals
Seventy-six juvenile male pigtailed macaques (Macaca nemestrina) were inoculated intravenously with the same stock of SIV inoculum containing neurovirulent clone SIV/17E-Fr and immunosuppressive swarm SIV/DeltaB670 as previously described; this SIV infection protocol leads to high levels of viremia in plasma and cerebral spinal fluid (CSF) and progression to acquired immune deficiency syndrome (AIDS)-defining criteria in 3 months [14]. Plasma and CSF viral loads were assessed across all years included in this analysis, and no changes to indicate a loss in virulence of the viral stock over time were found (P > .1, Spearman correlation) (Supplementary Figure S3). A summary of the covariates controlled for in this analysis can be found in Supplementary Table S1. All macaques were seronegative for SIV, simian T-cell leukemia virus, and simian type D retrovirus before study. All macaques tested negative for the major histocompatibility complex class I allele, Mane-A1*08401. Macaques were sedated intramuscularly with 10 mg/kg ketamine at 3 preinoculation time points that occurred at least 2 weeks apart and on days 7, 10, and 14 postinoculation to facilitate blood and CSF collection. On day 12, the majority of macaques started on ART (Supplementary Table S2); any animals that did not begin an ART intervention were excluded from the day 14 postinoculation analysis. Samples from each animal group were obtained at the 6 distinct pre- and postinoculation time points. The data for this analysis represent the combined data set of all the singly housed animal groups at each time point compared with the socially housed animal groups at each time point. Thirty-five macaques were singly housed upon assignment to study. These animals remained separated from direct physical contact with conspecifics before inoculation (approximately 2-month duration) and throughout the course of the study. All singly housed macaques were able to see, hear, and smell conspecifics in the same room although they did not share a cage with a conspecific. Forty-one macaques were socially housed (full contact in compatible pairs or trios) upon study assignment for at least 2 months before inoculation, including all preinoculation time points, and remained with their conspecific(s) after inoculation. The change from housing macaques singly to housing macaques in social groups occurred institutionally in response to the 2011 Guide for the Care and Use of Laboratory Animals emphasis that single housing should be the exception for macaques. Grouping of individuals was completed under oversight of an animal behaviorist to ensure social compatibility and reevaluated periodically throughout the study; no socially housed animals showed signs of incompatibility or had to be separated during the course of collecting these data. These data represent a retrospective analysis of a total of 18 studies conducted over a 10-year period, with the 9 studies involving the singly housed macaques occurring during the first 5-year period, and the 9 studies involving the socially housed macaques occurring during the second 5-year period (Supplementary Table S2). The number of animals from which data was used for this analysis for each parameter is listed in Supplementary Table S3. All procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee and conducted in accordance with guidelines in the Animal Welfare Regulations and the Guide for the Care and Use of Laboratory Animals. All animals consumed commercial macaque chow (Purina 5038) and water ad libitum throughout the study, and all macaques were provided daily enrichment by a behaviorist. These studies were conducted within a fully AAALAC-accredited facility.
Quantification of Simian Immunodeficiency Virus Viral Load
Viral ribonucleic acid (RNA) was isolated from plasma and CSF using the QuantiTech Virus Kit (QIAGEN). Viral loads were quantified by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) using primers in the SIV gag region: forward 5’-GTCTGCGTCATCTGGTGCATTC-3′; reverse 5’-CACTAGGTGTCTCTGCACTATCTGTTTTG-3′; 5’-FAM/3’-Black hole-labeled probe 5’-CTTCCTCAGTGTGTTTCACTTTCTCTTCTG-3′. All viral RNA extractions and PCR reactions over the 10-year period were prepared by 1 specific, skilled technician within the laboratory.
Complete Blood Count and Flow Cytometry
Citrated whole blood samples were used for complete blood count (CBC), which were completed on site using a Hemavet (Drew Scientific) hematology analyzer or a Procyte Dx Hematology Analyzer (IDEXX Laboratories) (Supplementary Table S2). Samples were excluded at select time points from this analysis if clotting was noted upon CBC analysis. To assess the lymphocyte composition of macaque whole blood, samples were stained with fluorochrome-coupled CD3, CD4, CD8a, and CD69 antibodies for 20 minutes at room temperature and fixed for 10 minutes with BD FACS Lysing Solution (Becton Dickinson, Franklin Lakes, NJ). The samples from all of the socially housed animals and 17 of the singly housed animals were stained using antibodies listed in panel A of Supplementary Table S4; the remaining 18 singly housed animals were stained using antibodies in panel B of Supplementary Table S4. The stained whole blood samples were analyzed by a single skilled technician using a BD LSRFortessa (panel A) or FACSCalibur (panel B) cytometer (Supplementary Table S2). The FACS data files were re-analyzed in FlowJo version 10 by another single blinded technician at the time of this retrospective analysis (Supplementary Figures S4 and S5). To calculate the circulating number of CD4 T cells, the percentage of CD3+CD4+ cells identified by FACS were multiplied by the total number of circulating lymphocytes quantified by CBC. Similarly, the circulating number of CD8 T cells were calculated by multiplying the percentage of CD3+CD8+ cells by the total number of circulating lymphocytes.
Data Management and Statistical Analysis
All retrospective macaque data were stored in a custom database built in FileMaker Pro 18 Advanced (Claris International Inc., Santa Clara, CA). A series of mixed-effects regression models were conducted in SAS PROC MIXED (version 9.4; SAS Institute Inc., Cary, NC) to examine whether social housing status affects acute SIV infection (Supplementary Table S5). On day 12, the majority of macaques started antiretroviral therapy (Supplementary Table S2); 15 animals from the singly housed group did not begin ART intervention on day 12 and thus they were excluded from the day 14 postinoculation analysis. The number of animals for each dataset in this study is detailed in the legends for each figure and in Supplementary Table S3. For some animals, data were unavailable due to sampling considerations during the time of the original study. The distributions of data for each parameter were examined, and those that were not normally distributed were log transformed before analysis. Mixed-effects regression models were used to accommodate repeated laboratory measurements across time that were correlated to different degrees. In addition, these models account for the fact that different animals were nested in different studies. The full SAS code used for this analysis is available upon request. Significance was set at P < .05 for a priori analyses, and for exploratory analyses a false discovery rate correction was used to control for multiple comparisons. Comparison of the aggregated standard deviation values for all variables included in this analysis (Figure 3) was done using a 2-tailed Wilcoxon matched-pairs signed-rank test with significance set at P < .05. Graphs were constructed using GraphPad Prism (version 8.3.0 for Windows; GraphPad Software, San Diego, CA [www.graphpad.com]). All data, including deidentified raw data on all parameters, are available upon request.
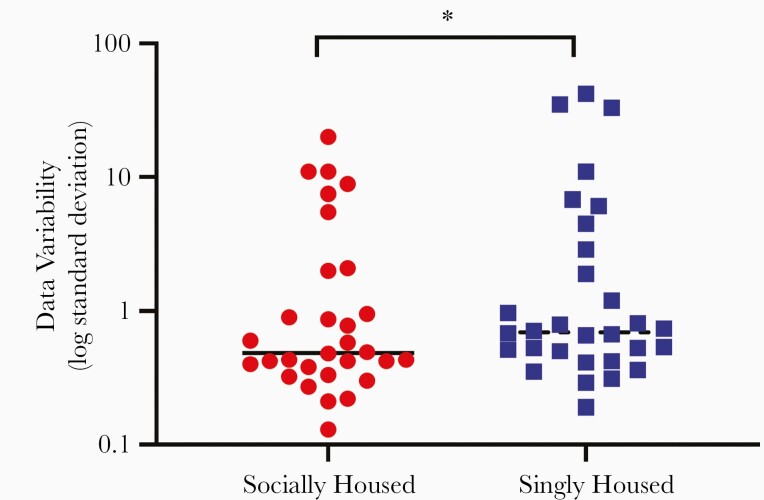
Figure 3.
Psychosocial stress increases the variability of data produced by the simian immunodeficiency virus (SIV)-infected macaque model during acute infection. The standard deviations for all time points (preinoculation, days 7, 10, and 14 post-SIV infection) of all data sets (plasma and cerebrospinal fluid viral load, CD4 T cell, CD8 T-cell, and lymphocyte numbers, CD4/CD8 ratio, and the percentage of CD69+CD8 T cells and the percentage of CD69+CD4 T cells; N = 30 parameters) for both the singly and socially housed macaque groups were compared in a pairwise fashion using a 2-tailed Wilcoxon matched-pairs signed-rank test. *, P = .02.
RESULTS
Psychosocial Stress Results in Elevated Viral Loads and Greater CD4+ T-Cell Decline During Acute Simian Immunodeficiency Virus Infection
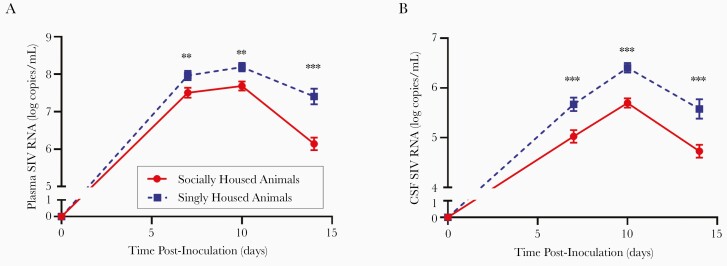
Viral load and CD4+ T-cell decline are 2 of the most frequent parameters used to assess clinical progression in PWH. To determine whether psychosocial stress directly influences progression of HIV, we used linear mixed-effects regression models to conduct a retrospective analysis of data generated throughout acute infection (days 7, 10, and 14 postinoculation) by singly housed (n = 35) compared with socially housed (n = 41) SIV-infected macaques. We assessed the SIV viral load in the plasma and CSF using qRT-PCR to detect SIV gag. Singly housed macaques had higher plasma viral loads than socially housed counterparts throughout acute infection (by 6.1%, 6.5%, and 21% on days 7, 10, and 14, respectively; P < .0001) (Figure 1A). Singly housed macaques also had higher viral loads in the CSF than socially housed macaques (13%, 12%, and 18% difference at the same time points; P < .0001) (Figure 1B). The magnitude of the difference in viral loads between the singly and socially housed macaques compounded over time in the plasma (P = .0007) but not the CSF.
Figure 1.
Psychosocial stress increases the plasma and cerebrospinal fluid (CSF) viral load in acute simian immunodeficiency virus (SIV) infection. Simian immunodeficiency virus viral load in plasma (A) and CSF (B) were quantified utilizing quantitative reverse-transcriptase polymerase chain reaction. Linear-mixed effects regression model. Statistics depicted in figure represent significance of difference between singly and socially housed animals at each time point. *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .001. Circles connected by a solid line (red) indicate socially housed animals (N = 41 for all time points), whereas squares connected by a dashed line (blue) represent singly housed animals (N = 35 for the preinoculation and days 7 and 10 postinoculation; N = 17 for plasma viral load and N = 14 for CSF viral load for day 14 postinoculation). Error bars represent the standard error of the least square mean estimates generated by the linear mixed-effects regression model. Antiretroviral therapy initiated on day 12 postinoculation.
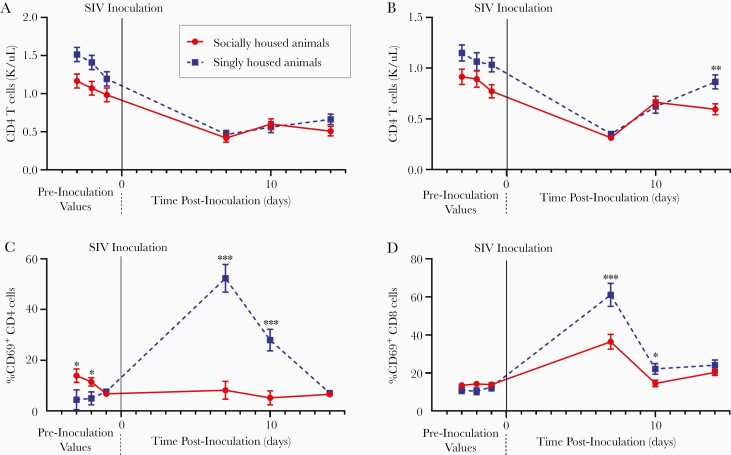
We used flow cytometry coupled with CBCs to examine absolute T-cell numbers in these animals. As expected, all macaques demonstrated CD4 T-cell decline upon infection (P < .0001) (Figure 2A). Singly housed pigtailed macaques had a 38.5% greater decline after SIV inoculation compared with socially housed animals (P = .0001) (Figure 2A). In addition, singly housed animals had a 34.1% greater decline in CD8 T cells (P = .0002) (Figure 2B) and a 44.4% greater decline in total circulating lymphocytes (P < .0001) (Supplementary Figure S1A). These differential declines originated preinoculation, during which CD4 T-cell numbers were 27.8% higher (P = .007) (Figure 2A), CD8 T cells were 25.9% higher (P = .0006) (Figure 2B), and total lymphocytes were 28% higher (P = .0004) (Supplementary Figure S1A) in singly housed compared with socially housed animals. A lower CD4 to CD8 T-cell ratio is associated with negative outcomes in PWH [15], and, as expected, infection led to lower CD4/CD8 ratios in all animals (P = .0004). However, the CD4/CD8 ratio was 18% lower in singly housed animals at day 14 after inoculation compared with socially housed animals (P = .04) (Supplementary Figure S1B) .
Figure 2.
Psychosocial stress increases the immune impact of acute simian immunodeficiency virus (SIV) infection. CD4 T cells (A) and CD8 T cells (B) in the peripheral blood of macaques pre- and postinoculation with SIV. Percentage of CD4 T cells expressing CD69 (C), and percentage of CD8 T cells expressing CD69 (D). Linear mixed-effects regression model. Statistics depicted in figure represent significance of difference between singly and socially housed animals at each time point. *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .001. Circles connected by a solid line (red) indicate socially housed animals, whereas squares connected by a dashed line (blue) represent singly housed animals. Solid vertical line at day 0 indicates time point of SIV inoculation. Error bars represent the standard error of the least square mean estimates generated by the linear mixed-effects regression model. Antiretroviral therapy initiated on day 12 postinoculation. CD4 T cells and CD8 T cells (1) social housed group N = 35 at preinoculation time point 1, N = 40 at preinoculation time point 3 and N = 41 at preinoculation time point 2 and all days postinoculation; (2) single housed group N = 34 at all preinoculation time points, N = 35 at day 7, N = 33 at day 10, and N = 17 at day 14 postinoculation. %CD69 (CD8 T cells) (1) social housed group N = 35 at preinoculation time point 1, N = 40 at preinoculation time point 3 and N = 41 at preinoculation time point 2 and all days postinoculation; (2) single housed group N = 17 at all preinoculation time points and at day 7 postinoculation, N = 14 at day 10 postinoculation and N = 11 at day 14 postinoculation. %CD69 (CD4 T cells) (1) social housed group N = 35 at preinoculation time point 1, N = 40 at preinoculation time point 3 and N = 41 at preinoculation time point 2 and all days postinoculation; (2) single housed group N = 17 at all preinoculation time points, days 7 and 10 postinoculation and N = 11 at day 14 postinoculation.
Psychosocial Stress Alters the Immune Response During Acute Simian Immunodeficiency Virus Infection
To further determine the effect of psychosocial stress on the immune response to SIV infection, we examined CD4 and CD8 T-cell activation. Activated CD4 T cells are susceptible to infection with HIV and are key producers of virus during acute infection [16, 17]. Singly housed macaques had 9.2-fold more CD69+CD4 T cells at peak activation in acute infection compared with preinoculation values (P < .0001) (Figure 2C), whereas socially housed animals demonstrated negligible activation. CD8 T cells are essential for viral control, and CD8 T-cell activation is an expected sequelae of acute HIV infection [18, 19]. All macaques had heightened CD8 T-cell activation during acute infection, demonstrated by increased percentage of cells expressing CD69, with peak activation on day 7 (P = .001) (Figure 2D). However, singly housed animals had 68% more CD69+CD8 T cells at peak activation compared with socially housed (P = .0007).
Psychosocial Stress Increases Variability of the Data Produced by Simian Immunodeficiency Virus-Infected Macaques
Reduction of exogenous stress through introduction of stress-reducing refinements has been shown to reduce variability of data in other macaque models [20]. To evaluate whether psychosocial stress similarly impacts data variability in this SIV-infected macaque model, we compared standard deviations of all data reported herein between socially and singly housed animals. Data produced by singly housed animals were overall more variable than data from socially housed animals (P = .02) (Figure 3 and Supplementary Figure S2).
DISCUSSION
Our data demonstrate that reducing psychosocial stress by providing social housing with compatible conspecifics lessens the immune impact of acute SIV infection in a pigtailed macaque model of HIV infection, resulting in lower plasma and CSF viral loads, less marked decline in circulating CD4 T cells, and reduction in CD4 and CD8 T-cell activation. This effect is observed while all other aspects of infection and care remained consistent between these groups (Supplementary Table S1), effectively removing the sociobehavioral confounders that affect access to care and adherence to treatment common in PWH. Thus, this study provides evidence that there is a direct pathophysiologic effect of psychosocial stress on the pathogenesis of SIV infection and implies that psychosocial stressors may have implications for the pathogenesis of other viral infections.
Single housing of macaques in the laboratory setting does not fully recapitulate the complexity of psychosocial stress in general human or macaque populations. One of the hallmarks of psychosocial stressors is psychological understanding and processing of a social stressor, the implications of which may be enhanced or mitigated based on internal factors of the individual perceiving the psychosocial stressor and their social environment [4]. Due to the multitude of factors that can cause psychosocial stress, much of the related literature focuses on a specific aspect of an individual’s environment, such as the level and perceived quality of social contact. Many psychosocial stressors in human society cannot be captured in a model system in the laboratory, such as stigma and socioeconomic status; however, changes to the housing of social species such as macaques can serve as a model of psychosocial stress in general, allowing for study of the mechanisms underlying the pathophysiologic impact of these stressors.
This analysis has implications beyond primary infection for PWH. Latent reservoirs established during acute infection remain the major barrier to cure [21, 22]. Our findings of higher viral loads in plasma and CSF in the context of psychosocial stress have potential long-term implications for PWH, because elevated plasma viral load early in infection in both PWH [23, 24] and SIV-infected rhesus macaques [25, 26] is associated with accelerated clinical course of disease progression. These higher viral loads may ultimately originate from the increased CD4 activation that we observed in singly housed animals, because activated CD4 T cells serve as permissive cells for virus infection and replication, thus potentiating viral spread and reservoir seeding [17]. It is interesting that negligible change after infection was observed in the percentage of CD4 T cells expressing CD69 in the socially housed group, whereas activation was noted in singly housed animals. CD69 is an early marker of T-cell activation and is transient compared with other markers of activation [27]; it is possible that CD4 T cells from socially housed animals had short-term increase in CD69 expression before day 7 postinoculation that was not captured by the sample collection schedule. The CD4/CD8 ratio was lower late in acute infection and CD4 decline greater in singly housed compared with socially housed animals. Lower CD4/CD8 ratio predictive for non-AIDS related morbidity and mortality in PWH [15], whereas low CD4 counts are a prognostic indicator of disease progression throughout infection [28]. Our observations in the context of psychosocial stress, while controlling for many other variables that could impact infection progression, provide important evidence that psychosocial stress may have a direct adverse effect on the immune response to HIV and, ultimately, viral production, establishment of latent reservoirs, and disease outcomes.
Due to the retrospective nature of our analysis, we did not evaluate the mechanism underlying the effect of psychosocial stress on SIV pathogenesis, yet work on the biology of stress responses of people with and without HIV provides insight. In uninfected people, chronic stress dysregulates immune function [29], leading to reduction in virus-specific T-cell activity [30]. Social isolation is associated with overexpression of proinflammatory genes concurrent with reduced expression of those involved in innate antiviral resistance through type I interferon responses [31], directly affecting immune response and viral control. Compounding these effects, PWH generally have increased plasma levels of the immunosuppressive glucocorticoid cortisol compared with uninfected controls [32, 33], potentially mediated by inflammatory cytokines interleukin-1 and HIV gp120 [34]. Clinical studies demonstrate that glucocorticoid receptors of PWH have decreased affinity for cortisol, resulting in glucocorticoid insufficiency despite increased circulating cortisol that is not fully attenuated through viral suppression with ART [35]. However, the findings from studies on the effect of social support on PWH have been equivocal, and any impact of psychosocial stress on disease progression and health outcomes in PWH is compounded by the fact that social isolation is more prevalent in PWH compared with uninfected controls [10]. In sum, psychosocial stressors affect immune function and HIV progression, and robust controlled studies are needed to better understand the pathophysiologic mechanisms underpinning these effects on PWH.
These data also have implications for the translational value and reproducibility of HIV research with macaque models. Macaque models are instrumental in achieving scientific advancement for treatment of PWH and continue to be at the forefront of discovery in HIV and in other infectious virus research [36–41]. However, an animal model’s value is only as great as the translational value of its data, and how we keep animals in research settings can impact translational value and reproducibility of generated data [42]. Excessive psychosocial stress as a result of housing conditions such as single housing has the potential to reduce the translational value of animal models. Macaques are social animals, and housing macaques individually results in psychosocial stress. Otherwise healthy macaques relocated from social to single housing show modulated immune responses for weeks to months thereafter, as demonstrated by decreases in total lymphocytes, CD8+ T cells, and CD4+ T cells [43] and by lower CD4/CD8 ratios and less robust proinflammatory cytokine responses [44, 45]. The mechanisms underlying the full impact of social environment on macaque immunological parameters is incompletely understood. Howver, because macaques are naturally social animals, the social context of macaques in biomedical research is an important area for future study due to its potential to impact the validity of data produced by the animals [46]. Such changes to the immune system driven by psychosocial stress may confound infectious disease research, including vaccine studies and trials [47]. Social instability, another psychosocial stressor, has previously been shown to be associated with increased risk of mortality from SIV infection in untreated macaques [48, 49]. Our data complement existing literature by demonstrating differences in immune response and viral loads between singly and socially housed SIV-infected macaques. Although we cannot directly demonstrate improvements in translation and reproducibility through this work, the magnitude of the decline in CD4 T-cell counts that we observed in socially housed macaques during acute infection (2-fold) is more analogous to that seen in humans during primary HIV infection [50] than the 3-fold change observed in singly housed macaques. Furthermore, decrease in variability of the data generated by our socially housed macaques bodes favorably for study reproducibility. Social housing of macaques is therefore an important refinement that may improve translational value and reproducibility of data obtained from macaque models of HIV infection, and caution must be taken when comparing the data from studies completed under different housing conditions.
CONCLUSIONS
We have demonstrated that psychosocial stress from single housing of macaques has direct impact on pathogenesis of acute SIV infection, leading to elevated peripheral and central nervous system viral loads, greater decline in circulating CD4 T cells, lower CD4/CD8 ratio, and increased T-cell activation. These data indicate that social support for PWH may have a direct positive impact on disease progression beyond the positive psychobehavioral effects and practical benefits, and they provide evidence supporting a direct pathophysiologic effect of social support on outcomes for PWH. Further research is needed to define the implications of psychosocial stressors on the pathogenesis of other viral infections. These data should furthermore be considered as evidence for the direct effect that social housing has on this vital animal model of HIV infection, because refinements such as social housing have the potential to improve reproducibility and translational value of biomedical research conducted with animal models.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the members of the Retrovirus Laboratory and Research Animal Resources at Johns Hopkins University, past and present, for their support, especially Samuel Brill, Alisa McNamara, Claire Lyons, Nadine Forbes, Rock Scarborough, Natalie Green, Coffy Bennis, Sara Flemming, and Eric Hutchinson. We acknowledge the generous antiretroviral therapy donation from Abbvie (Abbott), Bristol-Myers Squibb, Gilead, Merck, Janssen Pharmaceuticals, Roche, and ViiV Healthcare.
Author contributions. S. M. G.-M. and K. A. M. P. designed this retrospective study, planned, and oversaw all analyses, and wrote the manuscript. L. H. R. planned analyses, generated linear mixed model, and performed statistics. S. M. G.-M., L. H. R., E. N. S., S. E. Q., B. W. C., R. J. A., L. G., J. E. C., J. L. M., and K. A. M. P. provided input into the experimental design. S. M. G.-M., K. M. M., B. W. C., E. N. S., and S. E. Q. organized and analyzed data. E. N. S., S. E. Q., M. L., B. B., K. A. M. P., R. J. A., L. G., D. R. G., C. Z., J. E. C., and J. L. M. performed and analyzed the original studies that generated the data analyzed in this retrospective analysis. L. G., D. R. G., C. Z., J. E. C., and J. L. M. designed the original studies and procured resources for them.
Financial support. Funding for this project was provided by Grants for Laboratory Animal Science (GLAS to K. A. M. P.) and for the projects included in the retrospective analysis (to R. J. A., L. G., D. R. G., J. E. C., J. L. M., and K. A. M. P.) by the National Institutes of Health (P30 AI094189, P01 AI131306, K01 OD018244, T32 OD011089, RR00116, P40 OD013117/U42 OD013117, R01 NS089482, NS097221, NS055651, MH61189, MH070306, NS36911, and RR019995), Johns Hopkins Medicine Brain Science Institute (BSI), and the Blaustein Pain Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: American Association of Laboratory Animal Science National Symposium, October 2019, Denver, CO; JHU NIMH Center Symposium, October 2019, Baltimore, MD; AbbVie International Reproducibility Roundtable webinar series, September 2019, Online webinar, available internationally; American Society of Primatologists, August 2019, Madison, WI; Conference on Retroviruses and Opportunistic Infections, March 2019, Seattle, WA; National Center for AIDS Research Scientific Symposium, November 2018, Atlanta, GA; American Association of Laboratory Animal Science National Symposium, August 2018, Baltimore, MD; American Society of Primatologists Meeting, August 2018, San Antonio, TX; Symposium on Social Housing of Laboratory Animals, June 2018, Beltsville, MD; Center for Alternatives to Animal testing Symposium on Refinement, November 2017, Baltimore, MD.
References
- 1. Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol 2009; 60:501–24. [DOI] [PubMed] [Google Scholar]
- 2. Perez IR, Baño JR, Ruz MAL, et al. Health-related quality of life of patients with HIV: impact of sociodemographic, clinical and psychosocial factors. Qual Life Res 2005; 14:1301–10. [DOI] [PubMed] [Google Scholar]
- 3. Engelhard EAN, Smit C, van Dijk PR, et al. Health-related quality of life of people with HIV: an assessment of patient related factors and comparison with other chronic diseases. AIDS 2018; 32:103–12. [DOI] [PubMed] [Google Scholar]
- 4. Cruces J, Venero C, Pereda-Pérez I, De la Fuente M. The effect of psychological stress and social isolation on neuroimmunoendocrine communication. Curr Pharm Des 2014; 20:4608–28. [DOI] [PubMed] [Google Scholar]
- 5. Upton J. Psychosocial Factors. In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York, NY: Springer New York, 2013: pp 1580–81. [Google Scholar]
- 6. Kogler L, Müller VI, Chang A, et al. Psychosocial versus physiological stress - Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage 2015; 119:235–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajkumar RP. COVID-19 and mental health: a review of the existing literature. Asian J Psychiatr 2020; 52:102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the U.S. HIV epidemic. Am Psychol 2013; 68:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Audet CM, McGowan CC, Wallston KA, Kipp AM. Relationship between HIV stigma and self-isolation among people living with HIV in Tennessee. PLoS One 2013; 8:e69564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greysen SR, Horwitz LI, Covinsky KE, Gordon K, Ohl ME, Justice AC. Does social isolation predict hospitalization and mortality among HIV+ and uninfected older veterans? J Am Geriatr Soc 2013; 61:1456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ellis RJ, Iudicello J, Sun-Suslow N, et al. Social isolation is linked to inflammation in aging people with HIV and uninfected individuals. J Acquir Immune Defic Syndr 2021; 86:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marziali ME, McLinden T, Card KG, et al. Social isolation and mortality among people living with HIV in British Columbia, Canada. AIDS Behav 2021; 25:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deere JD, Schinazi RF, North TW. Simian immunodeficiency virus macaque models of HIV latency. Curr Opin HIV AIDS 2011; 6:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol 2008; 14:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 2014; 9:e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 1990; 61:213–22. [DOI] [PubMed] [Google Scholar]
- 17. Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J 1990; 9:1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 1994; 68:6103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McBrien JB, Kumar NA, Silvestri G. Mechanisms of CD8+ T cell-mediated suppression of HIV/SIV replication. Eur J Immunol 2018; 48:898–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graham ML, Rieke EF, Mutch LA, et al. Successful implementation of cooperative handling eliminates the need for restraint in a complex non-human primate disease model. J Med Primatol 2012; 41:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Queen SE, Mears BM, Kelly KM, et al. Replication-competent simian immunodeficiency virus (SIV) Gag escape mutations archived in latent reservoirs during antiretroviral treatment of SIV-infected macaques. J Virol 2011; 85:9167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siliciano JD, Siliciano RF. Recent developments in the search for a cure for HIV-1 infection: targeting the latent reservoir for HIV-1. J Allergy Clin Immunol 2014; 134:12–9. [DOI] [PubMed] [Google Scholar]
- 23. O’Brien TR, Blattner WA, Waters D, et al. Serum HIV-1 RNA levels and time to development of AIDS in the multicenter hemophilia cohort study. JAMA 1996; 276:105–10. [PubMed] [Google Scholar]
- 24. Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272:1167–70. [DOI] [PubMed] [Google Scholar]
- 25. Watson A, Ranchalis J, Travis B, et al. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol 1997; 71:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lifson JD, Nowak MA, Goldstein S, et al. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol 1997; 71:9508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cibrián D, Sánchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol 2017; 47:946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev 2013; 254:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol 2014; 26:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonneau RH, Sheridan JF, Feng NG, Glaser R. Stress-induced suppression of herpes simplex virus (HSV)-specific cytotoxic T lymphocyte and natural killer cell activity and enhancement of acute pathogenesis following local HSV infection. Brain Behav Immun 1991; 5:170–92. [DOI] [PubMed] [Google Scholar]
- 31. Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol 2007; 8:R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Membreno L, Irony I, Dere W, Klein R, Biglieri EG, Cobb E. Adrenocortical function in acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1987; 65:482–7. [DOI] [PubMed] [Google Scholar]
- 33. Chrousos GP, Zapanti ED. Hypothalamic-pituitary-adrenal axis in HIV infection and disease. Endocrinol Metab Clin North Am 2014; 43:791–806. [DOI] [PubMed] [Google Scholar]
- 34. Costa A, Nappi RE, Polatti F, Poma A, Grossman AB, Nappi G. Stimulating effect of HIV-1 coat protein gp120 on corticotropin-releasing hormone and arginine vasopressin in the rat hypothalamus: involvement of nitric oxide. Exp Neurol 2000; 166:376–84. [DOI] [PubMed] [Google Scholar]
- 35. Valdez AN, Rubin LH, Neigh GN. Untangling the Gordian knot of HIV, stress, and cognitive impairment. Neurobiol Stress 2016; 4:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abreu CM, Veenhuis RT, Avalos CR, et al. Infectious virus persists in CD4+ T cells and macrophages in antiretroviral therapy-suppressed simian immunodeficiency virus-infected macaques. J Virol 2019; 93:e00065–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moldt B, Le KM, Carnathan DG, et al. Neutralizing antibody affords comparable protection against vaginal and rectal simian/human immunodeficiency virus challenge in macaques. AIDS 2016; 30:1543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018; 392:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okoye AA, Hansen SG, Vaidya M, et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat Med 2018; 24:1430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huot N, Rascle P, Müller-Trutwin M. [Contribution of animal models to HIV research]. Virologie (Montrouge) 2019; 23:229–40. [DOI] [PubMed] [Google Scholar]
- 41. Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020; 368:1012–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grimm D. Are happy lab animals better for science? Science 2018; doi: 10.1126/science.aat2326 [DOI] [Google Scholar]
- 43. Gordon TP, Gust DA, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Social separation and reunion affects immune system in juvenile rhesus monkeys. Physiol Behav 1992; 51:467–72. [DOI] [PubMed] [Google Scholar]
- 44. Benton CG, West MW, Hall SM, Marko ST, Johnson JC. Effect of short-term pair housing of juvenile rhesus macaques (Macaca mulatta) on immunologic parameters. J Am Assoc Lab Anim Sci 2013; 52:240–6. [PMC free article] [PubMed] [Google Scholar]
- 45. Gust DA, Gordon TP, Wilson ME, Brodie AR, Ahmed-Ansari A, McClure HM. Removal from natal social group to peer housing affects cortisol levels and absolute numbers of T cell subsets in juvenile rhesus monkeys. Brain Behav Immun 1992; 6:189–99. [DOI] [PubMed] [Google Scholar]
- 46. Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, Capitanio J. Laboratory rhesus macaque social housing and social changes: implications for research. Am J Primatol 2017; 79:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pahar B, Baker KC, Jay AN, et al. Effects of social housing changes on immunity and vaccine-specific immune responses in adolescent male rhesus macaques. Front Immunol 2020; 11:565746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Capitanio JP, Lerche NW. Psychosocial factors and disease progression in simian AIDS: a preliminary report. AIDS 1991; 5:1103–6. [DOI] [PubMed] [Google Scholar]
- 49. Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Natl Acad Sci U S A 1998; 95:4714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.