Lay Summary
Being physically active has many social, emotional, and health benefits, but very few individuals are active enough to see those benefits. Using interventions that are tailored, in other words, individualized to a person’s characteristics, needs, preferences, and/or situation, may help improve physical activity participation rates. However, a better understanding of how to do tailoring is needed. Our collaboration reviewed the literature and convened to suggest two key opportunities to better understand how tailored approaches to physical activity can be done: (a) improve engagement of those who the research is intended for and (b) understand the ethical impacts and patient/provider experience of using technology to support tailoring.
Keywords: Physical activity, Behavior, Implementation, Intervention, Exercise
Abstract
A physically active lifestyle provides innumerable benefits; yet, few individuals are physically active enough to reap those benefits. Tailored physical activity interventions may address low rates of physical activity by offering individualized strategies that consider a person’s characteristics, needs, preferences, and/or context, rather than the traditional one-size-fits-all approach. However, the tailoring methodology is in its nascency, and an understanding of how best to develop such interventions is needed. In this commentary, we identify future directions to enhance the impact of tailored interventions designed to increase physical activity participation. A multi-country collaborative was established to review the literature and discuss an agenda for future research. Two overarching research opportunities are suggested for improving the development of tailored, behavioral physical activity interventions: (a) optimize the engagement of diverse knowledge users in intervention co-design and (b) examine ethical considerations that may impact the use of technology to support tailored physical activity delivery. Specifically, there is a need for better reporting and evaluation of knowledge user involvement alongside targeting diversity in the inclusion of knowledge users. Furthermore, while technology boasts many opportunities to increase the scale and precision of interventions, examinations of how it impacts recipients’ experiences of and participation in tailored interventions are needed to ensure the benefits of technology use outweigh the risks. A better understanding of these research areas will help ensure that the diverse needs of individuals are met, technology is appropriately used to support tailoring, and ultimately it improves the effectiveness of tailored physical activity interventions.
Implications.
Practice: Practitioners using technology to support physical activity behavior change should be aware of potential positive and negative patient experiences.
Policy: Policy-makers should explore strategies to involve those who are hardly reached in research in order to diversify the applicability of tailored physical activity interventions to marginalized populations.
Research: Future research should be aimed at understanding the ethical impacts of using technology to support tailored physical activity interventions while reporting and evaluating diverse knowledge user involvement in intervention design.
Introduction
Physical activity is a highly effective and practical strategy for maintaining well-being at all ages [1]. Overwhelming scientific evidence supports the universal prescription of physical activity to improve population health and well-being, but fewer than 20% of Canadians engage in enough physical activity to meet physical activity guideline recommendations [2–4]. The literature identifies many reasons for this level of inactivity that span individual through to policy-level barriers [5]. However, both academics and clinicians frequently describe a need for precision in prescribing and promoting physical activity to improve the current situation [6–8].
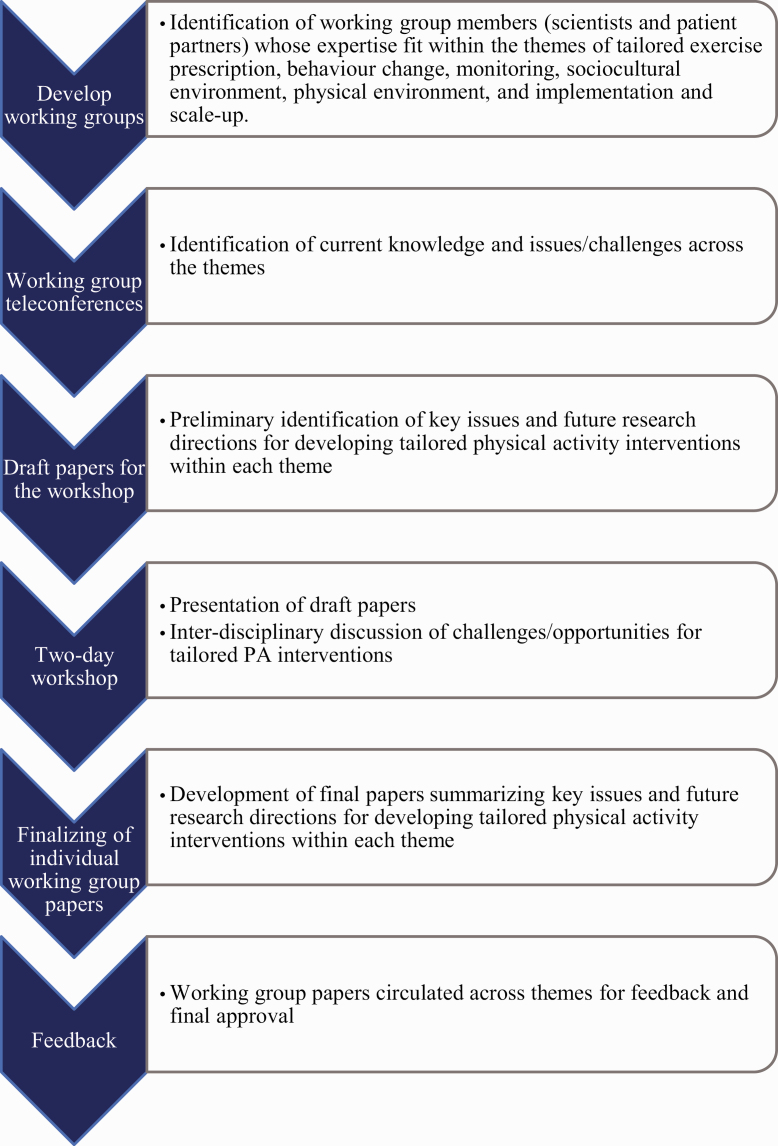
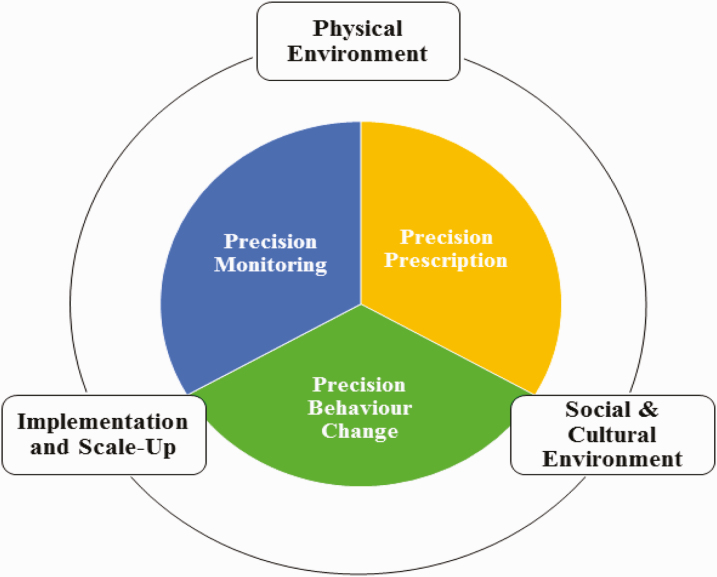
To address this challenge, scientists from medicine, population health, psychology, education, and computer science, as well as patient partners were invited as part of a university-based initiative to support inter-disciplinary research networks. Members were from Canada, Denmark, the UK, and the U.S. Group members collaborated over a 2-year period with an in-person workshop held on May 6–7, 2019, in Vancouver, Canada (Fig. 1). The group discussed how physical activity might be optimally prescribed, monitored, and promoted with better precision to enable implementation and scale up within and across different physical and sociocultural environments (Fig. 2). Their goal was to identify research opportunities to improve the tailored approach across these research areas. Their discussions and insights are summarized in six papers [9–13]. In this paper, we aim to identify future directions for improving the effectiveness of tailored interventions to increase physical activity participation.
Fig. 1.
Recommendation development process.
Fig. 2.
Depiction of the broader research group including three core research themes—precision monitoring, precision prescription, and precision behavior change—which are nestled within the context of three encompassing themes—social and cultural environment, physical environment, and implementation and scale up.
Tailoring in physical activity behavior change interventions: A growing area of opportunity
Tailoring can be defined as gathering or assessing information from a person to develop and deliver individualized intervention components. Unlike a one-size-fits-all approach, individualized strategies take into account a person’s characteristics, needs, preferences, and context [14]. In tailoring physical activity interventions, individual factors (e.g., psychosocial factors, disease symptoms, and physical activity history) are assessed by inquiry, observation, or monitoring and then used to select intervention components including behavior change techniques, intervention delivery parameters, and physical activity prescriptions to improve physical activity participation [8, 15].
While researchers have called for a need for tailored physical activity interventions [6–8], the evidence for the effectiveness of tailoring is mixed. On the one hand, evidence in support of tailoring shows that some of the most effective physical activity behavior change techniques include action planning, problem solving, and providing feedback on behavior, all of which rely on some form of tailoring [16–19]. Tailored interventions assessed using randomized controlled trials have demonstrated significant improvements in physical activity participation across populations with chronic disease and disability, with effects observed up to 3 years post-intervention [20–23]. A review of nurse-delivered physical activity interventions demonstrated that of 15 studies that improved physical activity, 11 used tailoring strategies such as providing motivation-specific strategies or setting individualized goals [24]. Furthermore, several moderators of physical activity that signal the value of tailored interventions have previously been identified, including gender, age, self-efficacy, personality traits, and proximity to recreation resources [10, 25, 26].
On the other hand, Conn et al. conducted a meta-analysis of 358 studies of interventions aimed at increasing physical activity among healthy adults [27]. A sub-analysis conducted within this review demonstrated that generic interventions (Cohen’s d = 0.2) were more effective than tailored interventions (Cohen’s d = 0.04) [27], but the robustness of the findings was questioned as 196 generic interventions were compared to only 10 tailored interventions [27]. Furthermore, the methods used for tailoring within the included interventions were not provided, making it challenging to determine the active ingredients of the tailoring interventions and if they were delivered as intended. In a meta-analysis of internet-based lifestyle interventions, a sub-analysis showed that the 11 interventions that used theory or behavioral predictors to tailor intervention components showed small effects (g = 0.21; 95% CI [0.07, 0.27]) [28]. It should be noted that support for the role of psychological theory in intervention effectiveness is mixed [26, 29, 30]. Likewise, a meta-analysis of 25 computer-tailored interventions demonstrated small effects on physical activity behavior (g = 0.16; 95% CI [0.10, 0.21]). However, these results may be a reflection of the remote-based delivery mode, as the overall effects across all interventions (including generic) were also small [18, 19]. Taken together, while these findings have their limitations, the evidence from available meta-analyses suggests that tailoring is either less effective than generic interventions or only produces small effects.
To date, methods for tailoring are ill-defined and haphazard. Limited evidence is available on which human and contextual factors should be assessed for tailoring, how the information assessed is then translated to tailored intervention components, and what intervention components should be tailored (e.g., frequency, delivery modes, and behavior change techniques) [31, 32]. In other words, to assess whether tailoring is effective, we need to understand “what, why, when, and how” interventions should be tailored (Ref. 30, p. 1). To this end, our group identified two research opportunities to address this need to increase the impact of tailored physical activity interventions: (a) optimize the engagement of diverse knowledge users in co-designing tailored interventions and (b) examine ethical considerations that may affect the use of technology to support tailored physical activity delivery (for a list of other research priorities identified in the collaboration process, please see Supplementary Appendix A).
Priority 1: Optimize the engagement of diverse knowledge users in co-designing tailored interventions
Knowledge users are defined as those who are the recipients of research impacts, such as patients, health professionals delivering an intervention, funders, and policy decision makers [33]. Support for patient/public engagement has built considerable momentum over the last two decades, with funding agencies recommending knowledge user partnerships as a means to improve research quality [34–36]. Co-developing research with knowledge users improves the applicability, impact, and translation of research findings [33, 37, 38]. Furthermore, many resources and guiding principles for engaging end-users in intervention development have been published [39–42]. Given the prerequisite for tailored physical activity interventions to address individual needs, it seems logical to engage knowledge users to optimize the impact of tailored physical activity interventions.
To capitalize on the value of knowledge user engagement, it is essential to focus on how and who we engage. How we optimally engage knowledge users in tailored intervention design requires a better understanding of the key ingredients for meaningful engagement [33]. We systematically reviewed tailored physical activity interventions designed for people with arthritis. Of the 37 interventions identified, 10 interventions involved knowledge users in some capacity of intervention design (Supplementary Appendix B). Importantly, how knowledge users were reported to be involved ranged widely and it is unclear whether knowledge user engagement had an impact on intervention effectiveness (Table 1). Indeed, the process and impact of knowledge user engagement are often under-reported and unexamined [43, 44]. The GRIPP 2 is one such example of a tool that can be used to improve knowledge user engagement reporting [45]. It comprises a checklist developed through international consensus that defines key items for reporting with the intent of improving the quality, transparency, and consistency of knowledge user engagement in research [45]. Evaluations of knowledge user engagement have been conducted previously in the broader literature including using interviews [46], reflections in casebooks [47, 48], and financial value estimates [49]. Borrowing from these examples and others who have established measuring the impact of knowledge user engagement as a research priority [38] may be a tangible next step in the co-development of tailored physical activity interventions. In summary, improved reporting and evaluation are needed to enhance knowledge user co-development of interventions and demonstrate the proposed value of involving those who the intervention is intended for.
Table 1.
Reporting of knowledge user involvement in the design of tailored physical activity interventions for people with arthritis
| Author, year | Reported knowledge user involvement in tailored intervention design |
|---|---|
| Allen et al., 2018 | A multidisciplinary team, including physical therapists, physicians and a software company developed the intervention. |
| Allen et al., 2020 | Input was captured from patients with osteoarthritis and their partners, primary care providers and representatives from community programs that provide physical activity resources appropriate for patients with osteoarthritis. |
| Bossen et al., 2013 | An iterative design methodology was used to test, analyze, and refine the intervention. Researchers conducted a focus group, in home observations, a pilot study, and interviews. Heuristic evaluation and a thinking aloud approach were used to determine the usability of the web-based program. End-users (patients with knee and/or hip osteoarthritis) were involved continuously throughout the development process. |
| Lamb et al., 2015 | Following a pilot study and therapist and patient recommendations, patient materials, exercise instructions and some trial procedures were modified. This included the assessment form, an intensity scale, and other forms used to document intervention delivery, instruction for exercise testing, and information provided to patients at discharge from treatment. |
| Lee et al., 2016 | The intervention was developed in consultation with a panel of experts including two physiotherapists, a medical officer, a sport scientist, a Traditional Chinese Medicine practitioner, a community geriatric nurse, and a social worker. |
| Li et al., 2017 | Patient collaborators were consulted during the study design process to provide insight into patient values and concerns about physical activity and the use of wearable activity trackers. |
| Li et al., 2018 | A physiotherapist involved in the feasibility study provided feedback to refine the intervention. |
| Li et al., 2020a | The intervention app was co-developed with three patient research partners and physiotherapists. |
| Li et al., 2020b | Patient partners provided feedback on the research questions and study design. Patient partners co-developed the intervention app, tested the beta-version, and assisted in participant recruitment through their affiliated organizations and social media contacts. |
| Quicke et al., 2018 | A networking meeting was held where national experts and patients advocates agreed upon the intervention. |
References provided in Supplementary Appendix B.
With respect to who we engage in intervention co-development, the individualized nature of tailored interventions begs the question, “who are the knowledge users?” Individuals experience a range of social factors that overlap and likely moderate tailored intervention effects (e.g., socio-economic status, social capital, geographic location, race, employment, religion, education, gender, and sexuality) [50]. Concerns about diversity of engagement have been raised previously, with those who face multiple and intersecting barriers to engagement often not being included in the research process [43]. For example, individuals in remote communities or who are unable to participate due to their health or life circumstances are under-represented in the current practice [51]. Indeed, engaging diverse and especially hardly reached individuals is a challenge all co-developed research faces, but is of particular importance for tailored interventions that are purported to meet the needs of individuals. Intersectionality explains social exclusion in terms of interconnected and overlapping systems of oppression and discrimination and may be an appropriate lens to examine how to foster diversity in knowledge user engagement [52]. An understanding of intersectionality could inform not only who should be at the table in intervention development but also how diverse characteristics may interact and influence the outcome of tailored physical activity interventions [11].
Take-home message for tailored physical activity intervention research
Engaging knowledge users may help identify and develop effective tailoring methods that take into account a broad range of human and contextual factors. We therefore need to both understand how to engage knowledge users in tailored intervention development through better reporting and evaluation and ensure that we target diversity in our inclusion of knowledge users. An understanding of how best to engage diverse knowledge users in intervention design may help refine a tailoring methodology that is appropriate for varying ages, genders, ethnicities, levels of education, and other individual factors that tailored interventions are uniquely positioned to consider.
Priority 2: Examine ethical considerations that may impact the use of technology to support tailored physical activity delivery
Many health technologies are available to equip health professionals to deliver tailored physical activity intervention to individuals in the community more effectively and efficiently [28, 53, 54]. Virtual coaching, mobile apps, and monitoring devices (e.g., accelerometers, GPS, and consumer wearables) extend the reach of behavior support programming to people living in rural areas or with limited access to transportation [55]. These programs may support personal feedback to users when face-to-face modes are not possible, while still providing a human connection, understanding, and personal accountability [56]. For example, fitness trackers and smartphone apps can be used to develop personalized activity goals, provide information about local opportunities for physical activity, send reminders about physical activity, deliver motivational messages, and monitor and give feedback on activity levels, heart rate, and distance walked [9, 57, 58]. Recent systematic reviews and meta-analyses of interventions using commercial wearable trackers to improve physical activity have shown positive, small- to large-sized effects and user acceptability across healthy to chronically ill groups [59–61]. Physicians, nurses, physical therapists, kinesiologists, and pharmacists are part of a growing list of health professionals play an integral role in delivering tailored physical activity interventions [24, 62–66]. The use of technology is has potential to support these clinicians and improve the feasibility of large-scale tailored physical activity interventions.
The use of artificial intelligence and machine learning represents some of the most recent applications of technology to tailor physical activity interventions [31]. The Human Behavior Change Project, for instance, is leveraging behavior change, computer science, and information science to synthesize data to predict “what works, compared with what, how well, with what exposure, with what behaviors (for how long), for whom, in what settings and why?” (Ref. 58, p. 1]. Zhou et al. employed machine learning to predict exercise relapse using accelerometry data [67]. The authors suggest that this application of machine learning could help clinicians and researchers to implement just-in-time adaptive interventions, whereby behavioral supports (e.g., financial incentives and restructuring of goals) are triggered in response to the objectively measured data from accelerometry and GPS [67, 68]. Likewise, Rabbi et al. tested an app that used machine learning to interpret physical activity patterns and generate physical activity recommendations that were tailored to their past behaviors and GPS-derived location [69]. A pilot test of the app demonstrated significant increases in walking behaviors among participants with chronic pain compared to controls [69]. Employing computational modeling, such as machine learning, presents a new approach to tailoring interventions that expands our ability to implement single case experimental designs (e.g., N-of-1 [cf. 70]) and consider temporal, contextual, and individual components [70].
While technology is rapidly advancing and it is exciting to consider the prospect of its role in improving patient–provider interactions and the tailoring methodology, we must not overlook the limitations of technology use. This includes the need to build capacity and skill to perform such data analytics [70, 71], potential increases in the ecological footprint of producing and using technologies like smartphones and wearables [71], and where we focus our discussion—the ethical considerations of incorporating technology into tailored physical activity interventions. Little is currently known about how patients engage with technologies recommended or prescribed by health professionals to support their physical activity behavior change, and about the full spectrum of positive and negative experiences they may encounter [72–74]. We are seeing evidence of unexpected negative impacts, such as increased physical pain from wearing these devices, feelings of guilt among patients if they do not meet their goals, drawing unwanted attention when using these devices in public, and concerns about privacy and data access [59, 75–78]. Additionally, both health professionals and patients have expressed concerns about the reliability and validity of measures derived from these technologies [79]. If such a technology is used to tailor an intervention, any associated inaccuracy may hamper results or participation. Furthermore, concerns over privacy, discomfort, and measurement inaccuracy may undermine shared decision-making, trust, and partnerships within patient–provider interactions, which are laden with power dynamics [75, 80–83]. An in-depth examination of end-users’ experiences (positive and negative) of using technology in tailored physical activity interventions would contribute to our understanding of potential challenges. Relational ethics, because of its particular focus on exploring ethical issues within interpersonal relationships in everyday life, offers an appropriate conceptual lens to guide examination of the impact of these technologies on patient–provider interactions [84].
Take-home message for tailored physical activity intervention research
Technology may help health professionals tailor physical activity by increasing reach and facilitating the monitoring and delivery of intervention components. Furthermore, the use of artificial intelligence and machine learning may help accelerate our understanding of the tailoring methodology. While the prospect of applying technology in these ways is exciting, we must examine how it impacts recipients’ experiences of and participation in tailored intervention to ensure the positives of the technology outweigh the negatives.
Limitations
It should be acknowledged that contributors to this paper are limited to representation from Canada, Denmark, the UK, and the USA. More work is needed to understand the unique priorities in tailored physical activity interventions within lower income countries, particularly with respect to the use/access of technology. The use of frameworks such as the PROGRESS-PLUS has been suggested previously as a practical tool for applying an equity lens to physical activity intervention development [85–87]. We also acknowledge that there are several priority areas for advancing the methodology and effectiveness of tailoring that may be borrowed from the broader health behavior literature and direct readers to such commentaries [70, 71, 88, 89].
Conclusion
This paper highlights the need for better refined methods of tailoring physical activity interventions and suggests future research priorities to advance research in individualized physical activity strategies. Future research should examine (a) how to optimize the engagement of diverse knowledge users in tailored physical activity intervention co-design and (b) how ethical considerations could impact the use of technology to support tailoring. These identified future directions may help advance this growing area and mobilize the impact of tailored physical activity interventions.
Supplementary Material
Acknowledgements
We would like to thank Dr. Ewa Roos for her review of the paper. The Physical Activity for Precision Health Research Cluster received funding from the Office of the Vice-President Research & Innovation, University of British Columbia. The Physical Activity for Precision Health Workshop was supported by the Research Cluster and a Canadian Institutes of Health Research grant (PCS-158389). J. K. Ma was supported by the Michael Smith Foundation for Health Research Trainee Award (#17936), the Arthritis Society Post-Doctoral Fellowship (TPF-18–0209), and the Canadian Institute of Health Research Post-Doctoral Fellowship (201910MFE-430114-231890). L. C. Li was supported by the Harold Robinson/Arthritis Society Chair in Arthritic Diseases. L. C. Li, M. de Vera, and T. Liu-Ambrose were supported by the Canada Research Chair Program.
Compliance with Ethical Standards
Conflict of Interest: None declared.
Primary Data: As indicated by TBM author guidelines, transparency statements are not needed for commentaries.
Ethical Approval: This article does not contain any studies with human participants performed by any of the authors. Also, this article does not contain any studies with animals performed by any of the authors.
Informed Consent: This article does not involve human participants and informed consent was therefore not required.
TRANSPARENCY STATEMENT
As indicated by TBM author guidelines, transparency statements are not needed for commentaries.
References
- 1. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity–A systematic review of longitudinal studies. BMC Public Health. 2013;13(1):813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian children and youth: Accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Reports- Statistics Canada. 2011;22(1):15–23. [PubMed] [Google Scholar]
- 3. Ross R, Chaput J, Giangregorio L, Janssen I, Saunders T, Kho M. Canadian 24-Hour movement guidelines for adults aged 18–64 years and adults aged 65 years or older: An integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metabol. 2020;102:57–102. [DOI] [PubMed] [Google Scholar]
- 4. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin Ginis KA, Ma JK, Latimer-Cheung AE, Rimmer JH. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psychol Rev. 2016;10(4):478–494. [DOI] [PubMed] [Google Scholar]
- 6. Martin Ginis KA, Ma JK, Stork MJ. Exercise psychology issues for people with physical disabilities. In: Anshel MH, Labb EE, Petrie TA, eds. APA Handbook of Sport and Exercise Psychology. American Psychological Association; 2018. [Google Scholar]
- 7. Estabrooks PA, Glasgow RE. Translating effective clinic-based physical activity interventions into practice. Am J Prev Med. 2006;31(4 Suppl):S45–S56. [DOI] [PubMed] [Google Scholar]
- 8. Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med. 2003;25(3 Suppl 2):172–183. [DOI] [PubMed] [Google Scholar]
- 9. Puterman E, Pauly T, Ruissen G, Faulkner G. Move more, move better: A narrative review of wearable technologies and its application to precision health. Under Review. n.d. [DOI] [PubMed] [Google Scholar]
- 10. Giles LV, Koehle MS, Saelens BE, Sbihi H, Carlsten C. A narrative review of physical activity and the physical environment: Precision health insights from the intersection. Under Review. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams J, Backman CL, Cox S, Hurd L, Loftsgard KC, Mahmood B. Sociocultural considerations for physical activity research and practice: A narrative review. Under Review. n.d. [Google Scholar]
- 12. Barha CK, Falck RS, Skou ST, Ambrose TL. Personalising exercise recommendations for healthy cognition and mobility in ageing: Time to consider one’s pre-existing function and genotype (Part 2). Br J Sports Med. 2020:1–2. doi: 10.1136/bjsports-2020-102865. [DOI] [PubMed] [Google Scholar]
- 13. Barha CK, Falck RS, Skou ST, Ambrose TL. Personalising exercise recommendations for healthy cognition and mobility in aging: Time to address sex and gender (Part 1). Br J Sports Med. 2020:1–2. doi: 10.1136/bjsports-2020-102864. [DOI] [PubMed] [Google Scholar]
- 14. Kreuter MW, Strecher VJ, Glassman B. One size does not fit all: The case for tailoring print materials. Ann Behav Med. 1999;21(4):276–283. [DOI] [PubMed] [Google Scholar]
- 15. Hill KD, Hunter SW, Batchelor FA, Cavalheri V, Burton E. Individualized home-based exercise programs for older people to reduce falls and improve physical performance: A systematic review and meta-analysis. Maturitas. 2015;82(1):72–84. [DOI] [PubMed] [Google Scholar]
- 16. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]
- 17. Olander EK, Fletcher H, Williams S, Atkinson L, Turner A, French DP. What are the most effective techniques in changing obese individuals’ physical activity self-efficacy and behaviour : A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2013;10(29):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009;28(6):690–701. [DOI] [PubMed] [Google Scholar]
- 19. Ma JK, Martin Ginis KA. A meta-analysis of physical activity interventions in people with physical disabilities: Content, characteristics, and effects on behaviour. Psychol Sport Exerc. 2018;37:262–273. [Google Scholar]
- 20. Ma JK, West CR, Martin Ginis KA. The effects of a patient and provider co-developed, behavioral physical activity intervention on physical activity, psychosocial predictors, and fitness in individuals with spinal cord injury: A randomized controlled trial. Sports Med. 2019;49(7):1117–1131. [DOI] [PubMed] [Google Scholar]
- 21. Li LC, Sayre EC, Xie H, et al. Efficacy of a community-based technology-enabled physical activity counseling program for people with knee osteoarthritis: Proof-of-concept study. J Med Internet Res. 2018;20(4):e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allen KD, Arbeeva L, Callahan LF, et al. Physical therapy vs internet-based exercise training for patients with knee osteoarthritis: Results of a randomized controlled trial. Osteoarthr Cartil. 2018;26(3):383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balducci S, D’Errico V, Haxhi J, et al. ; Italian Diabetes and Exercise Study 2 (IDES_2) Investigators . Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: The IDES_2 randomized clinical trial. JAMA. 2019;321(9):880–890. [DOI] [PubMed] [Google Scholar]
- 24. Richards EA, Cai Y. Integrative review of nurse-delivered physical activity interventions in primary care. West J Nurs Res. 2016;38(4):484–507. [DOI] [PubMed] [Google Scholar]
- 25. Rhodes RE, Dickau L. Moderators of the intention-behaviour relationship in the physical activity domain: A systematic review. Br J Sports Med. 2013;47(4):215–225. [DOI] [PubMed] [Google Scholar]
- 26. Bernard P, Carayol M, Gourlan M, et al. Moderators of theory-based interventions to promote physical activity in 77 randomized controlled trials. Health Educ Behav. 2017;44(2):227–235. [DOI] [PubMed] [Google Scholar]
- 27. Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: Meta-analysis of outcomes. Am J Public Health. 2011;101(4):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: A systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma JK, Martin Ginis KA. A meta-analysis of physical activity interventions in people with physical disabilities: Content, characteristics, and effects on behaviour. Psychol Sport Exerc. 2018. [Google Scholar]
- 30. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009;28(6):690–701. [DOI] [PubMed] [Google Scholar]
- 31. Michie S, Thomas J, Johnston M, et al. The human behaviour-change project: Harnessing the power of artificial intelligence and machine learning for evidence synthesis and interpretation. Implement Sci. 2017;12(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michie S, West R, Finnerty AN, et al. Representation of behaviour change interventions and their evaluation: development of the upper level of the behaviour change intervention ontology. Wellcome Open Res. 2020;5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jull JE, Davidson L, Dungan R, Nguyen T, Woodward KP, Graham ID. A review and synthesis of frameworks for engagement in health research to identify concepts of knowledge user engagement. BMC Med Res Methodol. 2019;19(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michael Smith Foundation for Health Research. Patient Engagement: How Can Research Help Us Get It Right? Vancouver, BC; 2014. [Google Scholar]
- 35. Canadian Institutes of Health Research. Strategy for Patient-Oriented Research - Patient Engagement Framework.Ottawa, Canada; 2014. Available at http://www.cihr-irsc.gc.ca/e/48413.html. [Google Scholar]
- 36. Patient Centred Outcomes Research Institute. Patient and Family Engagement Rbric.Washington, DC; 2017. Available at https://www.pcori.org/sites/default/files/Engagement-Rubric.pdf. [Google Scholar]
- 37. Kothari A, Regan S, Gore D, Valaitis R, Garcia J, Manson H, O’Mara L. Using an integrated knowledge translation approach to build a public health research agenda. Health Res Policy Syst. 2014;12(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Graham ID, Kothari A, McCutcheon C; Integrated Knowledge Translation Research Network Project Leads . Moving knowledge into action for more effective practice, programmes and policy: Protocol for a research programme on integrated knowledge translation. Implement Sci. 2018;13(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parry D, Salsberg J, Macaulay AC, FCPC C . A guide to researcher and knowledge-user collaboration in health research.Ottawa; 2009. Available at http://www.cihr-irsc.gc.ca/e/documents/Guide_to_Researcher_and_KU_Collaboration.pdf%5Cnhttp://www.leadershipenscience.irsc.gc.ca/e/documents/Guide%7B_%7Dto%7B_%7DResearcher%7B_%7Dand%7B_%7DKU%7B_%7DCollaboration.pdf [Google Scholar]
- 40. Glandon D, Paina L, Alonge O, Peters DH, Bennett S. 10 Best resources for community engagement in implementation research. Health Policy Plan. 2017;32(10):1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamzeh J, Pluye P, Bush PL, Ruchon C, Vedel I, Hudon C. Towards an assessment for organizational participatory research health partnerships: A systematic mixed studies review with framework synthesis. Eval Program Plann. 2019;73:116–128. [DOI] [PubMed] [Google Scholar]
- 42. Hamilton CB, Hoens AM, Backman CL, et al. An empirically based conceptual framework for fostering meaningful patient engagement in research. Health Expect. 2018;21(1):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Banner D, Bains M, Carroll S, et al. Patient and public engagement in integrated knowledge translation research: Are we there yet? Res Involv Engagem. 2019;5(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, et al. Patient and service user engagement in research: A systematic review and synthesized framework. Health Expect. 2015;18(5):1151–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. Res Involv Engagem. 2017;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. South A, Hanley B, Gafos M, et al. Models and impact of patient and public involvement in studies carried out by the Medical Research Council Clinical Trials Unit at University College London: Findings from ten case studies. Trials. 2016;17(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCutcheon C, Kothari A, Graham I.. How We Work Together: The Integrated Knowledge Translation Research Network Casebook, Vol. 1. Ottawa, Canada: IKT Research Network; 2019. [Google Scholar]
- 48. Kothari A, Mccutcheon C, Boland L, Graham ID.. How We Work Together: The Integrated Knowledge Translation Research Network Casebook. Ottawa, ON; 2020. Available at http://www.jla.nihr.ac.uk/jla-guidebook/downloads/Print-JLA-guidebook-version-7-March-2018.pdf. [Google Scholar]
- 49. Levitan B, Getz K, Eisenstein EL, et al. Assessing the financial value of patient engagement: a quantitative approach from CTTI’s patient groups and clinical trials project. Ther Innov Regul Sci. 2018;52(2):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. St Michael’s Hospital Knowledge Translation Program. Intersectionality & knowledge translation; 2020. Available at https://knowledgetranslation.net/portfolios/intersectionality-and-kt/.
- 51. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: A systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Calasanti T, Giles S. The challenge of intersectionality. Generations. 2018;41(4):69–75. [Google Scholar]
- 53. Free C, Phillips G, Watson L, et al. The effectiveness of mobile-health technologies to improve health care service delivery processes: A systematic review and meta-analysis. PLoS Med. 2013;10(1):e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tate DF, Lyons EJ, Valle CG. High-tech tools for exercise motivation: Use and role of technologies such as the internet, mobile applications, social media, and video games. Diabetes Spectr. 2015;28(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Phillips SA, Ali M, Modrich C, et al. Advances in health technology use and implementation in the era of healthy living: Implications for precision medicine. Prog Cardiovasc Dis. 2019;62(1):44–49. [DOI] [PubMed] [Google Scholar]
- 56. Geraedts H, Zijlstra A, Bulstra SK, Stevens M, Zijlstra W. Effects of remote feedback in home-based physical activity interventions for older adults: A systematic review. Patient Educ Counsel. 2019;91(1):13–23. [DOI] [PubMed] [Google Scholar]
- 57. McPhail S, Schippers M. An evolving perspective on physical activity counselling by medical professionals. BMC Fam Pract. 2012;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: A review of the evidence for increasing physical activity. Front Public Health. 2017;11(4):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davergne T, Pallot A, Dechartres A, Fautrel B, Gossec L. Use of wearable activity trackers to improve physical activity behavior in patients with rheumatic and musculoskeletal diseases: A systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2019;71(6):758–767. [DOI] [PubMed] [Google Scholar]
- 60. Brickwood KJ, Watson G, O’Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: Systematic review and meta-analysis. J Med Int Res. 2019;21(4). doi: 10.2196/11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ringeval M, Wagner G, Denford J, Paré G, Kitsiou S. Fitbit-based interventions for healthy lifestyle outcomes: Systematic review and meta-analysis. J Med Internet Res. 2020;22(10):e23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. AuYoung M, Linke SE, Pagoto S, et al. Integrating physical activity in primary care practice. Am J Med. 2016;129(10):1022–1029. [DOI] [PubMed] [Google Scholar]
- 63. Kunstler BE, Cook JL, Freene N, Finch CF, Kemp JL, Halloran PDO, Gaida JE. Physiotherapist-led physical activity itnerventions are efficacious at increasing physical activity levels: A systematic review and meta-analysis. Clin J Sports Med. 2018;28(3). [DOI] [PubMed] [Google Scholar]
- 64. Brawley LR, Gierc MS, Locke SR. Powering adherence to physical activity by changing self-regulatory skills and beliefs: Are kinesiologists ready to counsel? Kinesiology Rev. 2013;2(1):1–13. [Google Scholar]
- 65. Boardman HF, Avery AJ. Effectiveness of a community pharmacy weight management programme. Int J Clin Pharm. 2014;36(4):800–806. [DOI] [PubMed] [Google Scholar]
- 66. Um IS, Armour C, Krass I, Gill T, Chaar BB. Weight management in community pharmacy: What do the experts think? Int J Clin Pharm. 2013;35(3):447–454. [DOI] [PubMed] [Google Scholar]
- 67. Zhou M, Fukuoka Y, Goldberg K, Vittinghoff E, Aswani A. Applying machine learning to predict future adherence to physical activity programs. BMC Med Inform Decis Mak. 2019;19(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hardeman W, Houghton J, Lane K, Jones A, Naughton F. A systematic review of just-in-time adaptive interventions (JITAIs) to promote physical activity. Int J Behav Nutr Phys Act. 2019;16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rabbi M, Aung MS, Gay G, Reid MC, Choudhury T. Feasibility and acceptability of mobile phone-based auto-personalized physical activity recommendations for chronic pain self-management: pilot study on adults. J Med Internet Res. 2018;20(10):e10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chevance G, Perski O, Hekler EB. Innovative methods for observing and changing complex health behaviors: Four propositions. Transl Behav Med. 2021;11(2):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hekler EB, Klasnja P, Chevance G, Golaszewski NM, Lewis D, Sim I. Why we need a small data paradigm. BMC Med. 2019;17(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lupton D. The digitally engaged patient: Self-monitoring and self-care in the digital health era. Soc Theory Health. 2013;11(3):256–270. [Google Scholar]
- 73. Piwek L, Ellis DA, Andrews S, Joinson A. The rise of consumer health wearables: Promises and barriers. PLoS Med. 2016;13(2):e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ellis DA, Piwek L. Failing to encourage physical activity with wearable technology: What next? J R Soc Med. 2018;111(9):310–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Papi E, Belsi A, McGregor AH. A knee monitoring device and the preferences of patients living with osteoarthritis: A qualitative study. BMJ Open. 2015;5(9):e007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nebeker C, Murray K, Holub C, Haughton J, Arredondo EM. Acceptance of mobile health in communities underrepresented in biomedical research: Barriers and ethical considerations for scientists. JMIR Mhealth Uhealth. 2017;5(6):e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. JAMA. 2016;316(11):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mercer K, Li M, Giangregorio L, Burns C, Grindrod K. Behavior change techniques present in wearable activity trackers: A critical analysis. JMIR Mhealth Uhealth. 2016;4(2):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ma JK, Chan A, Sandhu A, Li LC. Wearable physical activity measurement devices used in arthritis. Arthritis Care Res (Hoboken). 2020;72 Suppl 10:703–716. [DOI] [PubMed] [Google Scholar]
- 80. Belsi A, Papi E, McGregor AH. Impact of wearable technology on psychosocial factors of osteoarthritis management: A qualitative study. BMJ Open. 2016;6(2):e010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leese J, Macdonald GG, Tran BC, et al. Using physical activity trackers in arthritis self-management: A qualitative study of patient and rehabilitation professional perspectives. Arthritis Care Res (Hoboken). 2019;71(2):227–236. [DOI] [PubMed] [Google Scholar]
- 82. Feehan LM, Geldman J, Sayre EC, et al. Accuracy of fitbit devices: Systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth. 2018;6(8):e10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Leese J, Geldmanc J, Zhu S, et al. The perspectives of persons with arthritis on the use of wearable technology to self-monitor physical activity: A qualitative evidence synthesis. Arthritis Care Res. 2021. doi: 10.1002/acr.24585. [DOI] [PubMed] [Google Scholar]
- 84. Moore J, Engel J, Prentice D. Relational ethics in everyday practice. Can Oncol Nurs J. 2014;24(1):31–39. [DOI] [PubMed] [Google Scholar]
- 85. Guillemin F, Carruthers E, Li LC. Determinants of MSK health and disability - Social determinants of inequities in MSK health. Best Pract Res. 2014;28(3):411–433. [DOI] [PubMed] [Google Scholar]
- 86. O’Neill J, Tabish H, Welch V, et al. Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- 87. Li LC, Feehan LM, Hoens AM. Rethinking physical activity promotion during the COVID-19 pandemic: Focus on a 24-hour day. J Rheumatol. 2021:jrheum.201595. doi: 10.3899/jrheum.201595. [DOI] [PubMed] [Google Scholar]
- 88. Hekler E, Tiro JA, Hunter CM, Nebeker C. Precision health: The role of the social and behavioral sciences in advancing the vision. Ann Behav Med. 2020;54(11):805–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Petersen C, Austin RR, Backonja U, et al. Citizen science to further precision medicine: From vision to implementation. JAMIA Open. 2021;3(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 91. Michie S, Johnston M. Optimising the value of the evidence generated in implementation science: The use of ontologies to address the challenges. Implementation Science. 2017;131:10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.