Abstract
Background
Bacterial vaginosis (BV) treatment failures and recurrences are common. To identify features associated with treatment response, we compared vaginal microbiota and host ectocervical transcriptome before and after oral metronidazole therapy.
Methods
Women with BV (Bronx, New York and Thika, Kenya) received 7 days of oral metronidazole at enrollment (day 0) and underwent genital tract sampling of microbiome (16S ribosomal RNA gene sequencing), transcriptome (RNAseq), and immune mediator concentrations on day 0, 15, and 35.
Results
Bronx participants were more likely than Thika participants to clinically respond to metronidazole (19/20 vs 10/18, respectively, P = .0067) and by changes in microbiota composition and diversity. After dichotomizing the cohort into responders and nonresponders by change in α-diversity between day 35 and day 0, we identified that transcription differences associated with chemokine signaling (q = 0.002) and immune system process (q = 2.5 × 10–8) that differentiated responders from nonresponders were present at enrollment. Responders had significantly lower levels of CXCL9 in cervicovaginal lavage on day 0 (P < .007), and concentrations of CXCL9, CXCL10, and monocyte chemoattractant protein 1 increased significantly between day 0 and day 35 in responders vs nonresponders.
Conclusions
Response to metronidazole is characterized by significant changes in chemokines and related transcripts, suggesting that treatments that promote these pathways may prove beneficial.
Keywords: bacterial vaginosis, vaginal microbiota, metronidazole, chemokines, ectocervical transcriptome
Clinical response to oral metronidazole treatment for bacterial vaginosis was more common among women in the Bronx, New York, compared to Thika, Kenya. Favorable response was characterized by microbiota changes, increases in chemokine signaling pathways, and chemokine concentrations in cervicovaginal secretions.
Bacterial vaginosis (BV), a clinical syndrome characterized by replacement of a Lactobacillus-dominant vaginal microbiota with diverse anaerobes, is a major contributor to preterm birth and human immunodeficiency virus (HIV) acquisition and transmission [1]. The prevalence of BV varies among different populations and affects approximately 30% of women in the United States (US) with rates as high as 55% in sub-Saharan Africa [2, 3]. BV is more common among black compared to white women independent of risk factors such as number of sexual partners [4]. This may reflect the observation that Lactobacillus iners, which is less stable and more likely to transition to BV, is more common than Lactobacillus crispatus or Lactobacillus jensenii in black women [5–8].
Globally, approximately 5500 women aged 15–24 years are newly infected with HIV weekly [9]. BV increases the risk for HIV acquisition (relative risk, 1.6 [95% confidence interval, 1.2–2.1]) and transmission (~3-fold increased risk) [10, 11]. The mechanisms linking BV with HIV are not fully defined but may reflect local inflammation [1, 7, 12]. The vaginal microbiome also modulates vaginal antiretroviral drug pharmacokinetics, which could affect local viral replication [13, 14]. For example, tenofovir gel was more effective in women with a Lactobacillus-dominant compared to a more diverse microbial community [13]. Subsequent mechanistic studies demonstrated that tenofovir uptake by human cells is reduced with increasing vaginal pH, a hallmark of BV, and is inhibited by adenine, which is actively secreted by Gardnerella vaginalis and other bacteria. Tenofovir also may be sequestered and/or metabolized by various vaginal bacteria [13–15].
Oral metronidazole is commonly prescribed to treat BV, but treatment failures and recurrences are well documented [16]. The reasons for this and whether therapy changes the mucosal immune environment are not fully understood. To address these questions, we compared bacterial composition, host gene expression in ectocervical biopsy tissue, and concentrations of immune mediators in cervicovaginal secretions in women with symptomatic BV before and after metronidazole treatment at 2 sites: Bronx, New York and Thika, Kenya. We hypothesized that site-specific differences in the microbiota and/or mucosal immune environment that characterize BV might result in differential responses to metronidazole. We also quantified the ability of vaginal secretions to inhibit Escherichia coli, a functional assay that may capture interactions between host and bacterial proteins [17]. Prior studies demonstrated that in healthy US adult women, vaginal secretions have potent anti–E. coli activity, which is associated with acid-producing lactobacilli; this activity is reduced in the setting of BV and lower in adolescents and other populations with different vaginal microbial communities [18–21].
MATERIALS AND METHODS
Study Design
Women 18 to 45 years of age with BV, diagnosed by demonstrating at least 3 of 4 Amsel criteria [22], were eligible for participation. The study was conducted in parallel at Albert Einstein College of Medicine–Montefiore Medical Center in Bronx, New York and Partners in Health Research and Development in Thika, Kenya between February 2018 and April 2019 and was approved by the Albert Einstein College of Medicine Institutional Review Board and the Kenya Medical Research Institute. All participants provided written informed consent.
Participants were evaluated at time of BV diagnosis (visit 1, day 0) and were provided with a 7-day course of oral metronidazole (500 mg twice daily). Follow-up visits were scheduled on day 14 (visit 2) and day 35 (visit 3). Exclusion criteria included metronidazole allergy, pregnancy, breastfeeding, menopause, HIV, and active sexually transmitted infections (STIs). At enrollment, participants had a gynecological examination; nucleic acid amplification testing for Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis (Gen-Probe, San Diego, California); urine pregnancy testing; and HIV testing (OraQuick ADVANCE Rapid HIV-1/2 antibody, OraSure Technologies, Bethlehem, Pennsylvania). Thika participants were also tested for syphilis (rapid plasma reagin). If clinically indicated, STI testing was repeated at subsequent visits and participants were replaced if an active STI was detected or if pregnant. Women diagnosed with vulvovaginal candidiasis (VVC) or urinary tract infection (UTI) were treated and remained in the study. Participants with persistent or recurrent BV at follow-up visits were retreated and remained in the study. Vaginal swabs and cervicovaginal lavage (CVL) with 10 mL normal saline were collected at all study visits for pH (Whatman pH paper), Nugent scoring, 16S ribosomal (rRNA) gene sequencing (swabs), quantification of immune mediators (CVL), and E. coli inhibitory activity (CVL). Ectocervical tissue biopsies were obtained at visits 1 and 3. Adherence to metronidazole was assessed via an on-site, web-based, self-administered survey.
16S rRNA Gene Polymerase Chain Reaction and Sequencing
DNA was extracted from vaginal swabs using the BiOstic Bacteremia DNA isolation kit (Qiagen, Germantown, Maryland). Controls included swabs without human contact and no template water controls. DNA samples were tested for polymerase chain reaction (PCR) inhibitors and 16S rRNA gene [23]. Broad-range PCR targeting the V3–V4 hypervariable region of the 16S rRNA gene coupled with sequencing on the Illumina MiSeq instrument (Illumina, San Diego, California) was performed [24]. Sequence reads were filtered for length, quality, and contaminants and classified using the phylogenetic placement tool, pplacer, and a reference set of vaginal bacteria [25, 26] (data has been deposited to the Natioanl Center for Biotechnology Information Short Read Archive [PRJNA730544]). Staff were blinded to participant status. Sequence read counts were converted to proportions and dominance defined as >50% relative abundance. Alpha diversity was quantified using the Shannon diversity index (SDI). The median change in SDI between visits 1 and 3 was used to dichotomize patients as metronidazole responders or nonresponders.
RNA-Seq Analysis of Ectocervical Biopsies
Ectocervical tissue was weighed, embedded in optimal cutting temperature media, flash-frozen in liquid nitrogen, and stored at –80°C; RNA-Seq and bioinformatics were performed at the Yerkes Nonhuman Primates Genomics Core, Emory University (Atlanta, Georgia). RNA was extracted and assessed as described [27]. One nanogram of total RNA was amplified using Clontech Smarter V4 kits followed by fragmentation, and barcodes were appended using Illumina’s Nextera XT kits [28]. Libraries were sequenced on an Illumina NovaSeq 6000 as 101-base single-read reactions with multiplexing to achieve read depths of approximately 20 M reads per sample. Reads were aligned to the Human Reference Genome Sequence and transcripts annotated with Genome Reference Consortium (GEO accession number [GSE174799]) Build 38 using STAR software (version 2.5.2b) [29].
Measurement of Immune Mediators and E. coli Inhibitory Activity
CVL samples were transported on ice and clarified by centrifugation at 750g for 10 minutes at 4°C, and supernatants were aliquoted and stored at −80°C. Samples from Thika were batched and shipped on dry ice. CVL concentrations of interleukin (IL) 1α, IL-1β, IL-6, IL-8, IL-10, IL-17α, macrophage inflammatory protein (MIP) 1β, regulated upon activation, normal T-cell expressed and secreted, granulocyte macrophage colony-stimulating factor, interferon-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), MIP-3α, CXCL9, and tumor necrosis factor α were quantified by multiplex proteome array with beads from Millipore (Billerica, Massachusetts), measured using Luminex MAGPIX (Luminex, Austin, Texas) and analyzed using MILLIPLEX Analyst (VigeneTech, Carlisle, Massachusetts). Commercial enzyme-linked immunosorbent assays were used to measure CVL concentrations of secretory leukocyte protease inhibitor (SLPI) (R&D Systems, Minneapolis, Minnesota), human neutrophil peptides 1–3 (HNP1-3) (HyCult Biotechnology, Uden, the Netherlands), immunoglobulin A (IgA), and immunoglobulin G (IgG) (Thermo Fisher Scientific, Waltham, Massachusetts). Mediators were selected for their association with inflammation, HIV risk, and/or endogenous antimicrobial activity [7, 30]. The ability of CVL supernatants to inhibit E. coli growth was measured as previously described and is presented as the mean percentage inhibition relative to bacteria treated with control buffer [31].
Statistical Analysis
Demographic and clinical characteristics were compared between Bronx and Thika women using t test for continuous variables or χ 2 test (or Fisher exact tests as appropriate) for categorical variables. Immune mediators, relative abundance of bacterial taxa, and α-diversity (SDI) were compared between groups using the nonparametric Mann–Whitney U test. Vaginal pH, Nugent score, cytokine and chemokine concentrations, and antimicrobial activity were compared by Friedman test or paired Wilcoxon test to examine changes over time while accounting for repeated measures taken from the same women. Holm–Bonferroni adjustments were applied for post hoc comparisons between study visits. Concentrations of mediators below the lower limit of detection (LOD) were set at 50% of the lower LOD. Concentrations above the upper LOD after dilution were set at the upper limit. Multidimensional scaling plots of distances between gene expression profiles were drawn using plotMDS function limma, and likelihood ratio test of logistic regression was used to examine the association between top 2 components and participants’ response. Heatmaps were drawn on the normalized expression matrix using the NMF package in R. Euclidean distance and complete linkage were used for hierarchical clustering. Differential gene expression analysis was performed with sample quality weights using the limma-voom package in R. Pathway analysis was performed using the gage package in R. Statistical analyses were performed using GraphPad Prism, version 8 (GraphPad Software, La Jolla, California) and R software.
RESULTS
Study Population
Thirty-four women were screened in the Bronx; 9 were ineligible (7 did not meet Amsel criteria and 2 had an STI), 1 decided not to participate, and 4 were lost to follow-up. Thika screened 26 women; 7 were ineligible due to STI and 1 decided not to participate. None were lost to follow-up. Four Thika participants were replaced at follow-up because of pregnancy or an active STI. The final study population included 20 Bronx (designated P) and 18 Thika (designated K) participants. Two participants at each site were treated for a UTI (1 after visit 1 and 1 at visit 2). In addition, 2 Bronx and 6 Thika women received treatment for VVC (Bronx: 1 each at visit 1 and visit 3; Thika: 2 at visit 2, 3 at visit 3, and 1 at both visits 1 and 3).
Demographic and clinical characteristics are summarized in Table 1. Thika participants were younger (P < .01) and differed from the Bronx cohort by race (P = .021) and ethnicity (P = .003). Additionally, Thika participants were less likely to smoke (P < .001) or to report a history of prior BV (P = .004), other STI (P = .015), or anal sex (P = .003). Conversely, Thika participants reported more frequent sex acts (P = .026) in the month prior to enrollment compared to Bronx participants.
Table 1.
Characteristics of Study Participants at Baseline Visit
| Characteristic | Bronx (n = 20) | Thika (n = 18) | P Valuea |
|---|---|---|---|
| Age, y, mean ± SD | 36.35 ± 7.49 | 29.50 ± 6.54 | .007 |
| Race.ethnicity | |||
| White | 5 (25) | 0 | .021 |
| Black | 13 (65) | 18 (100) | |
| Other | 2 (10) | 0 | |
| Latinx | 8 (40) | 0 | .003 |
| Education, y, mean ± SD | 13.93 ± 2.42 | 10.89 ± 3.45 | .013 |
| Birth control method | |||
| Hormonal contraceptive | 4 (20) | 6 (33) | .11 |
| Copper IUD | 1 (5) | 5 (28) | |
| Condoms | 5 (25) | 2 (11) | |
| Tubal ligation | 3 (15) | 0 | |
| None | 7 (35) | 5 (28) | |
| Regular menstrual cycle (21–35 d) | 19 (95) | 13 (72) | .083 |
| History of anal sex | 8 (40) | 0 | .003 |
| No. of vaginal sex acts past 30 days, mean ± SD | 2.70 ± 2.70 | 6.94 ± 7.02 | .026 |
| History of douchingb past 90 d, mean ± SD | 3 (15) | 5 (28) | .44 |
| History of BV | 13 (65) | 3 (17) | .004 |
| History of STI | 10 (50) | 2 (11) | .015 |
| Current smoker | 7 (35) | 0 | .009 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BV, bacterial vaginosis; IUD, intrauterine device; SD, standard deviation; STI, sexually transmitted infection.
aχ 2 test was used for race and birth control method; Fisher exact test was used for ethnicity, regular menstrual cycle, current smoking, and history of anal sex, douching, BV, and STI.
bBronx women reported douching with vinegar or powder and Thika women were more likely to report use of soap and a bathing towel.
Clinical Response and Changes in Vaginal Microbiota Following Metronidazole Treatment
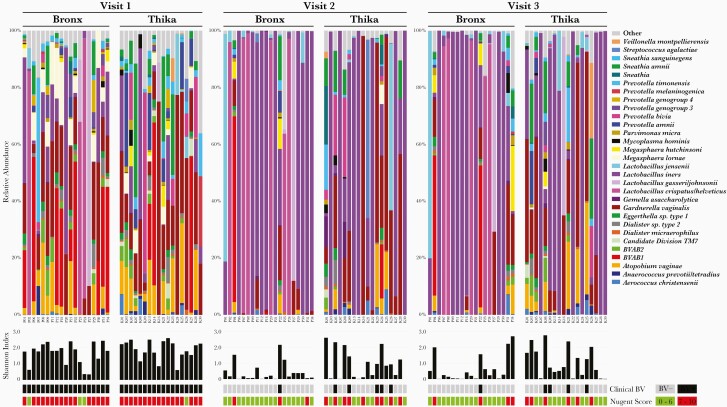
There were no differences in relative abundance of bacterial taxa, α-diversity (SDI), vaginal pH (5.44 ± 0.53 vs 5.49 ± 0.47), or Nugent scores (7.75 ± 2.48 vs 8.05 ± 1.08) (all mean ± standard deviation) at enrollment comparing Bronx and Thika participants (Figure 1 and Table 2). However, the clinical response to metronidazole differed with 19 of 20 (95%) Bronx vs 10 of 18 (55.5%) Thika women showing no clinical BV (<3 Amsel criteria) at visits 2 and 3 (P = .0067). Only 1 Bronx participant (P22) had persistent BV at both follow-up visits and was retreated each time; none had a recurrence. In contrast, 5 Thika women had persistent BV at visit 2 and received a second treatment course. At visit 3, 1 of these (K09) still had symptomatic BV and received a third treatment course and an additional 3 had a recurrence. Vaginal pH and Nugent scores declined following treatment, but the declines were more significant for the Bronx cohort (Table 2).
Figure 1.
Changes in microbiota following metronidazole treatment. Relative abundance of bacteria detected in vaginal swabs at each visit for Bronx (P) and Thika (K) participants. Bars show the top 30 most abundant bacteria across all samples in each participant at each visit. All other taxa are classified in the other category. Shannon diversity index (α-diversity), presence of clinical (red) bacterial vaginosis (BV) (defined by Amsel criteria), and Nugent scores are indicated below the heat map for each participant and visit.
Table 2.
Vaginal pH, Nugent Scores, and Concentrations of Immune Mediators (Log10 pg/mL) in Cervicovaginal Lavage Fluid at Each Visit
| Mediators | Bronx | Thika | ||||||
|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 3 | P Valuea | Visit 1 | Visit 2 | Visit 3 | P Valuea | |
| Vaginal pH | 5.44 ± 0.54 | 4.68 ± 0.80 | 4.68 ± 0.46 | .0005 | 5.49 ± 0.47 | 4.94 ± 0.77 | 5.08 ± 0.54 | .01 |
| Nugent score | 7.75 ± 2.42 | 2.98 ± 3.03 | 3.51 ± 3.31 | <.0001 | 8.05 ± 1.08 | 5.86 ± 2.07 | 5.32 ± 2.17 | .01 |
| IL-1α | 2.54 ± 0.68 | 1.93 ± 0.46 | 2.00 ± 0.76 | .01 | 2.47 ± 0.71 | 2.15 ± 0.55 | 2.11 ± 0.67 | .13 |
| IL-1β | 1.46 ± 0.55 | 0.78 ± 0.84 | 0.81 ± 0.86 | .02 | 1.60 ± 0.92 | 1.25 ± 1.06 | 1.11 ± 1.13 | .20 |
| IL-6 | 0.82 ± 0.54 | 0.69 ± 0.68 | 0.77 ± 0.64 | .91 | 0.69 ± 0.82 | 0.79 ± 0.73 | 0.42 ± 0.75 | .41 |
| IL-8 | 2.82 ± 0.47 | 2.71 ± 0.42 | 2.70 ± 0.53 | .64 | 2.86 ± 0.67 | 2.83 ± 0.67 | 2.87 ± 0.68 | .91 |
| IL-10 | 0.36 ± 0.44 | 0.17 ± 0.54 | 0.20 ± 0.46 | .13 | 0.38 ± 0.58 | 0.32 ± 0.53 | 0.25 ± 0.55 | .87 |
| TNF-α | 0.03 ± 0.47 | 0.18 ± 0.52 | 0.27 ± 0.35 | .08 | 0.01 ± 0.75 | 0.07 ± 0.69 | 0.24 ± 0.37 | .97 |
| CXCL10 | 1.89 ± 0.62 | 2.14 ± 0.51 | 2.29 ± 0.67 | .04 | 1.91 ± 0.84 | 2.15 ± 0.96 | 2.37 ± 0.67 | .18 |
| MCP-1 | 1.62 ± 0.65 | 2.02 ± 0.79 | 2.10 ± 0.63 | .04 | 1.61 ± 0.95 | 1.74 ± 0.73 | 1.87 ± 0.59 | .28 |
| MIP-1β | 0.39 ± 0.33 | 0.48 ± 0.42 | 0.46 ± 0.44 | .48 | 0.54 ± 0.42 | 0.63 ± 0.50 | 0.52 ± 0.36 | .34 |
| RANTES | 0.62 ± 0.71 | 0.68 ± 1.05 | 0.59 ± 0.93 | .40 | 0.76 ± 1.11 | 0.51 ± 0.84 | 0.78 ± 0.90 | .87 |
| MIP-3α | 1.79 ± 0.53 | 1.97 ± 0.65 | 1.95 ± 0.59 | .12 | 1.39 ± 0.81 | 1.55 ± 0.86 | 1.54 ± 0.69 | .83 |
| CXCL9 | 1.83 ± 0.81 | 2.36 ± 0.66 | 2.41 ± 0.75 | .03 | 2.02 ± 0.98 | 2.35 ± 0.95 | 2.42 ± 0.84 | .50 |
| HNP1-3 | 5.30 ± 0.59 | 5.34 ± 0.65 | 5.24 ± 0.64 | .33 | 4.98 ± 0.78 | 5.03 ± 0.92 | 4.98 ± 0.72 | .90 |
| SLPI | 5.21 ± 0.63 | 5.38 ± 0.43 | 5.43 ± 0.56 | .39 | 4.943± 0.70 | 5.07 ± 0.58 | 5.10 ± 0.52 | .20 |
| IgA, ng/mL | 3.40 ± 0.30 | 3.50 ± 0.60 | 3.54 ± 0.41 | .14 | 3.24 ± 0.54 | 3.29 ± 0.53 | 3.26 ± 0.42 | .68 |
| IgG, µg/mL | 0.54 ± 0.60 | 0.53 ± 0.79 | 0.50 ± 0.70 | .53 | 0.52 ± 0.73 | 0.82 ± 0.49 | 0.76 ± 0.50 | .74 |
Data are presented as mean ± standard deviation unless otherwise indicated.
Abbreviations: HNP1–3, human neutrophil peptides 1 through 3; IgA, immunoglobulin A; IgG, immunoglobulin G; IL, interleukin; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T-cell expressed and secreted; SLPI, secretory leukocyte protease inhibitor; TNF-α, tumor necrosis factor alpha.
a P value comparing changes over the 3 visits at each site by Friedman test.
The differences in metronidazole response were reflected by changes in the microbiota. In the Bronx cohort, there was a shift from a diverse to Lactobacillus-dominant microbiota: 8 (40%) women with acid-producing lactobacilli (L. crispatus, L. jensenii, and Lactobacillus gasseri) and 10 (50%) with L. iners at visit 2. Only 2 (10%) participants, including the one with persistent clinical BV, had a microbiota dominated by BV-associated bacteria (Figure 1). This favorable response persisted at visit 3, with 15 of 19 (79%) (1 sample was not available for sequencing) exhibiting Lactobacillus dominance (7 with acid-producing lactobacilli and 8 with L. iners). In contrast, only 8 (44%) of the Thika participants transitioned from a diverse to an L. iners–dominant microbiome at visit 2 and/or visit 3; none had a microbiota dominated by acid-producing lactobacilli at either follow-up visit.
Predictors of Metronidazole Response
To explore mechanisms that might contribute to the differential metronidazole response, we dichotomized participants based on the median change (Δ) in SDI between visits 1 and 3 [-log (SDI at visit 3 / SDI at visit 1)] as responders (Δ > 1.08) or nonresponders (Δ ≤ 1.08). Twelve of 18 (66.7%) Bronx participants (P04 did not have microbiota data at visit 1 and P02 did not have data for visit 3 and were excluded) were classified as responders compared to only 6 of 18 (33.3%) Thika participants (P = .09).
Many significant changes in relative abundance of bacteria (n = 44) were observed comparing visit 1 and visit 3 in the metronidazole responders, but few in the nonresponders (Table 3). These included an increase in L. iners (62%, P < .0003) and a decrease in diverse anaerobes. At visit 1, responders had significantly higher relative abundance of Megasphaera lornae (previously Megasphaera sp type 1 [32]), Maegeeibacillus indolicus, and Prevotella genotype group 4 and lower levels of L. gasseri compared to nonresponders, although each of these accounted for a very low proportion of total read counts (Supplementary Figure 1).
Table 3.
Percentage Change (Δ) in Relative Abundance of Indicated Taxa Between Visit 1 and Visit 3 in Metronidazole Responders and Nonresponders
| Taxa | Responders | Nonresponders | ||
|---|---|---|---|---|
| Δ Relative Abundance | P Value | Δ Relative Abundance | P Value | |
| Lactobacillus iners | +62% | .0003 | +13% | .045 |
| BVAB1 | –14% | .0080 | –7.6% | .20 |
| Gardnerella vaginalis | –18% | .0000153 | –0.75% | .57 |
| Atopobium vaginae | –8.6% | .00073 | +0.21% | .97 |
| Megasphaera lornae | –7.4% | .0011 | –1.9% | .035 |
| Eggerthella sp type 1 | –1.5% | .0025 | –0.21% | .36 |
| Sneathia amnii | –5.9% | .0025 | +0.93% | .84 |
| BVAB2 | –2.6% | .0025 | –0.11% | .31 |
| Megasphaera hutchinsoni | –0.44% | .036 | +1.3% | .59 |
| Prevotella timonensis | –5.2% | .00048 | +0.87% | .75 |
| Prevotella bivia | –1.6% | .019 | –4.2% | .41 |
| Mageeibacillus indolicus | –1.2% | .0059 | +0.12% | .42 |
| Parvimonas micra | –0.63% | .0011 | –0.11% | .68 |
| Prevotella amnii | –2.3% | .014 | –2.2% | .23 |
| Dialister sp type 2 | –2.1% | .00073 | –0.00632% | .78 |
| Anaerococcus prevotii/tetradius | –0.70% | .037 | +0.016% | .89 |
| Dialister micraerophilus | –0.28% | .0011 | –0.098% | .44 |
| Prevotella genogroup 3 | –1.43% | .022 | +0.13% | .86 |
| Peptoniphilus grossensis/harei | –0.75% | .0005 | –0.053% | .69 |
| Sneathia sanguinegens | –0.63% | .0059 | –0.15% | .92 |
| Gemella asaccharolytica | –0.16% | .0059 | +0.14% | .36 |
| Peptoniphilus lacrimalis | –0.18% | .0039 | –0.11% | .04 |
| Prevotella genogroup 4 | –1.7% | .014 | –0.040% | .79 |
| Mobiluncus curtisii | –0.16% | .014 | –0.056% | .72 |
| Prevotella | –0.42% | .036 | –0.15% | .097 |
| Lachnospiraceae | –0.14% | .022 | +0.029% | 1.00 |
| Porphyromonas uenonis | –0.22% | .014 | +0.032% | .83 |
| Arcanobacterium | –0.098% | .036 | +0.11% | .42 |
| Peptoniphilus coxii | –0.12% | .036 | –0.020% | 1.00 |
| Porphyromonas sp type 1 | –0.13% | .036 | –0.11% | .36 |
| Bulleidia | –0.030% | .022 | –0.014% | .58 |
| Prevotella buccalis | –0.074% | .022 | +0.016% | .94 |
Abbreviation: BVAB, bacterial vaginosis–associated bacteria.
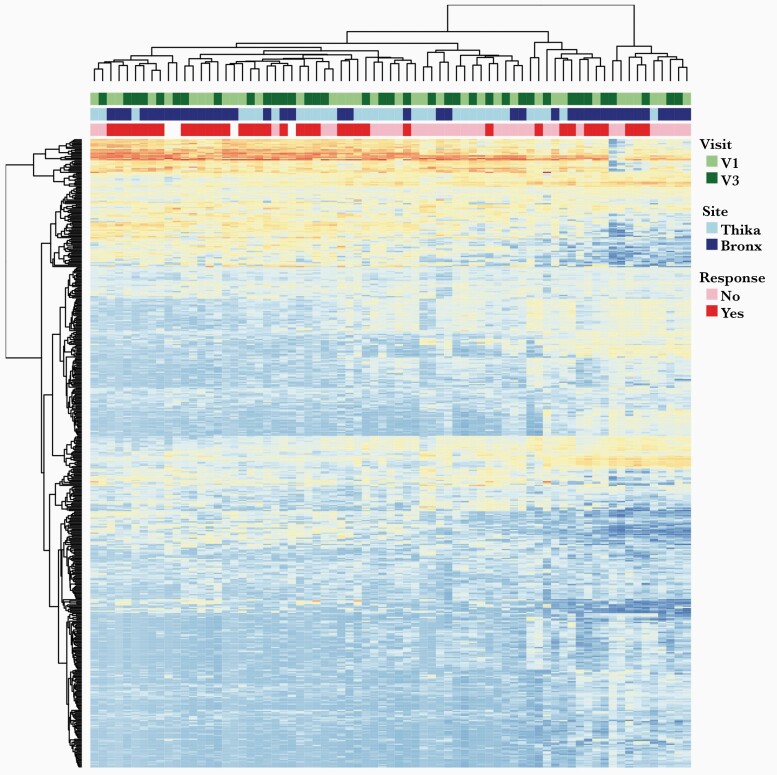
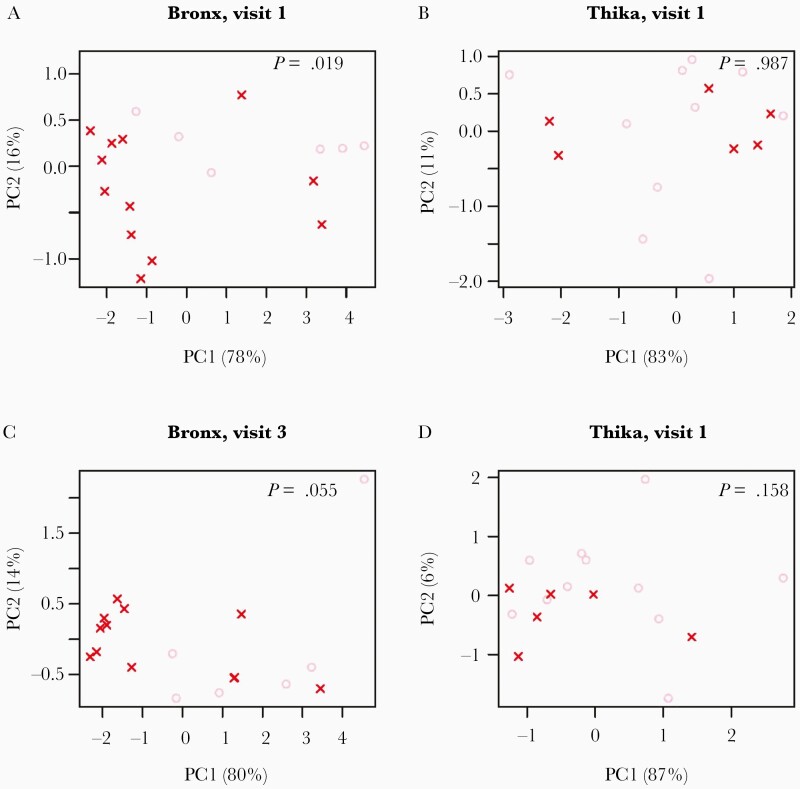
To explore factors that may contribute to differential treatment responses, we compared the host transcriptome in ectocervical tissue biopsies from visits 1 and 3 available for 20 Bronx and 17 Thika participants. A heatmap of the top 500 variable genes showed clustering of responders and nonresponders, independent of study visit (Figure 2). The top 2 components of the PCA were associated with the response to metronidazole in Bronx participants at visit 1 and visit 3 (P = .019 and P = .065, respectively; Figure 3). The change in SDI between visit 1 and visit 3 was correlated with the top 2 principal components in Bronx participants (PC1: r = –0.28, P = .35; PC2: r = –0.58, P = .01; Supplementary Figure 2), but did not differentiate Thika responders from nonresponders at either visit. KEGG pathways associated with differentially expressed genes at visit 1 comparing responders and nonresponders were cytokine–cytokine receptor interactions (q = .002), complement/coagulation (q = .002), and chemokine signaling (q = .002), and top gene ontology (GO) terms were immune response (q = 1.8 × 10–10), defense response (q = 1.5 × 10–8), and regulation of immune system process (q = 2.5 × 10–8). The differentially expressed genes contributing to these pathways are shown in Supplementary Table 1. Similarly, top KEGG pathways at visit 3 were also associated with complement/coagulation (q = .001) and cytokine–cytokine receptor interaction (q = .009), and top GO terms were immune response (q = 4.0 × 10–11), defense response (q = 2.8 × 10–10), and inflammatory response (q = 1.0 × 10 –11). Heat maps of the top 500 variably expressed genes at visit 1 and visit 3 show distinct clustering of responders and nonresponders (Supplementary Figure 3 and Supplementary Tables 2 and 3).
Figure 2.
Transcriptional profile of ectocervical biopsies at visit 1 and visit 3. Heatmap showing expression of top 500 variably expressed genes. Annotations indicate site (Bronx vs Thika), visit (visit 1 vs visit 3), and response to metronidazole based on change in Shannon diversity index between visits 1 and 3.
Figure 3.
Principal component (PC) analysis of ectocervical biopsy transcriptome differentiates Bronx, but not Thika, responders from nonresponders to metronidazole therapy. PC plots of RNAseq data for Bronx (A and C) and Thika (B and D) at visit 1 (A and B) and visit 3 (C and D) showing the top 2 components and percentage contribution to the variance. P values indicate the association between the top 2 components and participants’ response (likelihood ratio test of logistic regression).
Changes in Soluble Immune Mediators and E. coli Inhibitory Activity
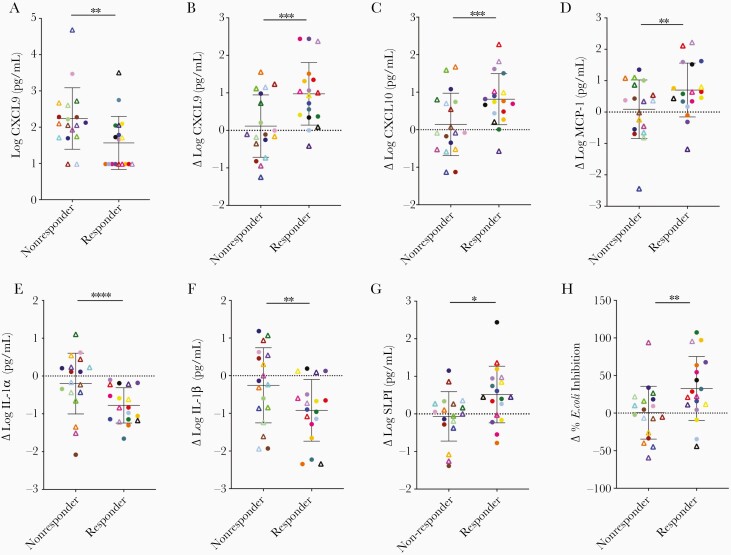
We measured select immune mediators and anti–E. coli activity in CVL at each visit. There was a significant decrease in IL-1α and IL-1β and an increase in several chemokines (CXCL-9, CXCL-10, and MCP-1) over the 3 visits in the Bronx cohort, but no significant changes over time in the Thika cohort (Table 2). There were also no significant changes over time in markers of mucosal defense including HNP1-3, SLPI, IgA, IgG, or anti–E. coli activity in either cohort. However, when participants were dichotomized by metronidazole response, additional differences were observed. At enrollment, responders had significantly lower levels of CXCL9 compared to nonresponders (P < .007; Figure 4A), a finding consistent with the transcriptome as multiple CXC chemokine family genes were downregulated in responders relative to nonresponders at visit 1. Moreover, the concentration of CXCL9, CXCL10, and MCP-1 increased significantly between visit 1 and visit 3 in responders (Figure 4B–D) as did the concentration of SLPI, an anti-inflammatory molecule, and the E. coli inhibitory activity. Conversely, there was a decrease in IL-1α and IL-1β (Figure 4E–H).
Figure 4.
Metronidazole treatment is associated with changes in concentrations of immune mediators in cervicovaginal lavage (CVL) fluid. Concentration of CXCL9 in CVL at visit 1 in patients who subsequently did or did not respond to metronidazole treatment as defined by change in Shannon diversity index (A). The change (Δ) between visit 3 and visit 1 in CVL fluid concentrations of CXCL9 (B), CXCL10 (C), monocyte chemoattractant protein 1 (MCP-1; D), interleukin 1α (IL-1α; E), interleukin 1β (IL-1β; F), secretory leukocyte protease inhibitor (SLPI; G), and Escherichia coli inhibitory activity (H) comparing responders and nonresponders to metronidazole. Circles indicate Bronx and triangles Thika participants. Data are presented as mean plus standard error and asterisks indicate differences between responders and nonresponders (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Discussion
Bronx women were more likely than women in Thika to respond to oral metronidazole treatment and these site-specific differences were more evident when response was defined by 16S rRNA gene sequencing data. The microbial composition among responders also differed with Thika participants shifting exclusive to L. iners dominance, whereas Bronx participants shifted either to L. iners or to acid-producing Lactobacillus-dominant communities (L. crispatus, L. jensenii, or L. gasseri). This difference is consistent with previous studies showing greater prevalence of L. iners–dominant microbiota in women in Kenya compared to the US, although black women in the US (65% of our Bronx cohort) are also more likely to have L. iners dominance [8, 33, 34]. The Bronx cohort was too small to address racial differences. The increase in the proportion of acid-producing lactobacilli correlated with an increase in E. coli inhibitory activity, which has been shown to be partially mediated by acid-producing lactobacilli [19].
To identify factors that might be associated with a favorable response to metronidazole, we dichotomized participants based on SDI score rather than relying on clinical criteria as the latter may mask differences in microbial composition that could impact the genital tract immune environment. This approach identified significant differences in the host transcriptome mapping to chemokine signaling, complement/coagulation pathways, and immune responses in ectocervical tissue at enrollment that were associated with a favorable response at visit 3, particularly among the Bronx participants. Moreover, the most upregulated pathway identified comparing the change in gene expression between visits 3 and visit 1 was the chemokine signaling pathway. Consistent with this transcriptome data, responders had lower CVL levels of CXCL9 protein at BV presentation, which increased significantly following metronidazole therapy. Response to metronidazole was also associated with increases in other chemokines (CXCL-10 and MCP-1) and in the anti-inflammatory molecule SLPI but a decrease in the proinflammatory cytokines IL-1α and IL-1β.
The observation that a favorable treatment response is associated with increased chemokine signaling is consistent with a prior study in Nairobi, Kenya, which also documented a significant increase in CXCL-9 and CXCL-10 and a decrease in IL-1α and IL-1β in the subset of patients who responded to metronidazole [35]. The concentrations of these chemokines correlated with the abundance of L. iners in a cohort of non-BV study participants [35]. The increase in chemokines (and decrease in IL-1α/β) observed in the current study was driven primarily by Bronx participants who also exhibited a significant increase in L. iners and a decrease in the relative abundance of multiple anaerobes between visits 1 and 3.
The association between metronidazole response and an increase in mucosal chemokines seems counterintuitive, as chemokines recruit immune cells that may be targets for HIV infection and dissemination. In a substudy of women who participated in the Centre for the AIDS Program of Research in South Africa (CAPRISA) tenofovir gel prevention trial, higher mucosal levels of several chemokines including IP-10, MIP-1β, IL-8, and MCP-1 were associated with HIV seroconversion [36]. However, a macaque study of simian immunodeficiency virus (SIV) found that CXCL-9 primarily recruited SIV-specific CD8+ T cells into the genital tract, which expressed higher levels of CXCR3 than peripheral blood CD8+ T cells [37]. The recruitment of this subpopulation may have contributed to viral control rather than promoting infection. Consistent with this latter finding, our gene ontology pathways analysis comparing changes in gene expression between visits 3 and 1 identified immune responses and lymphocyte activation pathways as most significantly different between responders and nonresponders. An increase in CD8+ T cells in the genital tract following metronidazole therapy for BV was observed in another small study [38].
The reason(s) for metronidazole failure, which was more common in Thika women, are unclear and likely multifactorial. It does not appear to reflect differences in adherence, which was assessed by computer-assisted self-interview. Only 2 participants at each site (P6, P18, K5, and K28) reported incomplete adherence and only 1 of these was a nonresponder. Douching behaviors, frequency of sex acts, or number of sex partners could contribute to treatment failures. Mechanistically, treatment failure could also reflect differences in microbial composition and metatranscriptome, which could modulate bacterial susceptibility and metronidazole pharmacokinetics. Similar to the observations with antiretroviral drugs [13–15], a recent study suggested that lactobacilli sequester metronidazole [39]. Drug efficacy decreased when the relative abundance of Lactobacillus was higher relative to BV-associated bacteria pretreatment. Another study found that CRISPR-associated genes of G. vaginalis, which are involved in DNA repair, were upregulated in nonresponding patients and speculated that these might interfere with the bactericidal effects of metronidazole, which is metabolized to short-lived free radical compounds that damage bacterial DNA [40]. Although we did not perform metatranscriptomics, there were differences in relative abundance of select bacteria at visit 1 comparing responders and nonresponders, which could impact drug pharmacokinetics and treatment responses. In addition, the more frequent transition to L. iners than to acid-producing lactobacilli in Thika participants compared with Bronx participants could also contribute to treatment failures as L. iners is less stable [5–8].
There are several limitations to our study including the small sample size, absence of samples from BV-negative women for comparison, and exclusion of women with HIV, STI, or pregnancy. Taken together, results of this study demonstrate that a favorable response to metronidazole is associated with a decrease in inflammatory cytokines and an increase in chemokine signaling pathways. Treatment failure is characterized by lack of significant changes in the levels of chemokines and related transcripts. The reasons why some patients fail to respond and whether adjunctive strategies that promote chemokine pathways may prove beneficial in restoring mucosal defenses will require further study.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the site staff and study participants.
Financial support. This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute for Allergy and Infectious Diseases, and the National Center for Advancing Translational Sciences at the National Institutes of Health (grant numbers R01 HD098977, R01 AI134367, U19 AI103461, P30 AI124414, and UL1 TR002556). The Yerkes Nonhuman Primate Genomics Core is supported in part by ORIP/OD P51OD011132.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis 2016; 214(Suppl 1):S29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007; 34:864–9. [DOI] [PubMed] [Google Scholar]
- 3. Torrone EA, Morrison CS, Chen PL, et al. STIMA Working Group . Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018; 15:e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 2013; 209:505–23. [DOI] [PubMed] [Google Scholar]
- 5. Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017; 46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011; 108(Suppl 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keller MJ, Guzman E, Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS 2007; 21:467–76. [DOI] [PubMed] [Google Scholar]
- 10. Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008; 22:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 2012; 9:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell C, Marrazzo J. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 2014; 71:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017; 356:938–45. [DOI] [PubMed] [Google Scholar]
- 14. Taneva E, Sinclair S, Mesquita PM, et al. Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI Insight 2018; 3:e99545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheu RK, Gustin AT, Lee C, et al. Impact of vaginal microbiome communities on HIV antiretroviral-based pre-exposure prophylaxis (PrEP) drug metabolism. PLoS Pathog 2020; 16:e1009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193:1478–86. [DOI] [PubMed] [Google Scholar]
- 17. Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol 2002; 187:561–8. [DOI] [PubMed] [Google Scholar]
- 18. Ghartey JP, Carpenter C, Gialanella P, et al. Association of bactericidal activity of genital tract secretions with Escherichia coli colonization in pregnancy. Am J Obstet Gynecol 2012; 207:297.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalyoussef S, Nieves E, Dinerman E, et al. Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions. PLoS One 2012; 7:e49506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valore EV, Wiley DJ, Ganz T. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 2006; 74:5693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madan RP, Carpenter C, Fiedler T, et al. Altered biomarkers of mucosal immunity and reduced vaginal Lactobacillus concentrations in sexually active female adolescents. PLoS One 2012; 7:e40415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 23. Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect Dis 2008; 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golob JL, Pergam SA, Srinivasan S, et al. Stool microbiota at neutrophil recovery is predictive for severe acute graft vs host disease after hematopoietic cell transplantation. Clin Infect Dis 2017; 65:1984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsen FA, Kodner RB, Armbrust EV. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics 2010; 11:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 2012; 7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keller MJ, Wood L, Billingsley JM, et al. Tenofovir disoproxil fumarate intravaginal ring for HIV pre-exposure prophylaxis in sexually active women: a phase 1, single-blind, randomised, controlled trial. Lancet HIV 2019; 6:e498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 2021; 184:460–75.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013; 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masson L, Passmore JA, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keller MJ, Madan RP, Torres NM, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 2011; 6:e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srinivasan S, Beamer MA, Fiedler TL, et al. Megasphaera lornae sp. nov., Megasphaera hutchinsoni sp. nov., and Megasphaera vaginalis sp. nov.: novel bacteria isolated from the female genital tract. Int J Syst Evol Microbiol 2019; 71. doi:10.1099/ijsem.0.004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jespers V, Kyongo J, Joseph S, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep 2017; 7:11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McClelland RS, Lingappa JR, Srinivasan S, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 2018; 18:554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joag V, Obila O, Gajer P, et al. Impact of standard bacterial vaginosis treatment on the genital microbiota, immune milieu, and ex vivo human immunodeficiency virus susceptibility. Clin Infect Dis 2019; 68:1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liebenberg LJ, Masson L, Arnold KB, et al. Genital-systemic chemokine gradients and the risk of HIV acquisition in women. J Acquir Immune Defic Syndr 2017; 74:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cromwell MA, Carville A, Mansfield K, et al. SIV-specific CD8+ T cells are enriched in female genital mucosa of rhesus macaques and express receptors for inflammatory chemokines. Am J Reprod Immunol 2011; 65:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thurman AR, Kimble T, Herold B, et al. Bacterial vaginosis and subclinical markers of genital tract inflammation and mucosal immunity. AIDS Res Hum Retroviruses 2015; 31:1139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee CY, Cheu RK, Lemke MM, et al. Quantitative modeling predicts mechanistic links between pre-treatment microbiome composition and metronidazole efficacy in bacterial vaginosis. Nat Commun 2020; 11:6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deng ZL, Gottschick C, Bhuju S, Masur C, Abels C, Wagner-Dobler I. Metatranscriptome analysis of the vaginal microbiota reveals potential mechanisms for protection against metronidazole in bacterial vaginosis. mSphere 2018; 3:e00262-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.