ABSTRACT
Interferon-γ-inducible protein 10 (IP-10) has been suggested as a marker for targeted viral load (VL) monitoring during antiretroviral treatment (ART). We aimed to determine the kinetics of IP-10 during the initial year of ART, with particular regard to the impact of tuberculosis (TB) co-infection on IP-10 secretion. Longitudinal plasma IP-10 levels were quantified in 112 treatment-naive HIV-positive adults at Ethiopian health centers, through enzyme-linked immunosorbent assay (ELISA) using samples obtained before and during the initial 12 months of ART. All participants underwent bacteriological TB investigation before starting ART. In virological responders (VRs; defined as VL < 150 copies/ml with no subsequent VL ≥ 1,000 copies/ml), IP-10 kinetics were analyzed using linear regression models. Among 91/112 (81.3%) participants classified as VRs, 17 (18.7%) had concomitant TB. Median baseline IP-10 was 650 pg/ml (interquartile range [IQR], 428–1,002) in VRs. IP-10 decline was more rapid during the first month of ART (median 306 pg/ml/month) compared with later time intervals (median 7-48 pg/ml/month, P < 0.001 in each comparison). Although VRs with TB had higher IP-10 levels at baseline (median 1106 pg/ml [IQR, 627–1,704]), compared with individuals without TB (median 628 pg/ml [IQR, 391–885]; P = 0.003), the rate of IP-10 decline during ART was similar, regardless of TB-status. During the initial year of ART, IP-10 kinetics followed a biphasic pattern in VRs, with a more rapid decline in the first month of ART compared with later time intervals. Baseline IP-10 was higher in individuals with TB versus individuals without TB, but the kinetics during ART were similar.
IMPORTANCE To reach the goal of elimination of HIV as public health threat, access to antiretroviral treatment (ART) has to be further scaled up. To ensure viral suppression in individuals receiving ART, novel and robust systems for treatment monitoring are required. Targeting viral load monitoring to identify individuals at increased likelihood of treatment failure, using screening tools, could be an effective use of limited resources for viral load testing. Interferon-γ-inducible protein 10 (IP-10), a host inflammation mediator, has shown potential for this purpose. Here, we have investigated IP-10 kinetics in Ethiopian adults with HIV during the initial year after ART initiation. IP-10 levels decreased in parallel with viral load during ART, and prevalent tuberculosis at ART initiation did not influence IP-10 kinetics. This study shows satisfactory performance for IP-10 as a surrogate marker for viral load in persons starting ART, with no influence of concomitant tuberculosis.
KEYWORDS: HIV, antiretroviral therapy, viral load, tuberculosis, IP-10, resource-limited settings
INTRODUCTION
WHO recommends universal virological monitoring for people with HIV (PWH) receiving antiretroviral treatment (ART) (1). However, access to viral load (VL) testing is limited in many areas, in particular low-income countries in sub-Saharan Africa (2). Clinical and immunological criteria, which are used for treatment monitoring if VL testing is not possible, have low predictive capacity for identification of treatment failure (3, 4). The use of algorithms or biomarkers to identify persons at increased likelihood of treatment failure, followed by VL confirmatory testing, has therefore been proposed as an alternative strategy (targeted VL testing) (5–11).
Among potential host biomarkers reflecting HIV replication, interferon-γ-inducible protein 10 (IP-10) has hitherto attracted most interest (12–14). IP-10, also known as C-X-C motif chemokine 10 (CXCL10), is a pro-inflammatory cytokine mainly secreted from monocytes and dendritic cells (15). Studies performed on ART recipients in Mozambique (16) and Ethiopia (17) have shown promising predictive capacity for identification of patients with elevated VL. However, several issues need to be resolved before IP-10 can be considered for clinical use. Patterns of IP-10 secretion may differ with regard to duration of ART, with anticipated higher levels during the initial phases of ART (18). Furthermore, several other conditions that are common in people with HIV in high-burden countries may affect IP-10 expression. In this context tuberculosis (TB), which is the leading opportunistic infection in PWH globally, is of particular importance. TB induces IP-10 secretion, and IP-10 has been proposed as a diagnostic marker for TB (19–21), as well as for monitoring of TB treatment (22). Yet, the impact of TB coinfection on IP-10 kinetics in PWH receiving ART has not been investigated.
Here, we present data on longitudinal kinetics of IP-10 expression during the initial year of ART, and the influence of TB co-infection and other patient characteristics on IP-10 kinetics. In addition, we have tested the performance of IP-10 as a screening tool for targeted VL testing during the first year after starting ART.
RESULTS
Participant characteristics.
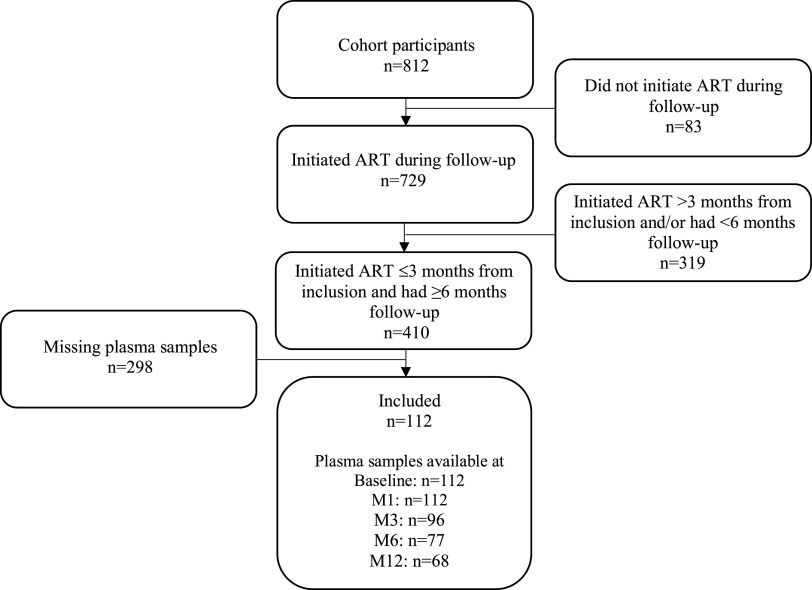
A flow chart of selection of participants for this study is shown in Fig. 1. Stored plasma samples (for IP-10 quantification) were available from 112 of 410 individuals who met study inclusion criteria. Plasma samples from the remaining 298 persons had been destroyed due to freezer failure in the study laboratory. Characteristics were similar for individuals with and without available samples, except for lower baseline CD4 counts among included participants (Table 1). In included individuals, median age was 33 years (IQR; 28–40) and 58% were female. Median baseline VL was log10 5.2 copies/ml (IQR, 4.8–5.6) and median baseline CD4 count was 156 cells/mm3 (IQR, 88–201). Eighteen persons (16%) were diagnosed with TB at baseline. No cases of incident TB were reported during the initial year of follow-up among those included in this study.
FIG 1.
Flow chart of study participant screening process. ART, antiretroviral therapy; M, month after ART initiation.
TABLE 1.
Characteristics of study participants and comparison of participants with and without available plasma samplesa
| Characteristic | Eligibleb (n = 410) | Excluded due to missing plasma samples (n = 298) | Included (n = 112) | P valuec |
|---|---|---|---|---|
| Age, yr | 33 (28–40) | 33 (28–40) | 33 (28–40) | 1.00 |
| Female | 235 (57.3%) | 170 (57.0%) | 65 (58.0%) | 0.86 |
| CD4 count, cells/mm3 | 166 (96–227) | 172 (103–241) | 156 (88–201) | 0.03 |
| MUAC, cm | 22.0 (20.4–24.0) | 22.0 (20.5–24.0) | 22.0 (20.0–24.5) | 0.74 |
| Concomitant TB | ||||
| All | 84 (20.5%) | 66 (22.1%) | 18 (16.1%) | 0.17 |
| Bacteriologically confirmed | 73/84 (86.9%) | 56/66 (84.8%) | 17 /18 (94.4%) | 0.38 |
| Clinically diagnosed | 11/84 (13.1%) | 10/66 (15.2%) | 1/18 (5.6%) | 0.17 |
| Baseline VL, log10 copies/ml | 5.2 (4.7–5.6) (n = 189) |
5.2 (4.7–5.6) (n = 109) |
5.2 (4.8–5.6) (n = 112) |
0.81 |
| ART regimen | ||||
| EFV + 3TC+TDF | 307 (74.9%) | 217 (72.8%) | 90 (80.4%) | 0.12 |
| EFV + 3TC+AZT | 20 (4.9%) | 11 (3.7%) | 9 (8.0%) | 0.07 |
| EFV + 3TC+d4T | 5 (1.2%) | 5 (1.7%) | 0 (0%) | 0.17 |
| NVP + 3TC+TDF | 45 (11.0%) | 38 (12.8%) | 7 (6.3%) | 0.06 |
| NVP + 3TC+AZT | 32 (7.8%) | 26 (8.7%) | 6 (5.4%) | 0.26 |
| NVP + 3TC+d4T | 1 (0.2%) | 1 (0.3%) | 0 (0%) | 0.54 |
Data presented as median (IQR) or n (%). MUAC, mid-upper arm circumference; TB, tuberculosis; VL, HIV viral load; ART, antiretroviral therapy; EFV, efavirenz; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate; AZT, zidovudine; d4T, stavudine; NVP, nevirapine.
ART initiation <3 months from inclusion and ≥6 months follow-up.
Comparing Excluded due to missing plasma samples with Included using the Mann-Whitney U test for continuous variables and Chi-square test for dichotomous variables.
Ninety-one participants (81.3%) were defined as VRs and 21 (18.7%) as virological nonresponders (VNRs). Seventeen VRs (18.7%) had TB; among these 10 (58.8%) started anti-TB treatment (ATT) before ART initiation, 4 (23.5%) started ATT within the first month after ART initiation and 3 (17.6%) started ATT at later time points during ART (Table S1 in the supplemental material).
Sixty VRs (65.9%) had complete IP-10 data up until month 6 (M6), and 41 VRs (45.1%) had complete IP-10 data up until month 12 (M12) excluding M6. These two subsets were used for longitudinal comparison of IP-10 levels and analyzed separately. Twenty-three VRs (25.3%) had complete IP-10 data at all time points and were included in both subsets.
IP-10 levels during ART.
IP-10 levels are presented in Table 2, with median IP-10 levels for VRs with M6 and M12 data, respectively, plotted longitudinally in Fig. 2. VRs with TB had higher IP-10 levels than individuals without TB at baseline (Mann-Whitney U test, P = 0.003) and month 1 (M1) (P = 0.036) but not at month 3 (M3) (P = 0.072), M6 (P = 0.209), or M12 (P = 0.411).
TABLE 2.
IP-10 levels during the initial 12 months of antiretroviral treatment in 112 HIV+ study participantsa
| IP-10 (pg/ml) |
|||||
|---|---|---|---|---|---|
| Participant sample | Baseline | M1 | M3 | M6 | M12 |
| All participants | 634 (436–971) n = 112 |
340 (236–582) n = 112 |
226 (145–342) n = 96 |
176 (127–300) n = 77 |
189 (117–302) n = 68 |
| VRs | |||||
| All | 650 (428–1,002) n = 91 |
337 (235–574) n = 91 |
218 (142–339) n = 78 |
165 (126–243) n = 68 |
159 (112–262) n = 51 |
| TB+ | 1,106 (627–1,704) n = 17 |
472 (307–737) n = 17 |
263 (189–670) n = 14 |
194 (148–333) n = 15 |
191 (132–213) n = 12 |
| TB- | 628 (391–885) n = 74 |
300 (215–515) n = 74 |
206 (136–317) n = 64 |
155 (125–231) n = 53 |
152 (105–269) n = 39 |
| VNRs | 557 (441–878) n = 21 |
406 (236–646) n = 21 |
253 (200–351) n = 18 |
319 (185–533) n = 9 |
343 (184–568) n = 17 |
Data presented as median (IQR). IP-10, interferon-γ-inducible protein 10; M, month after ART initiation; VRs, virological responders; VNRs, virological nonresponders; TB, tuberculosis.
FIG 2.
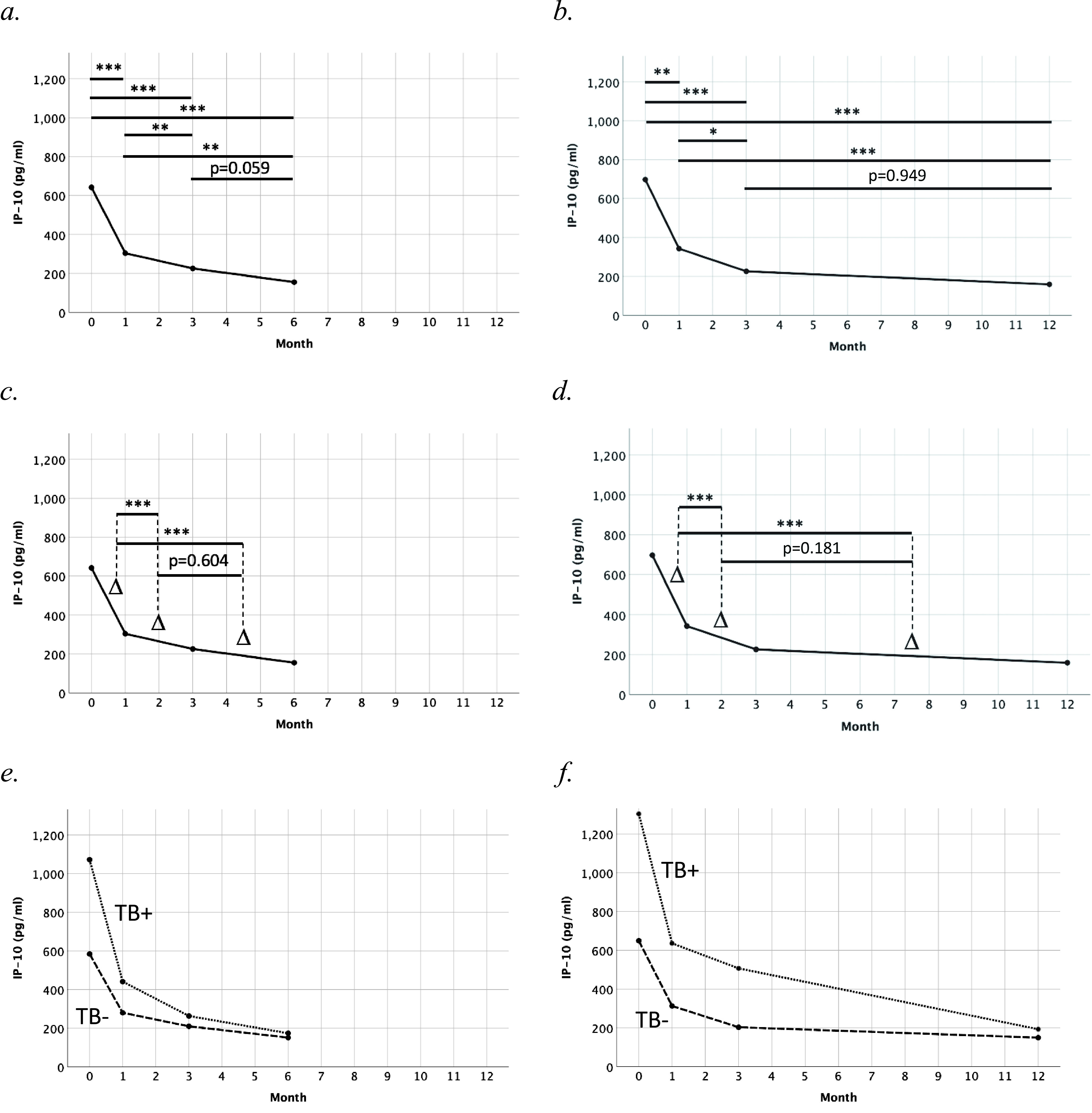
IP-10 kinetics during the initial 6–12 months of antiretroviral treatment in (a,c) VRs with IP-10 data available at B, M1, M3 and M6 (n = 60), (e) with (n = 12; dotted line) and without (n = 48; dash line) concomitant TB at baseline, and (b,d) VRs with IP-10 data available at B, M1, M3 and M12 (n = 41), (f) with (n = 9; dotted line) and without (n = 32; dash line) concomitant TB at baseline. Delta signs (Δ) represent IP-10 decline per month between two adjacent time points. P values correspond to pairwise comparisons from related-samples Friedman’s two-way ANOVA by ranks, adjusted by Bonferroni correction for multiple tests. IP-10, interferon-γ-inducible protein 10. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
For individuals with M6 data, pairwise comparison of time points showed lower IP-10 levels at later time points in all comparisons except for the comparison between M3 and M6 (Fig. 2a). For individuals with M12 data, pairwise comparison of time points showed lower IP-10 levels at later time points in all comparisons except for the comparison between M3 and M12 (Fig. 2b).
IP-10 decline between time points was compared similarly. For individuals with M6 data, pairwise comparison of time intervals showed faster IP-10 decline at baseline-M1 than M1–M3 and M3–M6 respectively, but no significant difference in IP-10 decline at M1–M3 compared with M3–M6 (Fig. 2c). For individuals with M12 data, pairwise comparison of time intervals showed faster IP-10 decline at baseline-M1 than M1–M3 and M3–M12 respectively, but no significant difference in IP-10 decline at M1–M3 compared with M3–M12 (Fig. 2d).
For descriptive purposes, median IP-10 levels for VRs with and without TB respectively, were plotted for individuals with M6 and M12 data respectively (Fig. 2e and f).
Baseline characteristics and IP-10 levels during ART.
Associations between baseline characteristics and IP-10 levels at baseline and follow-up time points were studied in VRs using linear regression models (Table 3). At baseline, concomitant TB (beta coefficient [B] 0.192, P < 0.01), VL (B 0.107, P < 0.01), and mid-upper arm circumference (MUAC; B -0.18, P = 0.03) were independently associated with IP-10 levels in multivariable analysis. No evidence of interaction was observed between TB, VL and MUAC (P > 0.5). At M1, baseline VL (B 0.137, P < 0.01) was the only variable independently associated with IP-10 levels in multivariable analysis. At M3 and M6, respectively, age (M3: B 0.010, P < 0.01; M6: B 0.008, P = 0.047) was the only variable independently associated with IP-10 levels in multivariable analysis. At M12, no significant association was detected in multivariable analysis.
TABLE 3.
Linear regression of baseline factors associated with log IP-10 levels at each clinical visit in virological responders (VRs)a
| Baseline |
Month 1 |
Month 3 |
Month 6 |
Month 12 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Uni. | Multi. | Uni. | Multi. | Uni. | Multi. | Uni. | Multi. | Uni. | Multi. |
| Age | 0.004* (0.003) |
0.004 (0.004) |
0.010*** (0.004) |
0.010*** (0.004) |
0.008** (0.004) |
0.008** (0.004) |
−0.004 (0.003) |
|||
| Gender (female) | −0.044 (0.061) |
0.038 (0.075) |
−0.062 (0.076) |
−0.057 (0.082) |
0.030 (0.072) |
|||||
| Baseline TB | 0.257*** (0.072) |
0.192*** (0.067) |
0.159* (0.094) |
0.181* (0.094) |
0.079 (0.097) |
0.065 (0.085) |
||||
| Log Baseline VL |
0.145*** (0.034) |
0.107*** (0.033) |
0.137*** (0.044) |
0.137*** (0.044) |
0.078* (0.042) |
0.052 (0.051) |
0.062* (0.045) |
|||
| Baseline CD4 cell count |
0.000* (0.000) |
0.000* (0.000) |
−0.001** (0.000) |
0.000 (0.000) |
−0.001* (0.000) |
|||||
| Baseline MUAC | −0.025*** (0.009) |
−0.018** (0.008) |
−0.022* (0.011) |
−0.016* (0.011) |
−0.016 (0.013) |
−0.002 (0.011) |
||||
| Intercept | 2.678*** (0.268) |
1.862*** (0.223) |
2.023*** (0.127) |
|||||||
| N | 91 | 91 | 78 | 68 | 51 | |||||
| R2 (adj.) | 0.250 | 0.089 | 0.080 | 0.044 | ||||||
Final multivariable models are shown, after stepwise removal of the least significant variable until only variables with P < 0.05 remained Date presented as unstandardized beta coefficient (standard error). Uni., univaritate; Multi., multivariate; TB, tuberculosis; VL, HIV viral load; MUAC, mid-upper arm circumference.
*, P < 0.2; **, P < 0.05; ***, P < 0.01.
Associations between baseline characteristics and IP-10 decline at specified time intervals (baseline–M1, M1–M3, M3–M6, and M6–M12) were also studied in VRs using linear regression models. Uni- and multivariable analyses were adjusted for IP-10 levels at the first time point of each analyzed time interval. No significant associations were observed, neither in uni- nor in multivariable analysis (Table S2 in the supplemental material).
Performance of IP-10 for targeted viral load testing.
We assessed the performance of IP-10 in identifying individuals in need of targeted VL testing (defined as individuals having VL ≥1,000 copies/ml) at different follow-up time points (in all study participants, i.e., VRs and VNRs). The numbers and proportions of participants with VL ≥1,000 copies/ml at different time points were 17/112 (15.2%) at M1, 6/95 (6.3%) at M3, 5/77 (6.5%) at M6, and 15/68 (22.1%) at M12. The corresponding area under the receiver operating characteristic (ROC) curve for IP-10 to classify participants with VL ≥1000 copies/ml were 0.75 (M1), 0.68 (M3), 0.56 (M6), and 0.79 (M12). The IP-10 threshold levels for identification of VL ≥1000 copies/ml were 276.5 pg/ml (M1), 260 pg/ml (M3), 140.5 pg/ml (M6), and 186 pg/ml (M12), respectively (Table S3 in the supplemental material).
DISCUSSION
In this study, we investigated plasma IP-10 levels in PWH during the initial year of ART. We observed a rapid IP-10 decline during the initial month after treatment initiation. Individuals with concomitant TB had significantly higher IP-10 levels before ART initiation, but both discrete IP-10 levels during ART and IP-10 decline were similar to those in persons without TB.
The marked initial IP-10 decline after starting ART corroborates findings from previous studies (23, 24). With our study design we could show that this decline mainly occurs during the initial month after ART initiation. Thereafter, IP-10 levels decline at a slower rate. This biphasic decline pattern resembles that of VL decline after ART initiation (25). Furthermore, we observed no significant difference between IP-10 levels at 3 months after ART initiation compared with 6 or 12 months, respectively, suggesting that IP-10 secretion is relatively stable after 3 months of suppressive ART. In a study conducted in Mozambique (16), median IP-10 levels for individuals with VL <150 copies/ml were markedly lower (38 versus 159 pg/ml) compared with previous data from this Ethiopian cohort (17). An obvious difference between the two studies is a longer median ART duration in the Mozambiquean study (41 months, versus 12 months in our material), which potentially could have explained the discrepancy in IP-10 levels; however, our current findings of IP-10 kinetics after 3 months of ART do not support that hypothesis.
IP-10 levels are elevated in active TB (26) and decline rapidly after initiation of ATT (27). In line with this, we found that concomitant TB was associated with higher IP-10 levels at baseline, but not at any time point after starting ART (14/17 VRs initiated ATT before or within 1 month after ART initiation). High levels of pro-inflammatory cytokines, including IP-10, are observed in TB-associated immune reconstitution inflammatory syndrome (TB-IRIS) (28–30), which is common in patients receiving concomitant ART and ATT. However, no cases of TB-IRIS occurred in the 18 persons with TB coinfection in this study. Interestingly, a pattern of IP-10 decline was found also in co-infected persons starting ART before ATT, suggesting that the inhibitory effect of blocked HIV replication on IP-10 levels is more pronounced than the stimulatory effect from ongoing TB infection (data not shown).
Previous studies have shown adequate predictive performance (sensitivity 91.5–91.7%; specificity 49.7–59.9%) for IP-10 at 12 months after ART initiation (17) and later (16) during ART. In this study, IP-10 showed similar performance at 12 months but lower performance at earlier time points during ART, albeit with wide confidence intervals (Table S3). Few individuals had VL ≥1,000 copies/ml at M3 (6.3%) and M6 (6.5%), respectively, limiting the precision of the statistical analyses on predictive performance at these time points.
With currently available technologies, and in view of the need for further scaling-up of ART delivery in resource-limited settings, there is a need to investigate alternatives to universal VL testing for ART monitoring (31). Targeting VL testing toward individuals with higher risk of viremia could potentially enable more efficient use of available resources. In particular, in settings with low frequency of virological failure, such an approach could be cost-saving with little effect on clinical outcome (8, 9). New techniques, such as GeneXpert, offer simplified alternatives to centralized HIV RNA quantification, but have certain limitations such as a low throughput capacity, relatively high cost and need for basic laboratory facilities. In this context IP-10, for which a point-of-care assay is under development, might be useful as a screening marker to identify persons at increased likelihood of having treatment failure. However, further studies are needed to determine optimal threshold levels and diagnostic performance in various settings.
This study has some limitations. Several participants from the source cohort could not be included because their stored samples had been destroyed due to freezer failure, leading to a relatively small sample size with a risk of type II error. Although the intensified TB case finding undertaken during this study suggests that the prevalence of undiagnosed TB was low in our material, several other infections that are common in PWH in sub-Saharan Africa, such as hepatitis B (32), hepatitis C (33, 34), malaria (35) and cryptosporidiosis (36) can also upregulate IP-10 expression. Systematic screening for these infections was not included in the cohort study protocol. Finally, our study participants started ART according to Ethiopian guidelines in use at the time of inclusion; hence, our findings cannot be generalized to individuals with less advanced immunosuppression at the time of starting ART.
In conclusion, IP-10 levels in patients starting ART showed a biphasic decline pattern, with a sharp decline during the initial month of ART. Persons with TB coinfection had higher IP-10 levels at baseline compared to persons without TB, but had similar kinetics during ART.
MATERIALS AND METHODS
Study population.
Participants were identified from a cohort study conducted 2011–2015 at five Ethiopian health centers (37, 38). For this cohort, consenting HIV-positive adults (≥18 years) who met criteria for starting ART (according to Ethiopian National ART guidelines 2012; CD4 count <350 cells/mm3 and/or WHO stage 4 disease) were included. Participants were followed for up to 4 years (1, 3, 6, and 12 months after ART initiation; and biannually thereafter), with venous blood sampling at each visit. Aliquots of plasma were stored at −80°C at the study laboratory (Adama Public Health Research and Referral Laboratory Center, Adama, Ethiopia). Plasma samples used in the current study had been collected at study inclusion (pre-ART); and at M1, M3, M6, and M12 after ART initiation.
At inclusion, all participants were investigated for active TB by smear microscopy, GeneXpert MTB/RIF, and liquid culture on two morning sputa and, in case of peripheral lymphadenopathy, fine-needle aspirates (39). Besides bacteriological testing, participants meeting clinical and radiological criteria (according to Ethiopian guidelines) were also diagnosed with TB. Bacteriological TB investigations were repeated during follow-up in case of clinical manifestations suggestive of active TB. Health center clinicians were responsible for starting ART and treating opportunistic infections (including TB) in accordance with national guidelines (40, 41).
For the current study, cohort participants who started ART ≤3 months after the inclusion visit, and who were followed for ≥6 months thereafter were included. In addition, available stored plasma samples from at least 3 time points (baseline; M1; and M6 or M12) were required for inclusion.
Study definitions.
To explore IP-10 kinetics after ART initiation, and the impact of patient characteristics, we included persons showing virological response to ART (VRs). VRs were defined as individuals having VL <150 copies/ml within 6 months of ART initiation (measured at month 1, 3 and/or 6), with at least one subsequent VL <1000 copies/ml, and no VL results ≥1000 copies/ml after reaching VL <150 copies/ml during the initial 12 months of ART. Participants who did not meet these criteria were defined as VNRs.
Due to a sample availability mismatch between M6 and M12, few individuals had complete IP-10 data at all time points (baseline, M1, M3, M6 and M12). Thus, longitudinal comparison of IP-10 levels was conducted separately for individuals with complete IP-10 data up until M6 (baseline, M1, M3, M6) and individuals with complete IP-10 data up until M12 excluding M6 (baseline, M1, M3, M12), respectively. Individuals with IP-10 data at all time points were included in both analyses.
Laboratory procedures.
HIV RNA quantification was performed in batches during the study period using Abbott Real-Time HIV-1 assay (Abbott Molecular Inc., Des Plaines, IL; lower detection limit 150 copies/ml). IP-10 levels were analyzed in duplicate on stored plasma, using the Human CXCL10/IP-10 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Samples with IP-10 concentration above the assay detection limit were rerun at higher dilutions. Duplicates with coefficient of variation (CV) ≥15% were also rerun. External controls were used to measure inter-assay variability.
Statistical analysis.
IP-10 levels at baseline and during ART were presented using medians and interquartile ranges (IQR). Plots were fitted showing IP-10 kinetics for individuals with M6 and M12 data, respectively. IP-10 levels at baseline and during follow-up were compared using related-samples Friedman’s two-way ANOVA by ranks, with pairwise comparisons adjusted by Bonferroni correction for multiple tests. IP-10 decline per month was calculated between time points (baseline–M1, M1–M3, and M3–M6 for individuals with M6 data; baseline–M1, M1–M3, and M3–M12 for individuals with M12 data) and compared longitudinally using the same test.
To study the impact of individual baseline characteristics on IP-10 levels at discrete time points, uni- and multivariable linear regression models were applied. Log transformed IP-10 levels at baseline, M1, M3, M6, and M12, respectively, were used as outcome variables in separate analyses. The following baseline characteristics were used as predictor variables: age, sex, prevalent active TB, CD4 count, log VL, and MUAC. Multivariable analysis was conducted using a backward stepwise approach in which variables with P < 0.2 in univariable analysis were included in the multivariable model, after which the least significant variable was removed until only variables with P < 0.05 remained. If two or more variables remained in the final model, we assessed whether there were interactions between the variables.
To study the impact of individual baseline characteristics on IP-10 decline a similar approach was used. Uni- and multivariable linear regression models were applied, with IP-10 decline at B-M1, M1–M3, M3–M6, and M6–M12, respectively, as outcome variables in separate analyses. The same baseline characteristics as above were used as predictor variables. These regression models were adjusted for IP-10 levels at the start of each analyzed time-interval (e.g., analysis with IP-10 decline at M1–M3 as outcome variable was adjusted for IP-10 levels at M1). Multivariable analysis was conducted using a backward stepwise approach as described above.
The performance of IP-10 for identification of individuals in need of targeted VL testing (defined as individuals having VL ≥1000 copies/ml) at different time points after ART initiation was investigated in all included participants, i.e., both VRs and VNRs, using ROC curves. Using the ROC curves, IP-10 threshold levels were determined for each time point. At these threshold levels, specificity, positive predictive value and negative predictive value with corresponding 95% confidence intervals were also calculated.
Ethical considerations.
Ethical approval was obtained from the National Research Ethics Review Committee, Ministry of Science and Technology, Addis Abeba, Ethiopia, and the Regional Ethical Review Board, Lund University, Sweden. Written informed consent was obtained from all participants before inclusion. Impartial witnesses confirmed consent from illiterate participants.
ACKNOWLEDGMENTS
We extend our gratitude to all study participants, and to the staff at the health centers as well as the staff at the Adama Public Health Research and Referral Laboratory Center for their contributions to this study.
Conceived and designed the study: J.T., P.B., D.N., P.M., A.R. Data collection: J.T., P.B., T.T.B., F.T., T.A., P.M., A.R. Analyzed the data: J.T., P.B., G.M., P.M., A.R. Wrote the paper: J.T., P.B., A.R. All authors read and approved the final manuscript.
This work was supported by Region Skåne (regional research grant); the Swedish State (under the agreement between the Swedish government ant the county councils, the ALF-agreement); The Royal Physiographic Society in Lund, Sweden; and through a private donation to Lund University.
Footnotes
Supplemental material is available online only.
Contributor Information
Johannes Thorman, Email: johannesthorman@gmail.com.
Anton Reepalu, Email: anton.reepalu@med.lu.se.
Yongjun Sui, National Institutes of Health.
REFERENCES
- 1.WHO. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed. https://www.who.int/hiv/pub/arv/arv-2016/en/. [PubMed]
- 2.Roberts T, Cohn J, Bonner K, Hargreaves S. 2016. Scale-up of routine viral load testing in resource-poor settings: Current and future implementation challenges. Clin Infect Dis 62:1043–1048. doi: 10.1093/cid/ciw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutherford GW, Anglemyer A, Easterbrook PJ, Horvath T, Vitoria M, Penazzato M, Doherty MC. 2014. Predicting treatment failure in adults and children on antiretroviral therapy: A systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS 28:S161–S169. doi: 10.1097/QAD.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 4.Gunda DW, Kidenya BR, Mshana SE, Kilonzo SB, Mpondo BCT. 2017. Accuracy of WHO immunological criteria in identifying virological failure among HIV-infected adults on First line antiretroviral therapy in Mwanza, North-western Tanzania. BMC Res Notes 10:45. doi: 10.1186/s13104-016-2334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meya D, Spacek LA, Tibenderana H, John L, Namugga I, Magero S, Dewar R, Quinn TC, Colebunders R, Kambugu A, Reynolds SJ. 2009. Development and evaluation of a clinical algorithm to monitor patients on antiretrovirals in resource-limited settings using adherence, clinical and CD4 cell count criteria. J Int AIDS Soc 12:3. doi: 10.1186/1758-2652-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abouyannis M, Menten J, Kiragga A, Lynen L, Robertson G, Castelnuovo B, Manabe YC, Reynolds SJ, Roberts L. 2011. Development and validation of systems for rational use of viral load testing in adults receiving first-line ART in sub-Saharan Africa. AIDS 25:1627–1635. doi: 10.1097/QAD.0b013e328349a414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans DH, Fox MP, Maskew M, McNamara L, MacPhail P, Mathews C, Sanne I. 2014. CD4 criteria improves the sensitivity of a clinical algorithm developed to identify viral failure in HIV-positive patients on antiretroviral therapy. J Int AIDS Soc 17:19139–19139. doi: 10.7448/IAS.17.1.19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mungwira RG, Divala TH, Nyirenda OM, Kanjala M, Muwalo F, Mkandawire FA, Choko A, Taylor TE, Mallewa J, van Oosterhout JJ, Laufer MK, Laurens MB. 2018. A targeted approach for routine viral load monitoring in Malawian adults on antiretroviral therapy. Trop Med Int Health 23:526–532. doi: 10.1111/tmi.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan V, Thai S, Koole O, Menten J, Meheus F, van Griensven J, Lynen L. 2013. Validation of a clinical prediction score to target viral load testing in adults with suspected first-line treatment failure in resource-constrained settings. J Acquir Immune Defic Syndr 62:509–516. doi: 10.1097/QAI.0b013e318285d28c. [DOI] [PubMed] [Google Scholar]
- 10.Van Griensven J, Phan V, Thai S, Koole O, Lynen L. 2014. Simplified clinical prediction scores to target viral load testing in adults with suspected first line treatment failure in Phnom Penh, Cambodia. PLoS One 9:e87879. doi: 10.1371/journal.pone.0087879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorman J, Björkman P, Tesfaye F, Jeylan A, Balcha TT, Reepalu A. 2019. Validation of the Viral Load Testing Criteria – an algorithm for targeted viral load testing in HIV‐positive adults receiving antiretroviral therapy. Trop Med Int Health 24:356–362. doi: 10.1111/tmi.13201. [DOI] [PubMed] [Google Scholar]
- 12.Malherbe G, Steel HC, Cassol S, de Oliveira T, Seebregts CJ, Anderson R, Cassol E, Rossouw TM. 2014. Circulating biomarkers of immune activation distinguish viral suppression from nonsuppression in HAART-treated patients with advanced HIV-1 subtype C infection. Mediators Inflamm 2014:1–7. doi: 10.1155/2014/198413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer-Smith T, Tedaldi EM, Rappaport J. 2008. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: Potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses 24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, Cohen MH, Zhang J, Greenblatt RM, Desai S, Wu S, Landay AL, Gange SJ, Norris PJ, Women's Interagency HIV Study. 2011. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS 25:1823–1832. doi: 10.1097/QAD.0b013e3283489d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons RP, Scully EP, Groden EE, Arnold KB, Chang JJ, Lane K, Lifson J, Rosenberg E, Lauffenburger DA, Altfeld M. 2013. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 27:2505–2517. doi: 10.1097/01.aids.0000432455.06476.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastor L, Casellas A, Rupérez M, Carrillo J, Maculuve S, Jairoce C, Paredes R, Blanco J, Naniche D. 2017. Interferon-γ–inducible protein 10 (IP-10) as a screening tool to optimize human immunodeficiency virus RNA monitoring in resource-limited settings. Clin Infect Dis 65:1670–1675. doi: 10.1093/cid/cix600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reepalu A, Arimide DA, Balcha TT, Björkman P, Medstrand. P. 2020. Interferon-γ-inducible protein 10 – a potential marker for targeted viral load monitoring of antiretroviral treatment? JAIDS 83:475–478. doi: 10.1097/qai.0000000000002292. [DOI] [PubMed] [Google Scholar]
- 18.Relucio KI, Beernink HT, Chen D, Israelski DM, Kim R, Holodniy M. 2005. Proteomic analysis of serum cytokine levels in response to highly active antiretroviral therapy (HAART). J Proteome Res 4:227–231. doi: 10.1021/pr049930y. [DOI] [PubMed] [Google Scholar]
- 19.Wergeland I, Pullar N, Assmus J, Ueland T, Tonby K, Feruglio S, Kvale D, Damås JK, Aukrust P, Mollnes TE, Dyrhol-Riise AM. 2015. IP-10 differentiates between active and latent tuberculosis irrespective of HIV status and declines during therapy. J Infect 70:381–391. doi: 10.1016/j.jinf.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Qiu X, Xiong T, Su X, Qu Y, Ge L, Yue Y, Zeng Y, Li W, Hu P, Mu D. 2019. Accumulate evidence for IP-10 in diagnosing pulmonary tuberculosis. BMC Infect Dis 19:924. doi: 10.1186/s12879-019-4466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrone L, Cannas A, Vanini V, Cuzzi G, Aloi F, Nsubuga M, Sserunkuma J, Nazziwa RA, Jugheli L, Lukindo T, Girardi E, Antinori A, Pucci L, Reither K, Goletti D. 2016. Blood and urine inducible protein 10 as potential markers of disease activity. Int J Tuber Lung Dis 20:1554–1561. doi: 10.5588/ijtld.16.0342. [DOI] [PubMed] [Google Scholar]
- 22.Tonby K, Ruhwald M, Kvale D, Dyrhol-Riise AM. 2015. IP-10 measured by dry plasma spots as biomarker for therapy responses in mycobacterium tuberculosis infection. Sci Rep 5:9223–9226. doi: 10.1038/srep09223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE, Feldpausch MN, Martin A, Melbourne K, Triant VA, Suchindran S, Lee H, Hoffmann U, Williams KC, Tawakol A, Grinspoon SK. 2016. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol 1:474–480. doi: 10.1001/jamacardio.2016.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Paula HHS, Ferreira ACG, Caetano DG, Delatorre E, Teixeira SLM, Coelho LE, João EG, de Andrade MM, Cardoso SW, Grinsztejn B, Veloso VG, Morgado MG, Guimarães ML, Côrtes FH. 2018. Reduction of inflammation and T cell activation after 6 months of cART initiation during acute, but not in early chronic HIV-1 infection. Retrovirology 15:76. doi: 10.1186/s12977-018-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenigl M, Chaillon A, Moore DJ, Morris SR, Mehta SR, Gianella S, Amico KR, Little SJ. 2016. Rapid HIV viral load suppression in those initiating antiretroviral therapy at first visit after HIV diagnosis. Sci Rep 6:1–5. doi: 10.1038/srep32947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan ZA, Wong EB, Ndung'u T, Kasprowicz VO, Bishai WR. 2015. Latent and active tuberculosis infection increase immune activation in individuals co-infected with HIV. EBioMedicine 2:334–340. doi: 10.1016/j.ebiom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Basteiro AL, Mambuque E, den Hertog A, Saavedra B, Cuamba I, Oliveras L, Blanco S, Bulo H, Brew J, Cuevas LE, Cobelens F, Nhabomba A, Anthony R. 2017. IP-10 kinetics in the first week of therapy are strongly associated with bacteriological confirmation of tuberculosis diagnosis in HIV-infected patients. Sci Rep 7:14302. doi: 10.1038/s41598-017-13785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conesa-Botella A, Meintjes G, Coussens AK, van der Plas H, Goliath R, Schutz C, Moreno-Reyes R, Mehta M, Martineau AR, Wilkinson RJ, Colebunders R, Wilkinson KA. 2012. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis 55:1004–1011. doi: 10.1093/cid/cis577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarti LA, Boucherie C, Bugault F, Cumont M-C, Roussillon C, Breton G, Patey O, Chêne G, Richert L, Lortholary O. 2014. Biomarkers of CD4+ T-cell activation as risk factors for tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 28:1593–1602. doi: 10.1097/QAD.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 30.Oliver BG, Elliott JH, Price P, Phillips M, Saphonn V, Vun MC, Kaldor JM, Cooper DA, French MA. 2010. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis 202:1728–1737. doi: 10.1086/657082. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Bärnighausen T, Eyal N. 2019. When global ART budgets cannot cover all patients, who should be eligible? J Acquir Immune Defic Syndr 81:134–137. doi: 10.1097/QAI.0000000000002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao K, Yang T, Sun M, Zhang W, An Y, Chen G, Jin L, Shang Q, Song W. 2017. IP-10 expression in patients with chronic HBV infection and its ability to predict the decrease in HBsAg levels after treatment with entecavir. Mol Cells 40:418–425. doi: 10.14348/molcells.2017.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patzwahl R, Meier V, Ramadori G, Mihm S. 2001. Enhanced expression of interferon-regulated genes in the liver of patients with chronic hepatitis C virus infection: Detection by suppression-subtractive hybridization. J Virol 75:1332–1338. doi: 10.1128/JVI.75.3.1332-1338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascia C, Lichtner M, Zuccalà P, Vita S, Tieghi T, Marocco R, Savinelli S, Rossi R, Iannetta M, Campagna M, Schiavone F, Mengoni F, Russo G, Mastroianni CM, Vullo V. 2017. Active HCV infection is associated with increased circulating levels of interferon-gamma (IFN-γ)-inducible protein-10 (IP-10), soluble CD163 and inflammatory monocytes regardless of liver fibrosis and HIV coinfection. Clin Res Hepatol Gastroenterol 41:644–655. doi: 10.1016/j.clinre.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Jain V, Armah HB, Tongren JE, Ned RM, Wilson NO, Crawford S, Joel PK, Singh MP, Nagpal AC, Dash AP, Udhayakumar V, Singh N, Stiles JK. 2008. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar J 7:1–15. doi: 10.1186/1475-2875-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H-C, Dann SM, Okhuysen PC, Lewis DE, Chappell CL, Adler DG, White AC. 2007. High levels of CXCL10 are produced by intestinal epithelial cells in AIDS patients with active cryptosporidiosis but not after reconstitution of immunity. Infect Immun 75:481–487. doi: 10.1128/IAI.01237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reepalu A, Balcha TT, Skogmar S, et al. 2014. High rates of virological suppression in a cohort of human immunodeficiency virus- positive adults receiving antiretroviral therapy in ethiopian health centers irrespective of concomitant tuberculosis. Open Forum Infect Dis 1. doi: 10.1093/ofid/ofu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reepalu A, Balcha TT, Sturegård E, Medstrand P, Björkman P. 2017. Long-term outcome of antiretroviral treatment in patients with and without concomitant tuberculosis receiving health center–based care—results from a prospective cohort study. Open Forum Infect Dis 4:ofx219. doi: 10.1093/ofid/ofx219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balcha TT, Sturegård E, Winqvist N, Skogmar S, Reepalu A, Jemal ZH, Tibesso G, Schön T, Björkman P. 2014. Intensified tuberculosis case-finding in HIV-positive adults managed at ethiopian health centers: diagnostic yield of xpert MTB/RIF compared with smear microscopy and liquid culture. PLoS One 9:e85478. doi: 10.1371/journal.pone.0085478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ethiopian Federal Ministry of Health. 2012. Guidelines for clinical and programmatic management of TB, Leprosy and TB/HIV in Ethiopia. https://etharc.org/index.php/resources/download/view.download/33/709.
- 41.Ethiopian Federal Ministry of Health. 2008. Guidelines for management of opportunistic infections and anti-retroviral treatment in adolescents and adults in Ethiopia. http://digicollection.org/hss/en/m/abstract/Js19146en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM01810-21_Supp_1_seq2.pdf, PDF file, 0.2 MB (180KB, pdf)