Abstract
Background
Autogenic drainage is an airway clearance technique that was developed by Jean Chevaillier in 1967. The technique is characterised by breathing control using expiratory airflow to mobilise secretions from smaller to larger airways. Secretions are cleared independently by adjusting the depth and speed of respiration in a sequence of controlled breathing techniques during exhalation. The technique requires training, concentration and effort from the individual but it has previously been shown to be an effective treatment option for those who are seeking techniques to support and promote independence. However, at a time where the trajectory and demographics of the disease are changing, it is important to systematically review the evidence demonstrating that autogenic drainage is an effective intervention for people with cystic fibrosis.
Objectives
To compare the clinical effectiveness of autogenic drainage in people with cystic fibrosis with other physiotherapy airway clearance techniques.
Search methods
We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched the reference lists of relevant articles and reviews, as well as two ongoing trials registers (02 February 2021).
Date of most recent search of the Cochrane Cystic Fibrosis Trials Register: 06 July 2021.
Selection criteria
We identified randomised and quasi‐randomised controlled studies comparing autogenic drainage to another airway clearance technique or no therapy in people with cystic fibrosis for at least two treatment sessions.
Data collection and analysis
Data extraction and assessments of risk of bias were independently performed by three authors. The authors assessed the quality of the evidence using the GRADE system. The authors contacted seven teams of investigators for further information pertinent to their published studies.
Main results
Searches retrieved 64 references to 37 individual studies, of which eight (n = 212) were eligible for inclusion. One study was of parallel design with the remaining seven being cross‐over in design; participant numbers ranged from 4 to 75. The total study duration varied between four days and two years. The age of participants ranged between seven and 63 years with a wide range of disease severity reported. Six studies enrolled participants who were clinically stable, whilst participants in two studies received treatment whilst hospitalised with an infective exacerbation. All studies compared autogenic drainage to one (or more) other recognised airway clearance technique. Exercise is commonly used as an alternative therapy by people with cystic fibrosis; however, there were no studies identified comparing exercise with autogenic drainage.
The certainty of the evidence was generally low or very low. The main reasons for downgrading the level of evidence were the frequent use of a cross‐over design, outcome reporting bias and the inability to blind participants.
The review's primary outcome, forced expiratory volume in one second, was the most common outcome measured and was reported by all eight studies; only three studies reported on quality of life (also a primary outcome of the review). One study reported on adverse events and described a decrease in oxygen saturation levels whilst performing active cycle of breathing techniques, but not with autogenic drainage. Seven of the eight included studies measured forced vital capacity and three of the studies used mid peak expiratory flow (per cent predicted) as an outcome. Six studies reported sputum weight. Less commonly used outcomes included oxygen saturation levels, personal preference, hospital admissions, intravenous antibiotics and pseudomonas gene expression. There were no statistically significant differences found between any of the techniques used with respect to the outcomes measured except when autogenic drainage was described as being the preferred technique of the participants in one study over postural drainage and percussion.
Authors' conclusions
Autogenic drainage is a challenging technique that requires commitment from the individual. As such, this intervention merits systematic review to ensure its effectiveness for people with cystic fibrosis, particularly in an era where treatment options are changing rapidly. From the studies assessed, autogenic drainage was not found to be superior to any other form of airway clearance technique. Larger studies are required to better evaluate autogenic drainage in comparison to other airway clearance techniques in view of the relatively small number of participants in this review and the complex study designs. The studies recruited a range of participants and were not powered to assess non‐inferiority. The varied length and design of the studies made the analysis of pooled data challenging.
Plain language summary
The autogenic drainage breathing technique for helping people with cystic fibrosis to clear mucus from their airways
Background
Cystic fibrosis affects the lungs by producing thick mucus lining the airways. This can lead to infection and inflammation causing lung damage. Physiotherapy can help to keep the airways clear of mucus and there are many methods used to do this including breathing techniques, manual techniques and mechanical devices. Autogenic drainage is a very controlled technique of breathing which uses different depths and speeds of exhaled breath to move mucus up the airways resulting in a spontaneous or voluntary cough. It can be used without help, but requires training, concentration and effort. We looked at the effect of using autogenic drainage on lung function measurements and quality of life in people with cystic fibrosis, to discover whether using autogenic drainage was better or worse than other existing physiotherapy techniques for clearing the lungs.
Search date
The evidence is current to: 06 July 2021.
Study characteristics
We searched the literature for studies comparing at least two sessions of autogenic drainage with other breathing techniques and devices which help to clear the lungs of mucus. We included eight studies in the review involving 212 people with cystic fibrosis, aged between seven and 63 years of age. People were randomly selected for one physiotherapy treatment or the other. The number of people in the studies ranged from 4 to 75, and had a wide range of disease severity. The studies lasted from four days to two years in total.
Key results
We did not find any clear evidence that autogenic drainage was better than the other techniques for lung function or quality of life in either the short‐term or long‐term studies. This was also true for our other outcome measures such as hospital admissions, additional antibiotic treatment, exercise tolerance and oxygen saturation, but people in one study autogenic drainage preferred autogenic drainage compared to postural drainage and percussion. The authors of this review wanted to compare exercise to autogenic drainage for airway clearance, but found no studies with this comparison, even though exercise is often used as an alternative by people with cystic fibrosis.
Certainty of the evidence
Overall, the certainty of the evidence from the studies was judged to be mainly low or very low. The main problems for this being the small numbers of participants in each study, the unclear reporting of results in the studies and the study design used. In one study, which was classed as having a high risk of bias due to incomplete results, those taking part had to change physiotherapy technique halfway through the study and there were many who dropped out and did not comply with the postural drainage and percussion treatment arm. Six of the eight studies used research staff to assess results who did not know which technique each person was using and this improved the quality of the evidence and reduced any bias in this respect.
Summary of findings
Background
Description of the condition
Cystic fibrosis (CF) is a genetic condition which is inherited in an autosomal recessive manner (two carrier parents have a one in four chance of a child with CF). It is more prevalent in populations of European descent (estimated incidence of between 1 in 3000 to 1in 6000 births (Scotet 2020)) but less prevalent in other populations around the world (Scotet 2020). The affected gene codes for the production of a protein that is involved in the movement of salt across cell walls. Infants born with CF often have minimal disease expression in their early weeks of life, but the abnormal salt transport predisposes them to a number of different problems; most commonly salt loss through abnormal sweat production, poor absorption of food through pancreatic dysfunction and airway infection and inflammation through dysfunction of the airway clearance mechanism that normally protects the lungs (Tiddens 2010).
Abnormal salt transport impacts on the production of airway surface liquid, which potentially disturbs the ability of the cilia to clear the airways (Boucher 2004). This is an important physiological process, called the mucociliary escalator, for protecting the airways. Disruption of this process makes the airways vulnerable to the unusual infections that characterise CF lung disease. Once established, airway infection and inflammation exacerbate the poor airway clearance. Together with increased production of airway mucus, this leads to a cycle of chronic infection, inflammation and airway damage (Cantin 2015; Konstan 1997). It is the impact of the CF defect on the airways that is the most significant cause of morbidity and ultimately early death for people with CF (Tiddens 2010).
Recently, cystic fibrosis transmembrane conductance regulator (CFTR) modulator medications which work to correct the basic genetic defect, correcting airway dehydration, thus preventing airway inflammation and infection, have been introduced to CF management. These medications have the potential to significantly change the disease trajectory and the demographics of the future CF population and their airway clearance needs (Nissenbaum 2020).
Description of the intervention
There is evidence from systematic reviews, including Cochrane Reviews, that exercise and airway clearance are important, even during early stages of the condition, for maintaining respiratory health (Flume 2009; ACPCF 2020). With more established airway infection, airway clearance techniques are critical to remove excess secretions which provide an ideal breeding ground for pathogens, helping to maintain respiratory function and prevent the deterioration associated with infection and inflammation.
Early introduction of CFTR modulators may prevent the development of lung disease, thus negating the necessity of regular airway clearance. In individuals with established lung damage and bronchiectasis, improved airway hydration due to modulator therapies appears to lead to a reduction in sputum volume and viscosity, again potentially reducing the need for daily airway clearance regimes. It may be that in the future, airway clearance techniques become part of a treatment toolbox for individuals with CF, utilised in times of illness and infection, rather than a daily essential therapy.
There are a number of different airway clearance techniques (including exercise) that exist and these have been evaluated by other Cochrane Reviews (Main 2005; McIlwaine 2019 ; Mckoy 2016; Morrison 2020; Radtke 2017; Warnock 2015; Wilson 2019). Historically, airway clearance involved percussion and postural drainage positioning, while today airway clearance options include the use of devices to help loosen secretions ranging from simple and cheap airway oscillating devices (OPEP), through devices generating positive expiratory pressure (PEP or Hi‐PEP) to high frequency chest wall oscillation (HFCWO) devices which have significant cost implications. Individuals can also use breathing exercises where they focus on appreciating and controlling their breathing pattern to augment airway clearance; these techniques include the active cycle of breathing (ACBT) and autogenic drainage (AD), the subject of this review. Exercise is commonly used as an adjunctive or possible alternative therapy to airway clearance by people with CF. It is thought that achieving effective airway clearance relies upon two factors ‐ ventilating behind obstructive secretions within lung areas and sufficient expiratory airflow to move secretions up the mucociliary escalator to enable expectoration or swallowing (ACPCF 2020; McIllwaine 2014).
Jean Chevaillier developed AD as an airway clearance technique in 1967 and AD is characterised by the individual with CF understanding and controlling their breathing (Chevaillier 1984). Secretions are cleared by adjusting the rate, depth and location of respiration in a sequence of controlled breathing techniques. The mechanism of mucus clearance rests on two different systems, the effect of the ciliary clearance and the effect of shearing forces induced by the airflow. To create the necessary shearing forces to clear the bronchi from secretions, it is essential to modulate the inspiratory and expiratory airflow. In order to do this, the individual inspires with a deeper than normal breath, described by Chevaillier as the functional tidal volume (1.5 to 2 times the size of normal tidal volume), and exhales in a gentle but active way as a sigh. Individuals breathe in with inspiratory pauses through an open glottis, allowing more time for obstructed areas of the lung to fill equally and air to move behind secretions. These secretions are mobilised from the periphery of the lungs to the mouth by adjusting the lung volume at which the individual is performing the AD‐style breathing in three distinct phases. In the first phase, known as the 'un‐sticking phase', repeated low‐lung volume breaths are used within the expiratory reserve volume, i.e. the individual will be instructed to breathe out as far as possible and then to breathe the functional tidal volume. To localize the secretions the three feedback signals (auditive, tactile and proprioceptive) are used, which informs the individual to move to the next phase. In the second phase (collective phase) a mid‐volume level of breathing is used, progressing into the inspiratory reserve and secretions are mobilised ready to be expectorated in the third (evacuation) phase using a huff (forced expiration technique) or controlled cough. The aim of breathing in this way is to achieve the highest possible expiratory air flow simultaneously in different generations of the bronchi, keeping bronchial resistance low, and avoiding bronchospasm and dynamic airway collapse. Under these circumstances, the speed of air flow may mobilise secretions by shearing them from the bronchial walls and transporting them from the peripheral to the central airways (IPG/CF 2019). The use of AD prevents airway collapse during forced expiratory maneuvers and it may consume less energy compared to other airway clearance techniques (Agostini 2007). In addition to the clinical benefit and improvement in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), a recent study in adults with CF has shown that AD improved inspiratory resistance in all airways except the distal small airways (Wallaert 2018).
How the intervention might work
The rationale behind airway clearance is simple; that removing infected secretions from the airway will improve ventilatory capacity and reduce direct inflammatory effects on the airway epithelia. There is convincing evidence that such a strategy is important and effective for people with chronic airway infection, but there is a less robust evidence base for those who do not have chronic airway infection and are not usually productive of sputum (McIllwaine 2014).
Why it is important to do this review
All airway clearance techniques are time‐consuming and require effort and commitment from the individual (Rand 2013). Some techniques have significant cost implications (Morrison 2020). While AD requires training and support from therapists, it is a popular technique with many people with CF. It allows independence from carers; requires no additional equipment; is recognised to be effective in the modulation of airflow and capable of augmenting the physiological process of the body's mucociliary escalator.
It is important that interventions which are a burden on the time of people with CF are systematically reviewed for evidence of efficacy and this will continue to be the case for the post‐CFTR modulator CF population.
This is an updated version of a previous review (McCormack 2017).
Objectives
To compare the clinical effectiveness of AD in people with CF with other physiotherapy airway clearance techniques.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Children and adults with CF with a diagnosis based on sweat testing or genetic testing or any combination of these.
Types of interventions
This review will compare AD to all other recognised airway clearance techniques either as a single technique or in combination with other techniques for at least two treatment episodes. Single treatment interventions were not considered.
Autogenic drainage (AD)
This airway clearance technique was developed by Jean Chevaillier in 1967. It is characterised by using breathing control to modulate inspiratory and expiratory airflow in order to mobilise secretions from peripheral to central airways. The central goal of AD is to generate optimum shearing forces in different generations of the bronchi to mobilise sputum, whilst keeping bronchial resistance low and avoiding dynamic airway collapse. Secretions are cleared independently by adjusting the rate, depth and speed of respiration in a sequence of controlled breathing techniques (IPG/CF 2019).
Conventional physiotherapy
Postural drainage and percussion (PD&P) was first introduced for the treatment of CF in the 1950s. Postural drainage (PD) has consisted of placing the individual in a position which allows gravity to assist in draining mucus from the periphery of the lungs centrally. In more recent years modified postural drainage has been adopted, which involves positioning without the use of head‐down tilt (Button 2016). Percussion, vibrations, clapping and shaking are manual techniques that can be used as an adjunct to PD and are directed over the chest wall. The techniques require external assistance and may also require deep breathing, huffing and directed coughing to complete the treatment (Main 2005). Many adolescents and adults with CF prefer alternative airway clearance techniques which afford them greater independence and can be more easily integrated into an active lifestyle.
Active cycle of breathing techniques (ACBT)
This technique combines breathing control, thoracic expansion exercises and forced expiratory techniques (FET) (Pryor 1999). Breathing control involves relaxed tidal volume breathing using diaphragmatic control, whereas thoracic expansion exercises focus on active inspiration to increase lung volumes. After one or more cycles of breathing control and thoracic expansion exercises, one or two FETs are encouraged, combined with breathing control. Forced expirations at low and mid lung volumes will help to mobilise secretions from smaller peripheral airways, whist those at high lung volumes (huffs) are used to clear secretions from larger central airways (IPG/CF 2019)The regimen is flexible and can be adapted to suit the individual (Button 2016). Chest wall manipulation and postural drainage may also be included along with this cycle.
Exercise
Physical exercise that increases minute ventilation leads to the mobilization of pulmonary secretions and enhances airway clearance. Physiological effects of exercise include reduced mechanical impedance of sputum, enhanced expiratory flow rates, increased lung recruitment and inducement of coughing (Button 2016; Dwyer 2011, IPG/CF 2019). Evidence from both short‐ and long‐term studies shows that exercise has a positive effect on lung function and well‐being (Radtke 2017). The latest Registry Data indicates that use of exercise as a form of airway clearance (whether primary or secondary) varies widely, ranging from 16.6% to 65.5% amongst children, 30% to 55.9% amongst adults and between 23.3% and 59.7% for the overall CF population (CFF 2020; Cystic Fibrosis NZ 2018; UK CF Trust 2020).
Positive expiratory pressure (PEP)
The PEP mask or mouthpiece contains a valve that increases resistance to expiratory airflow. The individual repeats 12 to 15 consecutive breaths through the flow resistor, creating mid‐expiratory positive pressures of 10 to 20 cm H₂O in the airways. The theoretical benefit of PEP therapy lies in its ability to enhance and promote mucus clearance by one or more mechanisms: by preventing small airway collapse through stenting of the airways; or, by enhancing lung recruitment distal to retained secretions using collateral ventilation (Andersen 1979; Groth 1985); or, by temporarily increasing functional residual capacity (McIlwaine 2019, IPG/CF 2019). The secretions mobilised can then be evacuated using a FET.
High‐pressure PEP (Hi‐PEP)
The Hi‐PEP mask physiotherapy employs forced expiratory manoeuvres against the PEP mask's expiratory resistor. An individual performs PEP breathing for eight to 10 cycles using moderately increased tidal breathing before inhaling to total lung capacity and performing a forced expiratory manoeuvre against the stenosis. Sustained expiratory pressures achieved usually range between 40 and 100 cm H₂O (Oberwaldner 1986; McIlwaine 2019; IPG/CF 2019).
Oscillatory devices
These devices combine positive expiratory pressure with intra‐ or extra‐thoracic high frequency chest wall oscillations.
The combination of oscillations of positive pressure in the airways and repeated accelerations of expiratory airflow have been shown to result in improved sputum clearance (Rogers 2005). Intermittent increases in endobronchial pressure splint the airways open, reducing airway collapsibility during exhalation. The vibrations generated by some oscillating devices may augment the respiratory system resonance frequency and reduce sputum viscoelasticity, thus loosening mucus from the airways and promoting upward transport along the tracheobronchial tree (Poncin 2020).
There are numerous devices employed for this purpose:
Flutter®
The Flutter VRP1 device comprises a mouthpiece, a plastic cone, a steel ball and a perforated cover. During exhalation through the device, the tracheobronchial tree undergoes internal vibrations, together with repeated changes of the expiratory airflow against the resistance (PEP component) and oscillations in endobronchial pressure (oscillatory component). This facilitates the mobilisation and loosening of secretions (Konstan 1994; Pryor 1999).
Acapella
The Acapella is a flow‐operated device that combines the principles of high‐frequency oscillation and PEP by employing a counterweighted lever and magnet. Exhaled gas passes through a cone, which is intermittently occluded by a plug attached to the lever, producing airflow oscillations. A dial located at the distal end of the device adjusts the proximity of the magnet and counterweighted plug, thereby adjusting the frequency, amplitude, and mean pressure (Volsko 2003).
Cornet®
The Cornet® is a horn‐shaped plastic tube which houses a rubber inner hose. Expiration through the Cornet® causes the hose to flex, buckle and unbuckle, causing oscillating positive pressure in the airways which fluctuates rapidly. The mouthpiece can be adjusted to produce the optimal resistance and oscillation (Pryor 1999).
Quake® (Thayer Medical, Tucson, Arizona, USA)
This device produces airway oscillation during both inspiration and expiration. The design consists of a manually turned outer barrel which rotates around an inner barrel. Airflow occurs only when vanes within the two barrels line up and is interrupted at regular intervals as the user turns the handle. Vibration is achieved as small bursts of air are inhaled and exhaled through the vanes of the device (Okeson 2007). As the resulting vibration is not flow‐dependent, the device may be helpful in reducing fatigue during airway clearance for some patients with severe obstructive lung disease (Morrison 2020).
The Aerobika®
The Aerobika® is hand‐held device which relies on oscillating positive expiratory pressure (OPEP) to mobilise secretions. The short pulses of resistance against an exhaled breath help to move secretions towards proximal airways so they can be cleared. The mechanism is not gravity‐dependent and can be used in combination with a nebuliser, which may improve aerosol deposition, reduce treatment burden and promote adherence.
Metaneb®
The MetaNeb® system alternates cycles of continuous positive expiratory pressure (CPEP) with cycles of continuous high frequency oscillation (CHFO) whilst delivering nebuliser treatment throughout. Flow, pressure and percussive rate are all adjustable to facilitate lung expansion and airway clearance (Patel 2013).
The VibraLung®
The VibraLung® is an acoustical percussor that uses sound waves applied directly to the lungs at adjustable frequencies covering many of the natural resonant frequencies of the human tracheobronchial tract (5 to 1,200Hz). The resulting vibrations at the mucus‐airway surface interface are thought to help dislodge sputum and aid expectoration. Exhalation through two small holes in the mouthpiece also provides a small amount of positive expiratory pressure (PEP) to prevent airway collapse (Wheatley 2018).
Intrapulmonary percussive ventilation (IPV)
This technique utilizes high frequency oscillatory ventilation to produce endotracheal percussion via the mouth using a device called the Percussionator. Percussive bursts of high‐flow respiratory gas are delivered throughout the entire respiratory cycle at high rates. These cause oscillatory airflow which vibrates the endobronchial walls to loosen and mobilize secretions towards the upper airways and oral pharynx (Homnick 1995).
High frequency chest wall oscillations (HFCWO)
HFCWO delivers external compression pulses to the chest wall through an inflatable vest connected to an air pulse generator (e.g., ThAIRapy™ Vest, The Vest™, Hayek Oscillator, InCourage system, SmartVest). The generator produces an alternating flow of air into, and out of, the vest that rapidly compresses and releases the chest wall within a range of selectable frequencies and pressures. The oscillatory compression imparted to the chest wall has been reported to thin viscous mucus, mobilise secretions and propel mucus to the major airways (Warwick 1991).
Types of outcome measures
We planned to assess the following outcomes in the review.
Primary outcomes
FEV1
-
Quality of life (QoL) as measured by any of the scales including:
Cystic Fibrosis Questionnaire‐Revised version (CFQ‐R) (Quittner 2009)
Cystic Fibrosis Quality of Life Questionnaire (CFQoL) (Gee 2000)
Quality of Well‐being (QWB) Scale (Kaplan 1989)
Nottingham Health Profile (NHP) (Hunt 1985)
any other validated QoL scale
Secondary outcomes
Participant preference
-
Exercise tolerance
six‐minute walk test
shuttle walk test
cardiopulmonary exercise testing (CPET)
any other validated exercise evaluation
Adverse effects (e.g. haemoptysis, bronchospasm, desaturation)
Number of admissions to hospital
Need for extra treatment
-
Other pulmonary function measurements
lung clearance index (LCI) (post hoc change)
FVC
forced mid‐expiratory flow between 25% and 75% of FVC (FEF25-75%)
-
Oxygen saturation
pulse oximeter
arterial blood gas analysis
Sputum weight
Survival
Search methods for identification of studies
There was no restriction on language or publication status.
Electronic searches
We identified relevant studies from the Group's Cystic Fibrosis Trials Register by using the term: autogenic drainage.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books relevant conferences, including three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group website.
Date of last search of the CF Register: 06 July 2021.
We also searched two online trials registries:
ClinicalTrials.gov (clinicaltrials.gov): date of last search 02 February 2021;
WHO ICTRP (http://apps.who.int/trialsearch/): date of last search 02 February 2021.
For details of our search strategies, please see Appendix 1.
Searching other resources
We checked the reference lists from the identified studies for further assessment. We also screened the references of all published Cochrane Reviews related to this title.
Data collection and analysis
Selection of studies
The authors (up to 2021: PM, PB and KWS; after 2021: PB, GS and RS) independently screened the results of the searches for relevant articles based on the title and abstract. They included the studies which any of them identified as relevant and reviewed the full text of those studies. They screened the full text articles to determine the eligibility of the study for inclusion in the review. If disagreement had occurred, the authors planned to seek resolution by consensus. For studies published in languages other than English, the authors planned to seek translation.
Data extraction and management
The authors (up to 2021: PM, PB and KWS; after 2021: PB, GS and RS) independently extracted the data using specifically formulated data extraction forms. The extracted data included characteristics of the participants, information on the study design (type of randomisation, type of allocation concealment, number of participants), aspects of the intervention (details of intervention and control intervention, duration of intervention, frequency of intervention, compliance with intervention, intensity of intervention and details of multifaceted interventions), outcome measures, adverse effects and dropouts.
The authors presented results separately for each comparison of techniques, i.e. AD versus conventional physiotherapy, AD versus ACBT, AD versus PEP, etc. We do not combine all oscillating devices together, instead present separate comparisons for AD versus Flutter®, AD versus Cornet® and AD versus intrapulmonary percussive ventilation (IPV) at low (200bpm) and high (400bpm) frequencies.
They compared the effect of treatment both in the short term and long term. For short‐term studies (up to one month), the authors reported outcomes of up to seven days, and from one to four weeks. Likewise, the outcome data for longer‐term studies were reported as those measured at one month, three months, six months, 12 months and annually thereafter. The authors also planned to consider any outcome data recorded at other time periods. The authors felt that it was difficult to assess the relevance of AD treatment after a single treatment intervention, so did not included these extremely brief studies in the review, setting instead a minimum requirement of two treatment sessions for inclusion.
Assessment of risk of bias in included studies
The authors (up to 2021: PM, PB and KWS; after 2021: PB, GS and RS) independently assessed the risk of bias from the included studies using the approach recommended in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). They planned to resolve any disagreements by consensus, but this was not necessary. The authors assessed and rated the following domains.
1. Generation of sequence
Low risk of bias: using a computerised random generator, random number tables, coin tossing or any other valid method. High risk of bias: sequence generation and allocation done by invalid methods such as using odd or even date of birth, or allocation by the judgement of the clinician. Unclear risk of bias: insufficient information provided about the sequence generation process.
2. Concealment of allocation sequence
Low risk of bias: allocation concealed so that neither the investigators or participants know group assignment at the time of study entry. Valid methods include central randomisation or serially numbered, opaque, sealed envelopes. High risk of bias: the method of allocation is not concealed (e.g. visible list of random numbers, unsealed or non‐opaque envelopes) leading to transparency in group assignments and thereby introducing selection bias. Unclear risk of bias: insufficient information provided about the concealment of allocation process.
3. Blinding of participants, personnel and outcome assessors
Note: we considered the risk of bias from blinding for the study overall rather than per outcome.
Low risk of bias: either participants or some key study personnel could not or were not blinded, but the outcome assessment was blinded and the non‐blinding of others is unlikely to introduce bias. High risk of bias: no blinding or incomplete blinding and the outcome measurement is likely to be influenced by lack of blinding. Unclear risk of bias: insufficient information or the study report did not mention it.
4. Incomplete outcome data
Low risk of bias: missing data have been included using appropriate methods such as intention‐to‐treat analysis. High risk of bias: authors did not include intention‐to‐treat analysis for missing data. Unclear risk of bias: insufficient reporting of attrition or exclusions, no reasons for missing data provided.
5. Selective outcome reporting
Low risk of bias: the published article(s) report(s) primary and secondary outcomes that are of interest to the review in the pre‐specified way. High risk of bias: pre‐specified outcomes not reported. Unclear risk of bias: insufficient information to permit judgement of low or high risk.
6. Other potential threats to validity
Low risk of bias: the study appears to be free of other sources of bias. High risk of bias: evidence of other potential sources of bias, e.g. there is bias pertaining to the study design (e.g. extreme baseline imbalance). Unclear risk of bias: insufficient information to assess whether any important risk of bias exist.
Authors previously stated that they would not be allowed to assess the risk of bias in included studies in which they were involved, but no such studies were relevant for this review. For studies published in languages other than English, authors fluent in that language would assess the risk of bias or the study would be translated; no such studies were relevant.
Measures of treatment effect
Where possible, for continuous outcomes (FEV1, QoL, exercise tolerance, number of admissions to hospital, LCI, FVC, FEF25-75%, pulse oximetry, arterial blood gas analysis and sputum weight) using the same unit of measurement, the authors reported the mean difference (MD) and 95% confidence intervals (CIs). They reported the standardised mean difference (SMD) with 95% CIs for continuous outcomes using different units of measurement. For dichotomous outcomes (participant preference, adverse effects, need for extra treatment and survival), the authors planned to report risk ratio (RR) and 95% CIs, however, no such outcomes have been analysed.
Unit of analysis issues
When combining the data from cross‐over studies, the authors planned to use the methods recommended by (Elbourne 2002). It is common that the analysis and presentation of results from cross‐over studies are often not appropriate or clear, leading to limited data being available for analysis (Nolan 2016). This was true for most of the studies included in this review and since only limited data were available, the authors used only the first‐arm data from the studies in order to avoid the carry‐over effect (Curtin 2002). As results were not presented from paired analyses for one study (Pfleger 1992), we treated this cross‐over study as if it was a parallel study, which is a conservative approach as it does not take into account within‐patient correlation.
Cluster‐randomised studies are not appropriate for this intervention. Where we have included studies with multiple treatment groups, each comparison is presented in a separate analysis.
Dealing with missing data
The review authors contacted the authors of included studies regarding all missing data. If the study authors had been unavailable or the additional data were insufficient for analysis, the review authors planned to include a narrative description of the study in the review. The review authors contacted a total of seven teams of investigators (Dingemans 2018; Helper 2020; McIlwaine 1991; Osman 2010; Prusak 2020; Sokol 2012; Sokol 2012a) and were able to obtain additional information from all of them, bar one (Prusak 2020).
Assessment of heterogeneity
For studies which investigated the effect of similar interventions on similar participants and assessed similar outcomes (clinically homogenous), the authors planned to pool the data in a meta‐analysis. However, it was not possible to combine data for any outcome measure. If there had been heterogeneity, the authors planned to assess this using the Chi² test and the I² statistic (with CIs) (Higgins 2003). The authors planned to regard heterogeneity as low if I² was less than 25%, moderate if I² was between 25% and 50% and substantial if I² was over 50%.
Assessment of reporting biases
The review authors planned to use funnel plots to assess any reporting bias if there had been a sufficient number of studies included (a minimum of 10 studies required for the assessment of biases). Had there been asymmetry in the funnel plot, the authors intended to explore the possibility of small study effects and heterogeneity as a cause, as well as outcome reporting bias.
Outcome reporting bias can occur when studies measure outcomes, but do not publish all of them, giving rise to misleading results (Kirkham 2010). The authors compared the 'Methods' section of each paper to the 'Results' section to ensure all outcomes were reported. If they had suspected outcome reporting bias, they would have contacted the study authors for the data.
Data synthesis
The authors analysed the data using a fixed‐effect model, since there was no evidence of substantial heterogeneity between the included studies. If they identify substantial heterogeneity in future updates of the review, they plan to use a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We were not able to combine data from multiple studies in an analysis, therefore an assessment of heterogeneity was not possible. In case of moderate to substantial levels of heterogeneity between the included studies, the authors planned to perform the following subgroup analyses:
age (paediatric, adolescent and adults as defined by the study investigators);
severity of the disease based on lung function (FEV1 % predicted: above 90%; 70% to 89%; 40% to 69%; under 40%);
participants with acute exacerbations in comparison with stable CF.
However, since we were unable to combine data from multiple studies, we have not undertaken any subgroup analysis.
Sensitivity analysis
If the authors had been able to combine studies and had established that some of these studies were judged to have a high risk of bias, in order to test the robustness of their findings they planned to undertake a sensitivity analysis excluding these studies as long as at least two studies would still be combined after any exclusions. However, since we were unable to combine data from multiple studies, we have not undertaken any sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
The current author team present summary of findings tables for each comparison of the review. The primary outcomes of the review and the first five secondary outcomes (participant preference, exercise tolerance, adverse effects (e.g. haemoptysis, bronchospasm, desaturation), number of admissions to hospital, need for extra treatment) are presented in the tables and the certainty of the evidence for each outcome of each comparison is assessed using GRADE methodology (Schünemann 2021).
Results
Description of studies
Please see the tables for additional information on the studies in this review (Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification).
Results of the search
The process of the search and study selection is documented in the PRISMA diagram (Figure 1).
1.
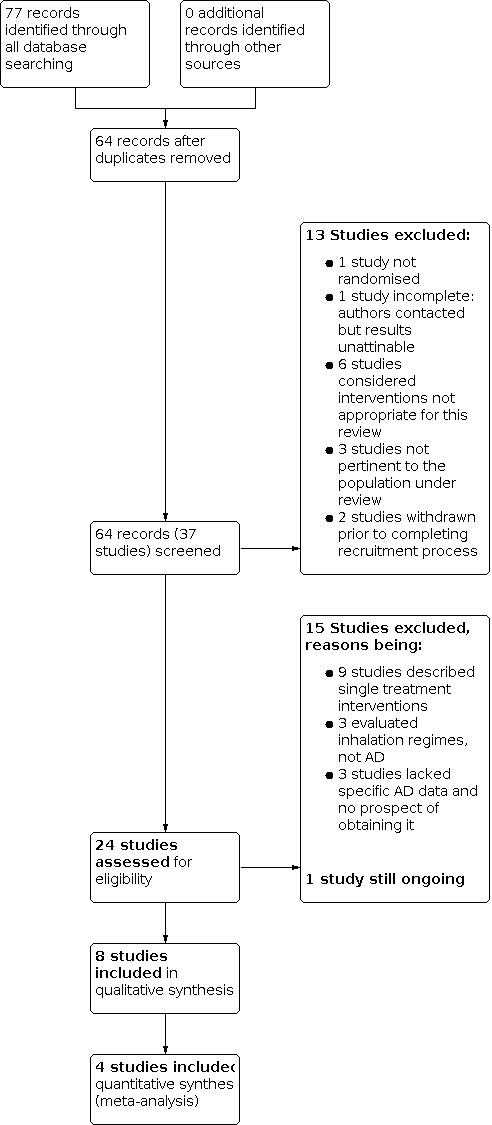
Study flow diagram.
A total of 64 references to 37 individual studies were retrieved through electronic searches. No additional records were identified by other sources. Eight of these studies were considered as eligible for inclusion following screening (App 1998; Dingemans 2018; McIlwaine 1991; McIlwaine 2010; Miller 1995; Osman 2010; Pfleger 1992; Pryor 2010). Of note, the authors have included one study (App 1998) using a German modification of the AD technique (David 1991). Whilst the intervention may not have been strictly to the guidance of Jean Chevaillier's description using three distinct breathing phases, it was felt the technique used was very similar and this study should be included in the evidence. A total of 28 studies were excluded (ACTRN12611000160932; Corten 2020; CTRI/2020/10/028509; Davies 2012; Giles 1995; Helper 2020; Herrero 2016; Lindemann 1992; NCT01854788; NCT02840136; NCT03522480; NCT03655249; NCT04187924; NCT04527796; Poncin 2017; Prusak 2020; Reix 2012; Roos 1987; San Miguel‐Pagola 2020; Skopnik 1986; Sokol 2012; Sokol 2012a; Stanford 2019; van Ginderdeuren 2001; van Ginderdeuren 2008; van Ginderdeuren 2011; Vendrusculo 2019; Warwick 1990). One further study remains ongoing (NCT04010253).
Included studies
Study characteristics
One randomised study was of parallel design (Pryor 2010). The remaining seven studies were of cross‐over design; in five of these a two‐arm design was used (App 1998; McIlwaine 2010; Miller 1995; Osman 2010; Pfleger 1992) and in two studies a three‐arm design was used (Dingemans 2018; McIlwaine 1991). A washout period was described in four of these studies, varying in length between one week (App 1998; Miller 1995), one month (McIlwaine 1991) and three months (Dingemans 2018). A total of 212 participants were randomised with participant numbers varying between studies; four participants in a small pilot study (Dingemans 2018) and 75 participants in the largest study (Pryor 2010). The total study duration varied between four days (Miller 1995) and two years (McIlwaine 2010). The majority of studies, seven in total, were single‐centre studies; three were based in the UK (Miller 1995; Osman 2010; Pryor 2010), two in Canada (McIlwaine 1991; McIlwaine 2010), one in Belgium (Dingemans 2018) and one in Austria (Pfleger 1992). The remaining study was a multicentre study based in Germany (App 1998).
Participants
One study enrolled children (McIlwaine 2010), three enrolled adults (Dingemans 2018; Osman 2010; Pryor 2010) and four enrolled both adults and children (App 1998; McIlwaine 1991; Miller 1995; Pfleger 1992). The age of participants ranged between seven years and 63 years. The gender of participants was reported in seven of the studies with a ratio of 109 males to 82 females (App 1998; Dingemans 2018; McIlwaine 2010; Miller 1995; Osman 2010; Pfleger 1992; Pryor 2010). The inclusion criteria in two studies stated a hospital admission with an infective pulmonary exacerbation requiring intravenous antibiotics (Dingemans 2018; Osman 2010), whereas in the remaining six studies participants were clinically stable. One study did not report any measure of disease severity of the included participants (App 1998). Lung function at baseline was described in four studies: one study reported a wide range in FVC (38% to 117%) (McIlwaine 1991); one pilot study involving 4 participants reported individual FEV1 and FVC values (Dingemans 2018); one measured FEV1 in litres with a range of 1.9 L to 2.6 L (Pryor 2010); and one study reported a mean FEV1 of 38% (Osman 2010). Four studies reported Shwachman scores as a measure of disease severity and each study reported participants with a wide range of scores (McIlwaine 1991; McIlwaine 2010; Miller 1995; Pfleger 1992).
Interventions
Each of the eight studies varied in their treatment comparisons. Three studies compared AD to PEP (McIlwaine 1991; Pfleger 1992; Pryor 2010), three studies compared AD to PD&P or just PD (McIlwaine 1991; McIlwaine 2010; Miller 1995), two studies compared AD to Flutter® (App 1998; Pryor 2010), one study compared AD to the Cornet® (Pryor 2010), two studies compared AD to ACBT (Miller 1995; Pryor 2010), one study compared participants' normal airway clearance technique (which included AD) to HFCWO (Pryor 2010) and one study compared AD alone to AD combined with IPV at either medium (200 bpm) or high (400 bpm) frequency (Dingemans 2018).
In four studies, the duration of each treatment arm was less than 10 days (Dingemans 2018; Miller 1995; Osman 2010; Pfleger 1992). In the remaining studies, the duration of each treatment arm ranged from four weeks to one year (App 1998; McIlwaine 1991; McIlwaine 2010; Pryor 2010).
Outcomes measured
Lung function, specifically FEV1, was the most common outcome measure used and was included in each of the eight studies. Seven of the eight studies also measured FVC and three of the studies used FEF25-75% as an outcome (McIlwaine 1991; McIlwaine 2010; Miller 1995). Six studies reported sputum weight or volume (App 1998; McIlwaine 1991; McIlwaine 2010; Miller 1995; Osman 2010; Pfleger 1992). Less commonly used outcomes were oxygen saturation (Miller 1995; Osman 2010), participant preference (McIlwaine 1991; McIlwaine 2010; Miller 1995; Osman 2010), QoL measures (McIlwaine 1991; Osman 2010; Pryor 2010), hospital admissions or intravenous antibiotic therapy (McIlwaine 2010; Pryor 2010). LCI has not been measured in any of the studies to date. One study also recorded bacterial load and gene expression of Pseudomonas aeuriginosa (Dingemans 2018).
Excluded studies
A total of 28 studies were excluded (ACTRN12611000160932; Corten 2020; CTRI/2020/10/028509; Davies 2012; Giles 1995; Helper 2020; Herrero 2016; Lindemann 1992; NCT01854788; NCT02840136; NCT03522480; NCT03655249; NCT04187924; NCT04527796; Poncin 2017; Prusak 2020; Reix 2012; Roos 1987; San Miguel‐Pagola 2020; Skopnik 1986; Sokol 2012; Sokol 2012a; Stanford 2019; van Ginderdeuren 2001; van Ginderdeuren 2008; van Ginderdeuren 2011; Vendrusculo 2019; Warwick 1990). Despite the specific search criteria employed, three studies were excluded on the grounds that they were not pertinent to the population under review (CTRI/2020/10/028509; NCT01854788; Poncin 2017). The authors felt it was difficult to assess the relevance of a single treatment session using AD and, consequently, excluded nine studies using this rationale (Giles 1995; Helper 2020; Herrero 2016; Lindemann 1992; NCT03655249; NCT04187924; Sokol 2012a; Stanford 2019; Vendrusculo 2019). One study had not been completed when the abstract was published and no further associated abstracts or papers were found despite correspondence with the study team (Roos 1987). In six studies the authors considered that the interventions were not appropriate for this review (Corten 2020; Prusak 2020; Reix 2012; Sokol 2012; van Ginderdeuren 2001; Warwick 1990). A futher three studies were excluded as they evaluated nebuliser inhalation regimens rather than AD (San Miguel‐Pagola 2020; van Ginderdeuren 2008; van Ginderdeuren 2011). One study had no evidence of randomisation (Skopnik 1986). Two studies were withdrawn by the investigators prior to completing recruitment (NCT02840136; NCT03522480) and the absence of specific and meaningful AD data meant that a final three studies were deemed unfit for inclusion (ACTRN12611000160932; Davies 2012; NCT04527796)
Risk of bias in included studies
We used the approach for assessing the risk of bias in included studies recommended by Cochrane and described above (Assessment of risk of bias in included studies; Higgins 2011).
The ‘Risk of bias graph’ illustrates the proportion of studies with each of the judgements for each entry in the tool (Figure 2), whilst the ‘Risk of bias summary’ presents the review authors' judgements in a cross‐tabulation of study by entry (Figure 3). Further details can be found in the risk of bias sections of the tables describing the Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
In four of the eight included studies, the authors failed to specify how the randomisation sequence was generated. These papers stated that participants had been randomly assigned to different treatment groups, but did not clearly define the means of doing so; hence the risk of bias for sequence generation was unclear in these studies (App 1998; McIlwaine 1991; Miller 1995; Pfleger 1992). Four studies were deemed to have a low risk of bias in this respect: three studies employed computer randomisation (McIlwaine 2010; Osman 2010; Pryor 2010) and a final study involved placing randomised treatment orders in sealed envelopes, which were then subsequently shuffled and handed out to determine treatment allocation (Dingemans 2018). The latter was confirmed following correspondence with the study authors.
Allocation concealment
Seven out of the eight included studies did not discuss allocation concealment and were judged to have an unclear risk of bias (App 1998; McIlwaine 1991; McIlwaine 2010; Miller 1995; Osman 2010; Pfleger 1992; Pryor 2010). Only one study was considered to carry a low risk of bias in this respect(Dingemans 2018); correspondence with the study authors confirmed that randomised treatment orders were written on index cards, which were then placed in sealed envelopes and subsequently shuffled.
Blinding
The airway clearance techniques being compared require the individual's participation and, on occasion, the use of manual techniques or mechanical devices. It is not possible to blind by design and, in this respect, all of the included studies were deemed to carry a similarly low risk of bias. Conversely, the extent to which the lack of blinding may have had an effect is unclear, particularly on the reporting of subjective outcomes such as individual preference (McIlwaine 1991; Miller 1995) or QoL (McIlwaine 1991; Osman 2010; Pryor 2010). It is feasible, however, to blind the individuals collecting data or assessing outcomes to the allocated treatment group.
Six studies identified that some or all of the outcome assessors had been blinded and were, therefore, considered to carry a low risk of bias in this respect (Dingemans 2018; McIlwaine 1991; McIlwaine 2010; Osman 2010; Pfleger 1992; Pryor 2010). In two studies the clinical assessment was carried out by a CF physician blind to the physiotherapy technique being performed (McIlwaine 1991; McIlwaine 2010). In another, both the physician and the pulmonary function technician had been blinded (McIlwaine 2010). Three papers stated that a blinded, independent investigator or observer had assessed one or more of the outcome measures (Dingemans 2018; Osman 2010; Pfleger 1992). Only one paper, however, noted that both the data collection and the statistical analysis had been performed by blinded observers (Pryor 2010). Two studies did not discuss the issue of blinding of outcome assessors and, thus, their risk of bias was deemed unclear (App 1998; Miller 1995).
Incomplete outcome data
Participant dropout was the primary reason for incomplete outcome data. Only a single study lasting four days had no reported withdrawals and all participants were analysed in the groups to which they were assigned (Miller 1995). The online supplementary material relating to one pilot study (Dingemans 2018) indicated that eight participants had been enrolled initially but only four of them were reported in the study; these withdrawals were not described as such and the study was, in this respect, deemed to have a high risk of bias. Reasons for withdrawals were described for the remaining studies and, with the exception of one other paper (McIlwaine 2010), were judged to have a low risk of bias in this respect.
In addition to the Miller study, only one other paper explicitly carried out an intention‐to‐treat analysis for the primary outcome of FEV1 (Pryor 2010). However, 13 participants in the Pryor study did not like the intervention to which they had been allocated and withdrew from the study; it is unclear whether these participants were included in the intention‐to‐treat group. The use of an intention‐to‐treat analysis was unclear for the remainder of the included studies (App 1998; Dingemans 2018; McIlwaine 1991; McIlwaine 2010; Osman 2010; Pfleger 1992).
All six studies reporting withdrawals gave reasons for these (App 1998; McIlwaine 1991; McIlwaine 2010; Osman 2010; Pfleger 1992; Pryor 2010). One pilot study did not report withdrawals per say, though these could be inferred from the supplementary material made available in the online version of the article (Dingemans 2018). Reported withdrawal rates ranged from 3.3% of participants (Osman 2010) to an overall attrition of 44.4% in the case of the longest study (McIlwaine 2010). It should be pointed out that in the McIlwaine study withdrawals at the end of the first year comprised 13.9% of the participants, but attrition increased to 33.3% of those remaining for the second year of the study (McIlwaine 2010). The reason for this increase following the crossing over to the alternate treatment was related to a large number of participants not returning for the PD&P arm of the study due to a preference to continue with AD. This, together with the strong cross‐over effect of a further seven participants who continued with the study whilst incorporating AD into PD&P, biased the second arm of the study.
Selective reporting
As the study protocols were unavailable for all but one article (Dingemans 2018), selective reporting was assessed in the remaining studies by comparing the outcomes listed in the 'Methods' section with those of the 'Results' section (App 1998; McIlwaine 1991; McIlwaine 2010; Miller 1995; Osman 2010; Pfleger 1992; Pryor 2010).
Two studies were considered as having a high risk of selective reporting ( McIlwaine 2010; Pryor 2010). In one study, relevant baseline characteristics such as FVC and Huang scores were omitted and adherence, which had been closely monitored throughout, was not reported (McIlwaine 2010). Similarly, the duration of hospital admissions was recorded but not reported. The Huang scoring system is applied pre‐ and post‐treatment to evaluate the therapeutic response to the intervention being studied, taking into account 20 separate items; 10 clinical, five radiographic and five pulmonary function parameters. The lower the score, the more severe the disease (Huang 1981). In the second study, lung function and BMI data were not reported at the six‐month time frame as had been stated in the 'Methods' for the study (Pryor 2010).
We judged two studies to have an unclear risk of selective reporting (App 1998; Dingemans 2018). In the former study, blood oxygen saturation levels were recorded during the study but were not commented on in the paper. As there is no published data available to reflect whether this parameter changed over the course of the study or as a result of any intervention received, the risk of selective reporting is deemed to be unclear (App 1998). In a second small pilot study, specific time points indicated in the protocol did not reflect those used in the full published study, though the outcomes listed in the `Methods´section did correspond to those of the `Results´section (Dingemans 2018). Similarly, lung function data relating to one of the treatment arms were absent for two out of the four participants but this was not commented upon. Correspondence with the study authors revealed that said participants were unable to complete their final treatment arm due to participation in other clinical trials. For these reasons, the review authors considered this study to have an overall unclear risk of selective reporting.
In the four remaining studies, all outcomes described in the 'Methods' section were reported in the 'Results' section, thus there is a low risk of bias from selective reporting associated with these studies (McIlwaine 1991; Miller 1995; Osman 2010; Pfleger 1992).
Other potential sources of bias
In one cross‐over study, those carrying out AD were asked to perform AD breathing exercises during the inhalation of their pre‐treatment nebuliser (Miller 1995). However, those performing ACBT were asked to breathe normally during the nebulisation period, potentially introducing bias in the form of an "extra" eight minutes of treatment time for the AD group. No statistically significant differences were found between the two treatment groups for any of the outcomes measured. Despite this, the risk of bias was deemed to be high as the stated treatment time for the two groups was unequal, favouring the AD group.
Out of seven cross‐over studies only four reported washout periods between treatment arms; these varied between one week (App 1998; Miller 1995) and three months (Dingemans 2018). The ideal length of washout periods is unknown, but the risk of bias due to carryover effects is certainly higher in short‐term studies lacking any washout period (Pfleger 1992; Osman 2010) and of less significance in long‐term studies lasting two years (McIlwaine 2010). However, in the case of those participating in a four‐day cross‐over study during an acute respiratory exacerbation, a washout period is likely to be impractical due to rapid clinical improvements during a hospital admission (Osman 2010). Another study also involved participants who were admitted to hospital for inpatient IV antibiotic treatment (Dingemans 2018); this study had the longest washout period between treatment arms but data collection time points varied both between patients and successive hospital admissions. Two out of the four participants did not take part in one of the three treatment arms; another participant received the same treatment on two admissions but only one single data set was presented. Taking into account the small sample size and the aforementioned issues, this study was deemed to have a high risk of bias.
One study was supported by Hill‐Rom (manufacturer of the oscillating VEST®) and a grant from the Robert Luff Foundation (Osman 2010). This may be considered as a source of bias. Although Hill‐Rom provided devices and equipment for the study, they did not participate in the design, collection, analysis, interpretation of data or in the writing of the manuscript. Thus, the risk of bias was deemed to be unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings 1. Autogenic drainage versus conventional physiotherapy.
| AD compared with conventional physiotherapy for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: AD Comparison: conventional physiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional physiotherapy | AD | |||||
|
FEV1 % predicted (change from baseline) Follow‐up: up to 12 months |
The mean change in FEV1 (% predicted) was 2.09% in the conventional physiotherapy group (also see comment). | The mean change in FEV1 (% predicted) was 1.12% lower (2.64% lower to 0.40% higher) in the AD group (also see comment). | NA |
54 participants (2 studies) |
⊕⊝⊝⊝
very lowa,b |
Data available for analysis for 31 participants from the first treatment period of one study. No significant difference in FEV1 between groups in the second study. |
|
QoL (Likert scale 0 ‐ 10) Follow‐up: up to 12 months |
See comment. | NA |
54 participants (2 studies) |
⊕⊝⊝⊝
very lowa,b,c |
Participants subjectively reported AD to be superior to conventional physiotherapy in terms of comfort, level of control and degree of interruption in their daily life. | |
|
Participant preference Follow‐up: 12 months |
See comment. | See comment. | NA |
36 participants (1 study) |
⊕⊝⊝⊝
very lowa,b,c |
All participants reported a preference for autogenic drainage and many refused to go back to conventional physiotherapy. |
| Exercise tolerance | Not reported. | NA | NA | NA | ||
| Adverse events | Not reported. | NA | NA | NA | ||
|
Number of admissions to hospital Follow‐up: 12 months |
There were 16 hospitalisations in the conventional physiotherapy group. | There were 13 hospitalisations in the AD group. | NA |
36 participants (1 study) |
⊕⊝⊝⊝
very lowa,b |
Unclear which treatment period of the cross‐over study these hospitalisations occurred in, so data not analysed. |
| Need for extra treatment | See comment. | NA |
36 participants (1 study) |
⊕⊝⊝⊝
very lowa,b |
No participants received home intravenous antibiotic treatment. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in one second; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision; small numbers of participants included in the comparison. b. Downgraded twice due to serious risk of bias; incomplete outcome data and selectively reported results. c. Downgraded once due to applicability; outcomes are recorded subjectively.
Summary of findings 2. Autogenic drainage versus spontaneous cough.
| AD compared with spontaneous cough for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: AD Comparison: spontaneous cough | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Spontaneous cough | AD | |||||
|
FEV1 % predicted Follow‐up: each treatment performed on 1 day |
See comment. | NA | 14 participants (1 study) | ⊕⊝⊝⊝ very lowa,b,c | There was no significant difference between groups in terms of FEV1 (% predicted). |
|
| QoL | Not reported. | NA | NA | NA | ||
| Participant preference | Not reported. | NA | NA | NA | ||
| Exercise tolerance | Not reported. | NA | NA | NA | ||
|
Adverse events Follow‐up: each treatment performed on 1 day |
See comment. | NA | 14 participants (1 study) | ⊕⊝⊝⊝ very lowa,b,c | No adverse events were reported during the study. | |
| Number of admissions to hospital | Not reported. | NA | NA | NA | ||
| Need for extra treatment | Not reported. | NA | NA | NA | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision; small numbers of participants included in the comparison. As results were not presented from paired analyses for one study, we treated the cross‐over studies as if they were parallel studies which is a conservative approach as it does not take into account within‐patient correlation. b. Downgraded once due to risk of bias; inconsistency between methods described and results reported regarding time for individuals to clear lungs. c. Downgraded once due to applicability; each treatment performed only once and very limited follow up (less than 1 week).
Summary of findings 3. Autogenic drainage versus active cycle of breathing technique.
| AD compared with ACBT for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: AD Comparison: ACBT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ACBT | AD | |||||
|
FEV1 (L) Follow‐up: up to 12 months |
The mean FEV1 was 1.94 L in the ACBT group (also see comment). | The mean FEV1 was 0.70 L higher (0.09 L lower to 1.49 L higher) in the autogenic drainage group (also see comment). | NA |
44 participants (2 studies) |
⊕⊕⊝⊝ lowa,b | Data were available for analysis for 26 participants from 1 study. A significant deterioration in FEV1 (% predicted) was also observed for the cohort of this study. No significant differences in pulmonary function tests in the other study. |
|
QoL (SF‐36 and CRQ) Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | There were no significant differences between groups in the mental and physical domains of the SF‐36. There were no significant differences between the dyspnoea, fatigue, emotion and mastery domains of the CRQ. | |
|
Participant preference Follow‐up: up to 12 months |
See comment. | NA |
44 participants (2 studies) |
⊕⊝⊝⊝
very lowa,b,c |
1 study reported that 9 participants preferred AD, 8 participants preferred ACBT and 1 participant had no preference. In the other study 13 out of the total of 75 participants (all treatments in the study) withdrew as they did not like the treatment they were randomised to (not specified by intervention). |
|
|
Exercise tolerance (modified shuttle test) Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | No significant difference between groups. | |
|
Adverse events Follow‐up: 2 days |
See comment. | NA |
18 participants (1 study) |
⊕⊝⊝⊝
very lowa,b,d |
1 study reported a decrease in oxygen saturation levels in 4 participants in the ACBT group but no participants experienced this during any AD sessions. | |
| Number of admissions to hospital | Not reported. | NA | NA | NA | ||
|
Need for extra treatment Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | The median number of antibiotics courses per treatment group ranged from 1.0 to 1.5 (no further information given). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; ACBT: active cycle of breathing technique; CRQ: Chronic Respiratory Questionnaire; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; NA: not applicable; QoL: quality of life; SF‐36: short form 36. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision; small numbers of participants included in the comparison. b. Downgraded once due to unclear risk of bias; many elements of study designs not clearly described. c. Downgraded once due to risk of bias; by design, study cannot be blinded and lack of masking may have influenced subjective outcomes. Further no details of treatment used prior to baseline reported, which may also have influenced subjective outcomes. d. Downgraded once due to applicability; each treatment performed only once and very limited follow up (less than one week).
Summary of findings 4. Autogenic drainage versus positive expiratory pressure.
| AD compared with PEP for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: AD Comparison: PEP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PEP | AD | |||||
|
FEV1 (L) Follow‐up: up to 12 months |
The mean FEV1 was 2.02 L in the PEP group (also see comment). | The mean FEV1 was 0.62 L higher (0.30 L lower to 1.54 L higher) in the AD group (also see comment). | NA | 62 participants (3 studies) | ⊕⊕⊝⊝ lowa,b | Data were available for analysis for 26 participants from 1 study. A significant deterioration in FEV1 (% predicted) was also observed for the cohort of this study. In the other 2 studies, there was there was no significant difference between groups in terms of FEV1. |
|
QoL (SF‐36 and CRQ) Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | There were no significant differences between groups in the mental and physical domains of the SF‐36. There were no significant differences between the dyspnoea, fatigue, emotion and mastery domains of the CRQ. | |
|
Participant preference Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | 13 out of the total of 75 participants (all treatments in the study) withdrew as they did not like the treatment they were randomised to (not specified by intervention). | |
|
Exercise tolerance: modified shuttle test Follow‐up: up to 12 months |
See comment. | NA | NA | NA | No significant difference between groups. | |
| Adverse events | Not reported. | NA | NA | NA | ||
| Number of admissions to hospital | Not reported. | NA | NA | NA | ||
|
Need for extra treatment Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | The median number of antibiotics courses per treatment group ranged from 1.0 to 1.5 (no further information given). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CRQ: Chronic Respiratory Questionnaire; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; NA: not applicable; PEP: positive expiratory pressure; QoL: quality of life; SF‐36: short form 36. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision; small numbers of participants included in the comparison. As results were not presented from paired analyses for one study, we treated the cross‐over studies as if they were parallel studies which is a conservative approach as it does not take into account within‐patient correlation. b. Downgraded once due to risk of bias; inconsistency between methods described and results reported regarding time for individuals to clear lungs and many elements of study designs not clearly described. c. Downgraded once due to risk of bias; by design, study cannot be blinded and lack of masking may have influenced subjective outcomes. Further no details of treatment used prior to baseline reported, which may also have influenced subjective outcomes.
Summary of findings 5. Autogenic drainage versus Cornet®.
| AD compared with Cornet® for CF | ||||||
|
Patient or population: adults with CF Settings: outpatients Intervention: AD Comparison: Cornet® | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cornet® | AD | |||||
|
FEV1 (L) Follow‐up: up to 12 months |
The mean FEV1 was 1.9 L in the Cornet® group (also see comment). | The mean FEV1 was 0.74 L higher (0.07 L lower to 1.55 L higher) in the AD group (also see comment). | NA |
27 participants (1 study) |
⊕⊕⊕⊝ moderatea |
Data for 27 participants were available for analysis. A significant deterioration in FEV1 (% predicted) was also observed for the cohort of this study. |
|
QoL (SF‐36 and CRQ) Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,b | There were no significant differences between groups in the mental and physical domains of the SF‐36. There were no significant differences between the dyspnoea, fatigue, emotion and mastery domains of the CRQ. | |
|
Participant preference Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,b | 13 out of the total of 75 participants (all treatments in the study) withdrew as they did not like the treatment they were randomised to (not specified by intervention). | |
|
Exercise tolerance: modified shuttle test Follow‐up: up to 12 months |
See comment. | NA | NA | NA | No significant difference between groups. | |
| Adverse events | Not reported. | NA | NA | NA | ||
| Number of admissions to hospital | Not reported. | NA | NA | NA | ||
|
Need for extra treatment Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,b | The median number of antibiotics courses per treatment group ranged from 1.0 to 1.5 (no further information given). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CRQ: Chronic Respiratory Questionnaire; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; NA: not applicable; QoL: quality of life; SF‐36: short form 36. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision; small numbers of participants included in the comparison. b. Downgraded once due to risk of bias; by design, study cannot be blinded and lack of masking may have influenced subjective outcomes. Further no details of treatment used prior to baseline reported, which may also have influenced subjective outcomes.
Summary of findings 6. Autogenic drainage versus Flutter®.
| AD compared with Flutter®for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: AD Comparison: Flutter® | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Flutter® | AD | |||||
|
FEV1 (L) Follow‐up:up to 12 months. |
The mean FEV1 was 0.21 L higher (0.64 L lower to 1.21 L higher) in the AD group in the parallel study The mean FEV1 was 0.10 L higher (0.95 L lower to 1.15 L higher) in the AD group in the cross‐over study |
NA | 39 participants (2 studies including one cross‐over study)d | ⊕⊕⊝⊝ lowa,b | A significant deterioration in FEV1 (% predicted) was also observed for the cohort of the parallel study. |
|
|
QoL (SF‐36 and CRQ) Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | There were no significant differences between groups in the mental and physical domains of the SF‐36. There were no significant differences between the dyspnoea, fatigue, emotion and mastery domains of the CRQ. | |
|
Participant preference Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | 13 out of the total of 75 participants (all treatments in the study) withdrew as they did not like the treatment they were randomised to (not specified by intervention). | |
|
Exercise tolerance: modified shuttle test Follow‐up: up to 12 months |
See comment. | NA | NA | NA | No significant difference between groups. | |
| Adverse events | Not reported. | NA | NA | NA | ||
| Number of admissions to hospital | Not reported. | NA | NA | NA | ||
|
Need for extra treatment Follow‐up: up to 12 months |
See comment. | NA |
30 participants (1 study) |
⊕⊕⊝⊝ lowa,c | The median number of antibiotics courses per treatment group ranged from 1.0 to 1.5 (no further information given). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CRQ: Chronic Respiratory Questionnaire; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; NA: not applicable; QoL: quality of life; SF‐36: short form 36. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision; small numbers of participants included in the comparison. b. Downgraded once due to unclear risk of bias; many elements of study designs not clearly described. c. Downgraded once due to risk of bias; by design, study cannot be blinded and lack of masking may have influenced subjective outcomes. Further no details of treatment used prior to baseline reported, which may also have influenced subjective outcomes. d. Data from the cross‐over study were analysed at the end of the first treatment period, before cross‐over occurred.
Summary of findings 7. Autogenic drainage versus high frequency chest wall oscillation.
| AD compared with HFCWOfor CF | ||||||
|
Patient or population: adults with CF Settings: hospital admission Intervention: AD Comparison: HFCWO | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| HFCWO | AD | |||||
| FEV1 | Not reporteda. | NA | NA | NA | ||
| QoL | Not reporteda. | NA | NA | NA | ||
| Participant preference | Not reporteda. | NA | NA | NA | ||
| Exercise tolerance (modified shuttle test) | Not reporteda. | NA | NA | NA | ||
| Adverse events | Not reporteda. | NA | NA | NA | ||
| Number of admissions to hospital | Not reporteda. | NA | NA | NA | ||
| Need for extra treatment | Not reporteda. | NA | NA | NA | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; HFCWO: high frequency chest wall oscillation; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. No outcome data presented as several interventions (AD, Flutter®, positive expiratory pressure and conventional physiotherapy) were grouped together as as "usual airway clearance techniques" and compared to HFCWO. Insufficient data comparing AD and HFCWO.
Summary of findings 8. Autogenic drainage versus intrapulmonary percussive ventilation (200 bpm).
| AD compared with IPV (200 bpm)for CF | ||||||
|
Patient or population: adults with CF Settings: hospital admission for respiratory exacerbation Intervention: AD Comparison: IPV (200 bpm) with AD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IPV plus AD | AD | |||||
|
FEV1% predicted (change from baseline) Follow‐up: 10 days |
Change in FEV1 % predicted was 3.8% higher in the AD group (0.57% lower to 8.17% higher) than in the IPC group. | N/A | 4 (1) |
⊕⊝⊝⊝ very lowa,b | The included trial is a cross‐over trial which has been analysed as a parallel trial; this means that the participants have been counted twice. | |
| QoL | This outcome was not reported. | |||||
| Participant preference | This outcome was not reported. | |||||
| Exercise tolerance (modified shuttle test) | This outcome was not reported. | |||||
| Adverse events | This outcome was not reported. | |||||
| Number of admissions to hospital | See comments. | Inclusion criteria for participants in the Dingemans study included hospitalisation for a respiratory infection (Dingemans 2018). | ||||
| Need for extra treatment | See comments. | All 4 participants were already receiving intravenous antibiotics as part of their medical management as inpatients during the course of this study (Dingemans 2018). | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; IPV: intrapulmonary percussive ventilation; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision caused by a very small number of participants.
b. Downgraded twice due to risk of bias from the design of the study. The study uses a cross over design whereby participants are randomised to a different treatment at each subsequent admission. It is unlikely that baseline values will be the same at the start of each treatment period. There is also a high risk of reporting bias and selective reporting of results.
Summary of findings 9. Autogenic drainage versus intrapulmonary percussive ventilation (400 bpm).
| AD compared with IPV (400 bpm)for CF | ||||||
|
Patient or population: adults with CF Settings: hospital admission for respiratory exacerbation Intervention: AD Comparison: IPV (400 bpm) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IPV | AD | |||||
|
FEV1 % predicted (change from baseline) Follow‐up: 10 days |
Change in FEV1 % predicted was 2.9% lower in the AD group (7.67% lower to 1.87% higher) than in the IPV group. | N/A | 4 (1) |
⊕⊝⊝⊝ very lowa,b | The included trial is a cross‐over trial which has been analysed as a parallel trial; this means that the participants have been counted twice. | |
| QoL | This outcome was not reported. | |||||
| Participant preference | This outcome was not reported. | |||||
| Exercise tolerance (modified shuttle test) | This outcome was not reported. | |||||
| Adverse events | This outcome was not reported. | |||||
| Number of admissions to hospital | See comments. | Inclusion criteria for participants in the Dingemans study included hospitalisation for a respiratory infection (Dingemans 2018). | ||||
| Need for extra treatment | See comments. | All 4 participants were already receiving intravenous antibiotics as part of their medical management as inpatients during the course of this study (Dingemans 2018). | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate or mean risk in the control group unless otherwise stated. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: autogenic drainage; CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume in 1 second; IPV: intrapulmonary percussive ventilation; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision caused by a very small number of participants.
b. Downgraded twice due to risk of bias from the design of the study. The study uses a cross over design whereby participants are randomised to a different treatment at each subsequent admission. It is unlikely that baseline values will be the same at the start of each treatment period. There is also a high risk of reporting bias and selective reporting of results.
Autogenic drainage versus conventional physiotherapy
Two studies (54 participants) reported on this comparison of AD versus PD&P (McIlwaine 1991; McIlwaine 2010). The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 1).
Primary outcomes
1. FEV1
Both studies measured FEV1 (McIlwaine 1991; McIlwaine 2010), but only data from the later study were available for our analysis (McIlwaine 2010). In this study, the rate of decline in FEV1 % predicted for each participant was determined over the one‐year study period. At the 12‐month time point, our analysis found no statistically significant difference between AD and PD&P, MD ‐1.12 (95% CI ‐2.64 to 0.40) (very low‐certainty evidence) (Analysis 1.1). In the earlier McIlwaine study, lung function was measured as % change from baseline for each of three two‐month treatment periods using AD, PEP and PD&P (results for the AD versus PEP arm are reported below). There were no statistically significant changes in FEV1/FVC between the AD and PD&P treatment periods (McIlwaine 1991).
1.1. Analysis.

Comparison 1: AD versus PD&P, Outcome 1: FEV₁ (change in % predicted)
2. QoL
Questionnaires incorporating a Likert scale 0 ‐ 10 were used to gauge comfort, level of control and degree of interruption in their daily life (very low quality evidence). Participants subjectively reported AD to be superior to PD&P (McIlwaine 1991). In the later study, the participants subjectively felt that AD "worked the best" and the authors reflected that, collectively, AD gave the participants more independence and a greater amount of freedom in performing their physiotherapy treatment when compared to PD&P (McIlwaine 2010).
Secondary outcomes
1. Participant preference
The later McIlwaine study reported a preference for AD by all participants in the study, with many participants refusing to go back to performing PD&P (very low‐certainty evidence) (McIlwaine 2010).
2. Exercise tolerance
Neither study reported on this outcome (McIlwaine 1991; McIlwaine 2010).
3. Adverse effects
Neither study reported on this outcome (McIlwaine 1991; McIlwaine 2010).
4. Number of admissions
Only the later study reported on this outcome and provided data to enter into our analysis (McIlwaine 2010). The authors did not specify the number of separate individuals admitted to hospital for pulmonary exacerbations, although they did state that the total number of hospitalisations per group by the 12‐month time point (13 for the AD group, 16 for the PD&P group) (very low‐certainty evidence). The published paper reported that mean number of hospital admissions was not significantly lower in the AD group compared to the PD&P group; however, in contrast, our analysis shows the mean number of hospital admissions during the first year of the study was significantly lower in the AD group, MD ‐0.24 (95% CI ‐0.42 to ‐0.06) (Analysis 1.2). The reason for this statistical discrepancy remains unclear and as we have been unable to further clarify this with the authors of the article, these results should be interpreted with caution.
1.2. Analysis.

Comparison 1: AD versus PD&P, Outcome 2: Hospital admissions
5. Need for extra treatment
The later McIlwaine study described 16 hospitalisations for pulmonary exacerbations in the PD&P group compared to 13 in the AD group in the first year of the study (there were 18 participants in each group), but the authors did not specify the number of separate individuals from each group who were hospitalised. The investigators did report that no participants received home intravenous antibiotic treatment (very low‐certainty evidence) (McIlwaine 2010).
6. Pulmonary function measurements
a. LCI
This outcome was not measured in either study (McIlwaine 1991; McIlwaine 2010).
b. FVC
Both studies measured FVC (McIlwaine 1991; McIlwaine 2010), but only data from the later study were available for our analysis (McIlwaine 2010). In this study, the change in FVC % predicted was determined over the 12‐month study period and analysed as a parallel study with no statistically significant changes being reported between the treatment methods. In contrast to the published paper, our analysis shows statistical significance in favour of AD, MD 1.88 (95% CI 0.68 to 3.08) (Analysis 1.3). The reason for this statistical discrepancy remains unclear and as we have been unable to clarify with the authors of the article, these results should be interpreted with caution. In the earlier McIlwaine study, FVC was measured as % change from baseline for each two‐month treatment period using AD and PD&P and there were no significant changes found between the treatment methods (McIlwaine 1991).
1.3. Analysis.

Comparison 1: AD versus PD&P, Outcome 3: FVC (change in % predicted)
c. FEF25 -75%
Both studies measured FEF25 -75% (McIlwaine 1991; McIlwaine 2010), but only data from the later study were available for our analysis (McIlwaine 2010). In this study, the change in FEF25 -75% predicted was determined over the 12‐month study period and analysed as a parallel study with no statistically significant changes being reported between the treatment methods. In contrast to the published paper, our analysis shows statistical significance in favour of PD&P, MD ‐7.54 (95% CI‐10.39 to ‐4.69) (Analysis 1.4). Once again, the reason for this statistical discrepancy remains unclear and as we have been unable to further clarify this with the authors of the article, these results should be interpreted with caution. In the earlier McIlwaine study, FEF25 -75% was measured as % change from baseline for each two‐month treatment period using AD and PD&P and there were no significant changes found between the treatment methods (McIlwaine 1991).
1.4. Analysis.

Comparison 1: AD versus PD&P, Outcome 4: FEF25-75% (change in % predicted)
7. Oxygen saturation
Neither study reported on this outcome (McIlwaine 1991; McIlwaine 2010).
8. Sputum weight
Only the earlier study measured sputum weight (McIlwaine 1991). The paper reported that the net weight of sputum produced during AD was significantly greater (P < 0.01) than that produced during PD&P, but data were not reported in sufficient detail to enter into our analysis (McIlwaine 1991). It was noted that sputum production whilst using AD was relatively consistent over the two‐month study period.
9. Survival
Neither study reported on this outcome (McIlwaine 1991; McIlwaine 2010).
Autogenic drainage versus spontaneous cough
One study (14 participants) used cough alone in a comparison with AD (Pfleger 1992). The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 2).
Primary outcomes
1. FEV1
There were no significant differences in FEV1 % predicted between AD and cough alone when measured at 30 minutes post physiotherapy, MD 3.00% (95% CI ‐11.08 to 17.08) (very low‐certainty evidence) (Analysis 2.1) (Pfleger 1992).
2.1. Analysis.

Comparison 2: AD versus spontaneous cough, Outcome 1: FEV₁ (% predicted)
2. QoL
The study did not report on this outcome (Pfleger 1992).
Secondary outcomes
1. Participant preference
The study did not report on this outcome (Pfleger 1992).
2. Exercise tolerance
The study did not report on this outcome (Pfleger 1992).
3. Adverse effects
No adverse effects were reported in this study (very low‐certainty evidence) (Pfleger 1992).
4. Number of admissions
The study did not report on this outcome (Pfleger 1992)
5. Need for extra treatment
The study did not report on this outcome (Pfleger 1992)
6. Pulmonary function measurements
a. LCI
This outcome was not measured in this study (Pfleger 1992).
b. FVC
There were no significant differences in FVC % predicted between the treatment groups when measured at 30 minutes post physiotherapy, MD 4.00% (95% CI ‐10.83 to 18.83) (Analysis 2.2) (Pfleger 1992).
2.2. Analysis.

Comparison 2: AD versus spontaneous cough, Outcome 2: FVC (% predicted)
c. FEF25 -75%
Pfleger did not report on this outcome (Pfleger 1992).
7. Oxygen saturation
The study did not report on this outcome (Pfleger 1992).
8. Sputum weight
Pfleger compared cough alone with AD and Hi‐PEP alone and in combination (Hi‐PEP results not reported here). It was reported that all four forms of physiotherapy used in this study produced significantly more sputum than spontaneous coughing alone (P < 0.001) and our statistical analysis corroborates this, MD 18.33 g (95% CI 3.11 to 33.55) (Analysis 2.3). However, sputum production with AD alone was the lowest and differed significantly from that of the other physiotherapy treatment groups (Pfleger 1992). .
2.3. Analysis.

Comparison 2: AD versus spontaneous cough, Outcome 3: Sputum weight (g)
9. Survival
The study did not report on this outcome (Pfleger 1992).
Autogenic drainage versus active cycle of breathing technique
Two studies (48 participants) reported on this comparison (Miller 1995; Pryor 2010). Although 75 participants were included overall in the Pryor study, only 15 were randomised to each of the five treatment groups; therefore the study only contributes 30 participants to this pair‐wise comparison (Pryor 2010). The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 3).
Primary outcomes
1. FEV1
Both studies reported on FEV1, but data were only available from one study for FEV1 (L) for our analysis (Pryor 2010). Pryor reported data at three time points over the 12‐month period of the study ‐ at the start, at six months and at 12 months (Pryor 2010). At the 12‐month time point, our analysis found no statistically significant difference between the AD and ACBT groups, MD 0.70 L (95% CI ‐0.09 to 1.49) (very low‐certainty evidence) (Analysis 3.1). Pryor also reported FEV1 % predicted and overall observed a significant deterioration in FEV1 % predicted over the 12‐month period for the entire cohort (‐1.8% predicted; P = 0.02), stating this decline was within the international average at the time of the study (Pryor 2010). However, recruitment was challenging for this long‐term study, meaning it was underpowered to detect such a change. Consequently, the results obtained may have over or underestimated any decline in lung function identified by the original authors.
3.1. Analysis.

Comparison 3: AD versus ACBT, Outcome 1: FEV₁ (L)
Miller (18 participants) measured lung function prior to and following each physiotherapy treatment over the four‐day period of the study, but FEV1 was not reported specifically (Miller 1995). The paper stated that taken overall, pulmonary function tests showed no significant difference between the two methods.
2. QoL
Health‐related QoL was measured in one study using the Short Form‐36 (Medical Outcomes Trust, Boston, USA), analysing the physical and mental domains of the participants (low‐certainty evidence) (Pryor 2010). There were no significant differences in the physical domain between the groups, though the paper observed that overall there was a trend towards deterioration over time reported (P = 0.05). Similarly, in the mental domain there were no significant differences found amongst the groups but there was a significant deterioration over time reported (P = 0.002).
Pryor also analysed data for the four domains of dyspnoea, fatigue, emotion and mastery in the Chronic Respiratory Questionnaire (CRQ) (Guyatt 1987); but found no significant differences in any domain, although there was a significant improvement in dyspnoea (P = 0.01) reported over time in the group as a whole (Pryor 2010).
Secondary outcomes
1. Participant preference
Miller reported that nine participants preferred AD, eight participants preferred ACBT, and one participant had no preference (Miller 1995). They went on to qualify that those who preferred AD to ACBT tended to be those who displayed a greater concentration and compliance with treatment. During the course of the Pryor study, 13 participants withdrew as they did not like the regimen to which they had been randomised and either reverted to their original preferred option or chose a different regimen; the intervention each participant was using was not identified (Pryor 2010). The quality of the evidence from both these studies was very low.
2. Exercise tolerance
The modified shuttle test was reported in one study (30 participants), but no data were available for our analysis (Pryor 2010). No significant difference was found between AD and ACBT (low‐certainty evidence).
3. Adverse effects
Miller described a decrease in oxygen saturation levels whilst performing ACBT in the moderate to severe group of participants, but not during any AD sessions (Miller 1995). The authors did not quantify the extent but did report that in three participants, one episode was observed and in a fourth participant two episodes were reported (both morning and afternoon ACBT sessions) (very low‐certainty evidence).
4. Number of admissions
Neither study reported on this outcome (Miller 1995; Pryor 2010).
5. Need for extra treatment
In the Pryor study, some participants in each of the regimens required intravenous antibiotics during the course of the study; the median number of courses per group ranged from 1.0 to 1.5 (low quality evidence) (Pryor 2010). The number of participants and allocated treatment arm was not specified.
6. Pulmonary function measurements
a. LCI
This outcome was not measured in either study (Miller 1995; Pryor 2010).
b. FVC
Neither study reported this outcome in sufficient detail to enter into our analysis, but both studies reported there was no statistically significant difference between the two methods (Miller 1995; Pryor 2010). However, Miller reported that more participants demonstrated an improved FVC with ACBT than AD (Miller 1995).
c. FEF25 -75%
This outcome was reported in one study (18 participants), but not in sufficient detail for inclusion in our analysis (Miller 1995). The investigators stated that more participants had an improved FEF25-75% with AD than with the ACBT.
7. Oxygen saturation
Oxygen saturation levels were reported in one study (18 participants), but no data were available for our analysis (Miller 1995). There was no difference found in mean saturation levels of oxygen between the treatment methods over the four study days. However, four participants with moderate to severe disease decreased their oxygen saturation levels during the morning ACBT session, and one also demonstrating a decrease in the afternoon session. Participants maintained their oxygen saturation levels during AD sessions.
8. Sputum weight
Sputum weight was an outcome used in one study (18 participants), but no data were available for our analysis (Miller 1995). Sputum was collected and weighed during one hour following physiotherapy treatment. There was no significant difference found between the AD and ACBT groups.
9. Survival
One participant died during the course of the Pryor study and the allocated treatment arm was not specified; however, the investigators stated that the death was unlikely to have been caused by any intervention under evaluation (Pryor 2010).
Autogenic drainage versus positive expiratory pressure
A total of three studies (62 participants) reported on this comparison (McIlwaine 1991; Pfleger 1992; Pryor 2010). Although 75 participants were included overall in the Pryor study, only 15 were randomised to each of the five treatment groups; therefore the study only contributes 30 participants to this pair‐wise comparison (Pryor 2010). Two studies compared AD with PEP (McIlwaine 1991; Pryor 2010) and one study compared AD to Hi‐PEP (Pfleger 1992). The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 4).
Primary outcomes
1. FEV1
All three studies (62 participants) reported on FEV1 as an outcome, but used different units of measurement and we were unable to combine any data (McIlwaine 1991; Pfleger 1992; Pryor 2010).
Pryor reported FEV1 (L) at the start and end of the 12‐month study period (Pryor 2010). At the 12‐month time point, our analysis found no statistically significant difference between the AD and PEP groups, MD 0.62 L (95% CI ‐0.30 to 1.54) (low‐certainty evidence) (Analysis 4.1).
4.1. Analysis.

Comparison 4: AD versus PEP, Outcome 1: FEV₁ (L)
Pryor also reported FEV1 % predicted and, overall, observed a significant deterioration in FEV1 % predicted over the 12‐month period for the entire cohort (‐1.8% predicted; P = 0.02), stating this decline was within the international average at the time of the study (Pryor 2010). However, recruitment was challenging for this long‐term study, meaning it was underpowered to detect such a change. Consequently, the results obtained may have over or underestimated any decline in lung function identified by the original authors. Pfleger reported FEV1 % predicted was measured repeatedly before, during and after physiotherapy treatments over the five‐day study period (Pfleger 1992); our analysis found no statistically significant difference between AD and Hi‐PEP at 30 minutes following physiotherapy, MD 2.00% predicted (95% CI ‐12.45 to 16.45) (Analysis 4.2). Finally, in the three‐arm study, McIlwaine measured FEV1 % predicted at the outset and at the beginning and end of each of the three two‐month study periods; investigators reported no significant difference in FEV1/FVC when each group performed either AD or PEP, but no data were available for our analysis (McIlwaine 1991).
4.2. Analysis.

Comparison 4: AD versus PEP, Outcome 2: FEV₁ (% predicted)
2. QoL
Pryor (n = 30) evaluated QoL as an outcome using the Short Form‐36 and CRQ (low quality evidence) (Pryor 2010). For the Short Form‐36 there were no significant differences in the physical domain between the two groups, but overall the paper reported that there was a significant trend towards deterioration over time; similarly, in the mental domain there were no significant differences found, but there was a significant deterioration reported over time. For the CRQ there were no significant differences found for dyspnoea, fatigue, emotion or mastery between the two groups. Overall, there was a significant improvement in dyspnoea (P = 0.01) reported over time in the group as a whole (Pryor 2010).
Secondary outcomes
1. Participant preference
During the course of the Pryor study (n = 30), 13 participants withdrew as they did not like the regimen to which they had been randomised and either reverted to their original preferred option or chose a different regimen (low‐certainty evidence). The intervention each was using at the time was not identified (Pryor 2010).
2. Exercise tolerance
The modified shuttle test was reported in one study (30 participants), but no data were available for our analysis (Pryor 2010). No significant difference was reported between AD and PEP.
3. Adverse effects
None of the studies reported on this outcome (McIlwaine 1991; Pfleger 1992; Pryor 2010).
4. Number of admissions
None of the studies reported on this outcome (McIlwaine 1991; Pfleger 1992; Pryor 2010).
5. Need for extra treatment
Pryor reported some participants in each of the regimens required intravenous antibiotics during the course of the study. The number of participants and allocated treatment arm was not specified (Pryor 2010). The median number of courses per group ranged from 1.0 to 1.5, though statistical analysis was not carried out due to the small numbers and scattered nature of the data (low‐certainty evidence).
6. Pulmonary function measurements
a. LCI
This outcome was not measured in any study (McIlwaine 1991; Pfleger 1992; Pryor 2010).
b. FVC
All three studies (62 participants) measured FVC, but data were only available from one study for FVC % predicted which was measured 30 minutes following physiotherapy (Pfleger 1992). Our analysis found no statistically significant difference between the AD and Hi‐PEP groups, MD 1.00% (95% CI ‐13.45 to 15.45) (Analysis 4.3). There were no significant changes in FVC found in either of the remaining studies when using AD or PEP (McIlwaine 1991; Pryor 2010).
4.3. Analysis.

Comparison 4: AD versus PEP, Outcome 3: FVC (% predicted)
c. FEF25 -75%
One study (18 participants) reported FEF25-75%, but not in sufficient detail to include in our analysis; the investigators reported no statistically significant changes between AD and PEP (McIlwaine 1991).
7. Oxygen saturation
None of the studies reported on this outcome (McIlwaine 1991; Pfleger 1992; Pryor 2010).
8. Sputum weight
Two studies (32 participants) used sputum weight as an outcome (McIlwaine 1991; Pfleger 1992). Data were only available from one study (14 participants) for our analysis (Pfleger 1992). The review authors estimated this data from a bar chart in the published article, demonstrating that AD showed the lowest sputum production and PEP the highest (Pfleger 1992). Our analysis of sputum weight following physiotherapy treatment showed a numerical advantage to PEP, but found no statistically significant differences between the AD and PEP groups, MD ‐15.00 g (95% CI ‐35.46 to 5.46) (Analysis 4.4). This, however, contrasts with the published paper, which states statistical significance in favour of PEP (Pfleger 1992). The data extracted were approximate and measured from the graph of sputum production, so this probably accounts for the discrepancy with the results in the published paper.
4.4. Analysis.

Comparison 4: AD versus PEP, Outcome 4: Sputum weight (g)
In one of the three published abstracts relating to the McIlwaine study (presented at the 17th European Cystic Fibrosis Conference), the authors reported the net weight of sputum obtained was significantly greater (P < 0.01) with AD compared to PEP, but the remaining two abstracts do not state this (McIlwaine 1991). The unpublished paper which we obtained from the authors does not fully clarify the matter and we will attempt to address these discrepancies in a future update. However, it was noted that sputum production whilst using AD was relatively consistent over the two‐month study period.
9. Survival
One participant died during the course of the Pryor study and the allocated treatment arm was not specified; however, the investigators stated that the death was unlikely to have been caused by any intervention under evaluation (Pryor 2010).
Autogenic drainage versus Cornet®
One study (30 participants) reported on this comparison (Pryor 2010). Although 75 participants were included overall in the Pryor study, only 15 were randomised to each of the five treatment groups; therefore the study contributes 30 participants (Pryor 2010). The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 5).
Primary outcomes
1. FEV1
Pryor reported FEV1 (L) data at the start and end of the 12‐month study period that was available to enter into our analysis (Pryor 2010). At the 12‐month time point our analysis found no statistically significant difference between the AD and Cornet® groups, MD 0.74 L (95% CI ‐0.07 to 1.55) (moderate‐certainty evidence) (Analysis 5.1). Pryor also reported FEV1 % predicted and, overall, observed a significant deterioration in FEV1 % predicted over the 12‐month period for the entire cohort (‐1.8% predicted; P = 0.02), stating this decline was within the international average at the time of the study (Pryor 2010). However, recruitment was challenging for this long‐term study, meaning it was underpowered to detect such a change. Consequently, the results obtained may have over or underestimated any decline in lung function identified by the original authors.
5.1. Analysis.

Comparison 5: AD versus Cornet®, Outcome 1: FEV₁ (L)
2. QoL
Pryor used the Short Form‐36 questionnaire and the CRQ and found no significant difference in the domains between the two groups (low‐certainty evidence) (Pryor 2010). The results of the CRQ reported minimal clinically important differences (improvements) in dyspnoea in the AD group, but not the Cornet® group over the 12‐month study period. However, there was an overall significant improvement in dyspnoea (P = 0.01) reported over time in the entire cohort.
Secondary outcomes
1. Participant preference
During the course of the Pryor study, 13 out of 75 participants withdrew as they did not like the regimen to which they had been randomised and either reverted to their original preferred option or chose a different regimen (low quality evidence) (Pryor 2010). The intervention each was using at the time was not identified.
2. Exercise tolerance
Pryor measured exercise tolerance using the Modified Shuttle Test and no significant difference was found between AD and Cornet® groups (Pryor 2010). No detailed data were available for our analysis.
3. Adverse effects
Pryor did not report this outcome (Pryor 2010).
4. Number of admissions
Pryor did not report this outcome (Pryor 2010).
5. Need for extra treatment
Pryor reported that some participants in each of the regimens required intravenous antibiotics during the course of the study (Pryor 2010). The median number of courses per group ranged from 1.0 to 1.5, though statistical analysis was not carried out due to the small numbers and scattered nature of the data (low‐certainey evidence). The number of participants and allocated treatment arm was not specified.
6. Pulmonary function measurements
a. LCI
Pryor did not report this outcome (Pryor 2010).
b. FVC
Pryor reported no significant difference in FVC (L) between AD and Cornet®, but no detailed data were available for our analysis (Pryor 2010).
c. FEF25 -75%
This outcome was not reported in this study (Pryor 2010).
7. Oxygen saturation
The study only reported oxygen saturation levels at baseline in the participant demographics (Pryor 2010).
8. Sputum weight
This outcome was not reported (Pryor 2010).
9. Survival
One participant died during the course of the Pryor study and the allocated treatment arm was not specified; however, the investigators stated that the death was unlikely to have been caused by any intervention under evaluation (Pryor 2010).
Autogenic drainage versus Flutter®
Two studies (47 participants) reported on this comparison (App 1998; Pryor 2010). Although 75 participants were included overall in the Pryor study, only 15 were randomised to each of the five treatment groups; therefore the study only contributes 30 participants to this pair‐wise comparison (Pryor 2010). The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 6).
Primary outcomes
1. FEV1
Both studies reported data for FEV1 (L) which we could enter into our analysis (App 1998; Pryor 2010). App recorded lung function before and after four weeks of treatment using each study intervention (App 1998). Only the first‐arm data from this cross‐over study were used as the authors felt there would be a carryover effect into the second arm of the study. There was no statistical difference found between AD and Flutter® at one month, MD 0.10 L (95% CI ‐0.95 to 1.15) (App 1998) or at 12 months MD 0.21 L (95% CI ‐0.64 to 1.06) (low‐certainty evidence) (Pryor 2010) (Analysis 6.1). Pryor also reported FEV1 % predicted and, overall, observed a significant deterioration in FEV1 % predicted over the 12‐month period for the entire cohort (‐1.8% predicted; P = 0.02), stating this decline was within the international average at the time of the study (Pryor 2010). However, recruitment was challenging for this long‐term study, meaning it was underpowered to detect such a change. Consequently, the results obtained may have over or underestimated any decline in lung function identified by the original authors.
6.1. Analysis.

Comparison 6: AD versus Flutter®, Outcome 1: FEV1 (L)
2. QoL
One study measured QoL using the Short Form‐36 questionnaire and the CRQ (Pryor 2010). Investigators found no significant difference in the domains between the two groups (low‐certainty evidence). However, the latter questionnaire reported an overall significant improvement in dyspnoea (P = 0.01) over time in the group as a whole.
Secondary outcomes
1. Participant preference
During the course of one study, 13 out of 75 participants withdrew as they did not like the regimen to which they had been randomised and either reverted to their original preferred option or chose a different regimen (low quality evidence) (Pryor 2010). The intervention each was using at the time was not identified.
2. Exercise tolerance
The modified shuttle test was reported in one study (30 participants) and no significant difference was found between AD and Flutter®. No detailed data were available for our analysis (Pryor 2010).
3. Adverse effects
Neither study reported on this outcome (App 1998; Pryor 2010).
4. Number of admissions
Neither study reported on this outcome (App 1998; Pryor 2010).
5. Need for extra treatment
One study reported some participants in each of the regimens required intravenous antibiotics during the course of the study (low‐certainty evidence) (Pryor 2010). The median number of courses per group ranged from 1.0 to 1.5, though statistical analysis was not carried out due to the small numbers and scattered nature of the data. The number of participants and allocated treatment arm was not specified. The second study reported that two participants required Intravenous antibiotic treatment (one from from each group) for an acute exacerbation and were withdrawn from the study. We are unable to present these data in the graphs as the paper did not clarify which treatment group the participants were in when they required the antibiotics (App 1998).
6. Pulmonary function measurements
a. LCI
This outcome was not measured in either study.
b. FVC
Both studies measured FVC (App 1998; Pryor 2010), but only one study (17 participants) provided data for our analysis (App 1998). There was no statistical difference found between AD and Flutter® at one month, MD ‐0.30 L (95% CI ‐1.50 to 0.90) (Analysis 6.2). Pryor reported no significant difference in FVC between AD and Flutter® (Pryor 2010).
6.2. Analysis.

Comparison 6: AD versus Flutter®, Outcome 2: FVC (L)
c. FEF25 -75%
Neither study reported on this outcome (App 1998; Pryor 2010).
7. Oxygen saturation
In both studies oxygen saturation levels were only reported at baseline as part of the participant demographics (App 1998; Pryor 2010).
8. Sputum weight
Only one study (17 participants) reported on this outcome with data we could use in our analysis (App 1998). There was no statistical difference found in sputum weight between AD and Flutter® at one month, MD ‐0.90 g (95% CI ‐3.52 to 1.72) (Analysis 6.3).
6.3. Analysis.

Comparison 6: AD versus Flutter®, Outcome 3: Sputum volume wet (g)
9. Survival
One participant died during the course of one study and the allocated treatment arm was not specified; however, the investigators stated that the death was unlikely to have been caused by any intervention under evaluation (Pryor 2010).
Autogenic drainage versus high frequency chest wall oscillation
One study reported on this comparison (Osman 2010). However, as a consequence of the investigators grouping several interventions (AD, Flutter®, PEP and PD&P) as "usual airway clearance techniques" when comparing them to HFCWO we have limited data. After contacting Leyla Osman, additional raw data was obtained which identified eight participants using AD alone as their 'normal' airway clearance technique as a comparison to HFCWO. This study was performed over four consecutive days alternating two treatment techniques. Due to the study design it was felt inappropriate to present this data in the analysis given the carry‐over effect. The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 7).
Primary outcomes
1. FEV1
There was no significant change found in FEV1 % predicted after either HFCWO or usual airway clearance techniques compared to baseline (Osman 2010).
2. QoL
Perceived efficacy and comfort of each airway clearance techniques and the incidence of urinary leakage during treatment were measured using 10 cm visual analogue scales (VAS). There was no significant difference in self‐reported comfort and urinary leakage after either HFCWO or usual airway clearance techniques. Participants scored perceived efficacy of their usual airway clearance techniques significantly higher than for HFCWO (Osman 2010).
Secondary outcomes
1. Participant preference
Of the 29 participants who completed the study, 17 (55%) expressed a preference for their usual airway clearance technique over HFCWO (Osman 2010).
2. Exercise tolerance
This outcome was not reported in this study (Osman 2010).
3. Adverse effects
One participant was withdrawn due to a hypoglycaemic episode. It is not clear in which treatment arm of the study this event occurred (Osman 2010).
4. Number of admissions
Inclusion criteria for participants in the Osman study included hospitalisation with an infective pulmonary exacerbation (Osman 2010).
5. Need for extra treatment
All 29 participants were already receiving intravenous antibiotics as part of their medical management as inpatients during the course of this study (Osman 2010).
6. Pulmonary function measurements
Osman did not report on LCI, FVC or FEF25 -75% (Osman 2010).
7. Oxygen saturation
There was no significant change found in oxygen saturation levels after either HFCWO or usual airway clearance techniques compared to baseline, but no information was provided for the comparison between groups (Osman 2010).
8. Sputum weight
Significantly more sputum was expectorated with usual airway clearance techniques than with HFCWO during both a single treatment session and over a 24‐hour period, MD 4.4 g and 6.9 g respectively (P < 0.001) (Osman 2010).
9. Survival
This outcome was not reported in this study (Osman 2010).
Autogenic drainage versus intrapulmonary percussive ventilation (IPV)
One pilot study (four participants) reported on this comparison (Dingemans 2018). The aims of this study were to evaluate the effect of IPV on bacterial load in CF sputum, lung function and P aeruginosa gene expression. The GRADE judgements and the reasons for these are presented in the summary of findings tables (Table 8; Table 9).
Primary outcomes
1. FEV1
One study reported on the change from baseline in FEV1 % predicted (Dingemans 2018). The authors reported that change in FEV1 % predicted was lower in the group treated with AD alone compared to high frequency IPV at 400 bpm, MD ‐2.90% (95% CI ‐7.67 to 1.87; P < 0.05) (Analysis 7.1), and AD, in turn, was observed to significantly improve lung function in FEV1 % predicted when compared to IPV at a lower frequency of 200 bpm, MD 3.80% (95% CI ‐0.57 to 8.17; P < 0.05) (Analysis 7.1). This suggests that the IPV frequency used might influence the efficacy of this treatment modality (Dingemans 2018). However, both of these results were graded as having very low‐certainty evidence.
7.1. Analysis.

Comparison 7: AD versus IPV , Outcome 1: AD versus IPV 200 FEV1 % predicted (change from baseline)
2. QoL
This outcome was not reported in this study (Dingemans 2018).
Secondary outcomes
1. Participant preference
This outcome was not reported in this study (Dingemans 2018).
2. Exercise tolerance
This outcome was not reported in this study (Dingemans 2018).
3. Adverse effects
This outcome was not reported in this study (Dingemans 2018).
4. Number of admissions
Inclusion criteria for participants in the Dingemans study included hospitalisation for a respiratory infection (Dingemans 2018).
5. Need for extra treatment
All four participants were already receiving intravenous antibiotics as part of their medical management as inpatients during the course of this study (Dingemans 2018).
6. Pulmonary function measurements
The authors of this study reported on the change from baseline in FVC % predicted (Dingemans 2018), and found that participants treated with AD alone showed a lower change in FVC compared to high frequency IPV at 400 bpm, MD ‐4.40% (95% CI ‐8.15 to ‐0.65; P < 0.05) (Analysis 7.1), whereas AD was observed to improve FVC % predicted when compared to IPV at a lower frequency of 200 bpm, MD 5.60% (95% CI 1.19 to 10.01; P < 0.05) (Analysis 7.1). Similar to the primary outome of FEV1, this result suggests that IPV frequency might influence the efficacy of this treatment modality (Dingemans 2018).
7. Oxygen saturation
This outcome was not reported in this study (Dingemans 2018).
8. Sputum weight
The Dingemans study also evaluated the effect of IPV and AD treatments on bacterial load and P aeruginosa gene expression in CF sputum, but these outcomes were outside the scope of this review (Dingemans 2018).
9. Survival
This outcome was not reported in this study (Dingemans 2018).
Discussion
Summary of main results
The aim of this review was to determine the effectiveness of AD, particularly the impact on lung function and QoL in people with CF compared to other airway clearance techniques or no physiotherapy. Single‐treatment studies were excluded because the short‐term outcomes measured were not of relevance to people with CF.
We identified eight studies eligible for inclusion in this review; seven were published as full papers and one as an abstract only. The authors of the abstract have kindly provided the full report of that study (McIlwaine 1991). The included studies compared AD to one or more recognised airway clearance techniques including PEP, ACBT, conventional physiotherapy (PD&P) and oscillatory devices (Flutter®, Cornet®, IPV and HFCWO). These techniques have been evaluated by other reviews (Main 2005; McIlwaine 2019; Mckoy 2016; Morrison 2020).
A total of 212 participants were randomised in the eight studies (numbers ranging from 17 to 75). The length of individual studies varied from four days to two years. Six studies enrolled clinically stable people with CF and two enrolled participants experiencing an exacerbation of their chest condition. Due to the heterogeneity of the studies, data analysis was not possible for most outcomes.
In terms of primary outcome measures, FEV1 was reported in all eight included studies. Changes in FEV1 were not significantly different for AD compared to other airway clearance techniques. The rate of decline in FEV1 in participants using AD over the course of a year‐long parallel study was comparable to that of a group using a variety of airway clearance techniques (ACBT, PEP, Cornet® and Flutter®) (Pryor 2010). However, recruitment was challenging for this long‐term study which meant it was underpowered to detect such a change and consequently any results may have under or overestimated any decline in lung function identified by the original authors.
Three of the eight studies measured the impact of airway clearance on health‐related QoL, but only one study used validated scales (Pryor 2010). Measures of QoL such as dyspnoea in the AD group were comparable with those observed in the other treatment groups (Pryor 2010). Similarly, when using a non‐validated Likert scale, there was evidence to suggest that AD, together with PEP treatment modalities, may be seen as preferable to PD&P in terms of QoL measures (McIlwaine 1991). One study compared AD and a variety of other airway clearance techniques to HFCWO and reported no significant difference in comfort and urinary leakage (Osman 2010). Participants in this study scored perceived efficacy of their usual airway clearance techniques, including AD, significantly higher than for HFCWO (Osman 2010).
Personal preference was assessed in two studies where participants were older children or adults (McIlwaine 2010; Miller 1995). Participants in one study preferred AD over PD&P (McIlwaine 2010), but the second study showed no difference between AD and ACBT (Miller 1995). Personal preference is associated with greater adherence to therapy, but is also subject to variability over the course of a lifetime (Flume 2009). A transient fall in oxygen saturation levels was reported for ACBT in one study but not for AD (Miller 1995).
With respect to other secondary outcomes, one study assessed exercise tolerance (Pryor 2010). Investigators found no significant differences between the treatment groups (Pryor 2010). Analysis of the data from a single long‐term study of people with CF with stable disease which compared AD to PD&P demonstrated a reduced number of mean (SD) hospital admissions over 12 months in the 12 to 18 years age group undertaking AD (1.00 (0.32) versus 0.76 (0.18)) resulting in MD ‐0.24 (95% CI ‐0.42 to ‐0.06). In contrast, this was reported as non‐significant by the study investigators (McIlwaine 2010). Seven of the eight included studies reported FVC and three of the studies reported FEF25-75%; results of these outcome measures showed AD was not significantly different to any of the other treatments under investigation in either short‐ or long‐term studies. One study suggested better sputum production with AD (McIlwaine 1991), but not consistently compared to other techniques (Pfleger 1992). It is difficult to assess the impact of sputum production on people with CF, particularly those with mild disease. These studies describe wet weight of sputum which can be unreliable taking into account underestimating due to swallowing sputum or overestimating due to inclusion of saliva.
There is no evidence that AD is superior to other airway techniques when considering the primary and secondary outcomes assessed in the review.
Overall completeness and applicability of evidence
The literature includes representation from both adults and children (range seven to 63 years); five out of eight studies included participants under the age of 16. Studies recruited participants with mild to severe disease. Three cross‐over studies were considered short term (less than seven days duration). A further four cross‐over studies and one of parallel design were considered long‐term and ranged from eight weeks to two years.
The literature is relevant and representative of the majority of airway clearance techniques currently available to people with CF; three studies compared AD to PD&P, two studies used AD versus ACBT as a comparison, three studies compared AD to PEP and a total of five studies compared AD to an oscillating device ‐ one study compared AD to the Cornet®, two studies compared it to Flutter®, one study compared AD to HFCWO and one other study compared AD to intrapulmonary percussive ventilation (IPV) combined with AD. There were no studies comparing AD to acapella, MetaNeb®, Aerobika®, VibraLung® or Quake® devices; or to exercise.
The applicability of the available evidence needs to be considered in light of the fact that some of the studies were undertaken over 20 years ago. General improvements in clinical condition of people with CF need to be taken into account as there have been well‐documented improvements in respiratory condition.
It should also be noted that the most recent national annual CF registry reports cite exercise as one of the most frequently used primary or secondary forms of airway clearance amongst both adult and paediatric populations (CFF 2020; Cystic Fibrosis NZ 2018; UK CF Trust 2020). Whilst this is not necessarily representative of current international practice outside the aforementioned countries, it is, nonetheless, a form of treatment which is likely to be available to the majority of people with CF. In this review, none of the included studies used exercise as a comparator intervention and only one study measured exercise capacity as an outcome measure (Pryor 2010).
Quality of the evidence
We have included eight RCTs, enrolling 212 participants. Seven studies were published as full papers and one in abstract form only. A copy of the unpublished paper was obtained following correspondence with the authors (McIlwaine 1991). Studies compared AD to a variety of airway clearance techniques and seven studies used a cross‐over design. A recent study examining cross‐over studies in Cochrane Reviews found that the studies' analysis and presentation of results were often not appropriate or clear, with less than a third of studies presenting results that could be included in a meta‐analysis (Nolan 2016) . Validated QoL measures were not available for the earlier studies (Gee 2000; Quittner 2009).
Overall, the certainty of the evidence from the studies was judged to be mainly low or very low (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9). We judged only one lung function outcome for one comparison (AD versus Cornet®) to have moderate‐certainty evidence (Table 5). The main reasons for downgrading the levels of evidence were the small numbers of participants, the lack of clarity of the reporting in the studies and the inability to blind participants.
With regard to study design, while the blinding of participants or research staff is challenging for this intervention, blinded outcome assessors were used in all but two of the studies (App 1998; Miller 1995), improving the certainty of the evidence gathered and reducing the risk of detection bias. One study reported the use of a blinded statistician (Pryor 2010). Three studies describe appropriate methods of random sequence generation and carry a low risk of bias in this respect (McIlwaine 2010; Osman 2010; Pryor 2010); but none of the included studies reported on the allocation concealment process. Half of the cross‐over studies described using a washout period, raising the potential for carryover effects and may influence outcomes recorded in the second arm of a study.
Of note regarding reporting issues, one two‐year cross‐over study was judged to have a high risk of bias due to incomplete outcome data (Figure 3). The authors acknowledged that data from the second arm of the study was affected by high dropout rates (59%) and non‐adherence (41%) in the PD&P arm (McIlwaine 2010). In addition, this study was considered to have selective reporting bias, as FVC, chest X‐ray scores and hospital admissions were measured, but not reported (McIlwaine 2010).
Furthermore, the tools used to record personal preference in the included studies were generally not well‐described or validated; and no study incorporated measures of adherence.
Potential biases in the review process
Adequate searches identified relevant studies with relatively limited participant numbers. Four studies were conducted more than 20 years ago and additional data requested from the authors were not available. In one study, AD was included with a number of other airway clearance techniques and compared to HFCWO, which limited the data available for this review (Osman 2010).
All three authors (PB, GS and RS) use AD in their clinical practice, but are not sponsored by any institution and have not been paid to provide training on this technique.
Agreements and disagreements with other studies or reviews
Previous Cochrane Reviews of conventional physiotherapy (Main 2005), ACBT (Mckoy 2016), PEP therapy (McIlwaine 2019) and oscillating devices (Morrison 2020) have not identified one technique to be significantly superior and this is consistent with the current review. There is no clear evidence to support the use of one airway clearance technique over another, but there is a reasonable evidence base to support some form of airway clearance, particularly in productive people with CF (Warnock 2015).
A Canadian team undertoook a systematic review of AD in 2015 and arrived at similar conclusions to this review, albeit by a slightly different route (Morgan 2015). Their published paper outlines the appropriate methodology they have employed and the majority of studies they selected are the same as in this review. They did not include one study which is included in this review as they felt the approach to AD was distinct (App 1998). Whilst the intervention may not have been strictly to the guidance of Jean Chevaillier's description, we felt it important to include this evidence (App 1998). They also included one study which assessed outcomes after a single treatment (Giles 1995). We decided not to select single treatment studies, for two reasons. Firstly, we did not feel these studies examined outcomes that were of relevance to people with CF and secondly, a single treatment does not enable the individual to establish confidence and expertise with the technique. A separate South African team undertook a systematic review evaluating the effect of AD and assisted AD compared to no physiotherapy, sham physiotherapy, or other methods of physiotherapy in children with CF (Corten 2017). Assisted AD is a passive technique used with babies and young children involving manual compression over the chest wall during expiration. We did not include this technique, as it is quite distinct from AD. Seven studies were identified in the Corten review, which concluded there was insufficient evidence to determine the efficacy and safety of AD and assisted AD in children (Corten 2017).
Authors' conclusions
Implications for practice.
Cystic fibrosis (CF) care and management has changed significantly within the last 20 years. The introduction of newborn screening and consequently a patient cohort with greatly improved clinical status combined with the significant development of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies have changed the face of CF care. Identifying and thus correcting the basic genetic defect has meant a reduction in excess mucus production, improved airway hydration and will consequently have an impact on the need for daily airway clearance routines in the future. At a time where the trajectory and demographics of the disease is changing (Nissenbaum 2020), autogenic drainage (AD) has been shown to be an effective treatment option for those individuals who are seeking techniques to support and promote independence.
However, although approximately 19% of those with CF use AD as a method of airway clearance (UK CF Trust 2020), it is a technique which requires time and appropriate professional input and training in order to use it proficiently. It requires a certain level of body awareness so as to effectively modulate the breath and ensure the highest possible appropriate expiratory airflow while avoiding bronchospasm and dynamic airway collapse (Agostini 2007). Evidence shows that AD is comparable to other airway clearance techniques (Wallaert 2018), but it demands time and concentration. Within the paediatric population there has been very little evidence to either support or refute the use of AD for CF (McIlwaine 1991; Corten 2017). It can be difficult for children to concentrate for the required period of time in order to learn and perform the technique correctly but if achieved, can provide an effective, independent method of airway clearance. Often personal preference and individual beliefs are central to the selection of airway clearance techniques and, therefore, it should not be discounted as an option (Flume 2009).
For those to whom AD is a new technique, the understanding and practice of it can be more complicated in airways that are generally clearer and where reducing lung volumes to terminal airways takes time and patience. This is especially true as the introduction of CFTR modulator therapies have resulted in airways that are better hydrated and secretions that are less viscous. However, there is still a large cohort of individuals for whom hypersecretion due to existing airway damage is still an issue, along with roughly 10% of the CF population for whom there is currently no appropriate modulator available due to their underlying genotype. For these individuals, continuing to evaluate the effectiveness of airway clearance techniques such as AD is essential.
Although the significant advances in CF care over recent years have correlated with an improvement in life expectancy and quality of life, these advances may require increased time and effort from the individuals with CF and, consequently, demand strategies to self‐manage. This can be equally challenging while simultaneously balancing work, family life and education (Sawicki 2009). As such, it is important that this intervention is continually reviewed in large scale, robust trials to ensure that the time spent carrying AD out results in a noticeable benefit to the individual.
Implications for research.
As CFTR modulator therapy continues to revolutionise the treatment of CF and transform the lives of many people with CF (Scotet 2020), understanding the effects on these small airways through more sensitive outcome measures and larger scale trials would seem appropriate. The majority of the studies in this review used forced expiratory volume in one second (FEV1) as their main outcome measure. This is primarily due to the fact that it is a good indicator of lung health and correlates well with survival and quality of life, factors which are important to those with CF. However, as children and adults with CF continue to benefit from the advances in medical management, presenting with more stable disease and reduced lung damage, it could be suggested that FEV1 is no longer sensitive enough to detect changes in small airways and thus unable to assess the effectiveness of airway clearance techniques in general. In light of the many variables which influence the measurement of FEV1 in the short term, some studies are focusing on frequency of exacerbation and time to next exacerbation as primary outcome measures (Konstan 2007; VanDevanter 2015).
Given that the proposed physiological impact of AD is to augment airway clearance, particularly in the small airways, measures of ventilatory capacity such as lung clearance index (LCI) are attractive and potentially may provide more sensitive identification of early lung disease and response to interventions such as AD. Currently, there is insufficient external validity of this measure to include it as a primary outcome, but it will represent an important secondary outcome in future reviews. Furthermore, the studies included in this Cochrane Review did not compare AD to exercise alone, although exercise is regularly used as an alternative to more formal airway clearance techniques (Ward 2019; UK CF Trust 2020). Further studies are required to evaluate the effectiveness of exercise alone for airway clearance, compared to all other techniques. A Cochrane Review of the current literature is underway (Patterson 2019).
The majority of studies in this review were of cross‐over design and several of these described changing from one technique to another with no washout period. The magnitude and duration of carry‐over effects are unknown in the CF population, but can influence the second arm of a study (Nolan 2016; Southern 2003). It must be noted that, especially in cross‐over studies, participant preference can also impact upon withdrawals and may limit the overall quality of a body of evidence (Pryor 2010). For this reason, future studies examining AD should avoid a cross‐over design where possible, or should be designed to include an adequate washout period.
Incorporating a validated personal preference tool, measures of adherence and health‐related quality of life in future research would promote a patient‐centred approach to clinical practice and would provide the clinical insight to respond to the needs of the individual. The acquisition of meaningful data from further long‐term, randomised controlled studies utilising large cohorts to control for participant variability when comparing airway clearance modalities is required to rigorously evaluate AD and other airway clearance techniques.
What's new
| Date | Event | Description |
|---|---|---|
| 28 October 2021 | New citation required but conclusions have not changed | The previous lead author of the original review Pam McCormack and fellow co‐author Kevin W Southern have stepped down and two new authors, Gemma Stanford and Ruth Stewart have joined the team. Our conclusions have remained the same. |
| 28 October 2021 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Register and two online trials registries (Appendix 1) identified 29 individual references to 24 studies potentially eligible for inclusion in this review. One new study (two references) has been included in the updated review (Dingemans 2018). Three references were additional references to already included studies (McIlwaine 1991; Osman 2010; Pryor 2010). Three were additional references to already excluded studies (Reix 2012; San Miguel‐Pagola 2020; Stanford 2019). We identified the full papers to two previously excluded abstracts; these have been added to the previous references and remain excluded in this updated review (San Miguel‐Pagola 2020; Stanford 2019). There were 17 references to 14 newly excluded studies (ACTRN12611000160932; Corten 2020; CTRI/2020/10/028509; Helper 2020; NCT01854788; NCT02840136; NCT03522480; NCT03655249; NCT04187924; NCT04527796; Poncin 2017; Prusak 2020; Sokol 2012; Sokol 2012a). Three references (one full paper and two abstracts) were additional references to two studies previously listed as 'Awaiting classification' (Davies 2012; Vendrusculo 2019). Following assesment for elegibility, both studies have now been excluded in this updated review. One newly identified study has been listed as "Ongoing" and will be assessed for elegibility once results are published (NCT04010253). We have updated the "Types of interventions" section and have added three relatively new oscillating devices not previously included. The Aerobika® is a hand‐held oscillating positive expiratory pressure device. The VibraLung® is an acoustic percussor incorporating positive expiratory pressure. Finally, the MetaNeb® is a pneumatic compressor system delivering continuous high frequency oscillation and positive expiratory pressure. |
History
Protocol first published: Issue 1, 2012 Review first published: Issue 10, 2017
Notes
A new author team took on this review after the protocol had been published.
Acknowledgements
The authors of the review update in 2021 would like to thank the original contact author Pam McCormack, as well as fellow co‐author of the original review, Kevin W Southern, for their inestimable contributions to this Cochrane Review.
The review authors would also like to acknowledge the kind and patient assistance of our Managing Editors, Nikki Jahnke (whose invaluable support and encouragement saw the original review and the current update through to completion) and Tracey Remmington, and Sarah Nevitt for her help with the summary of findings table in the original review. Similarly, the authors would also like to thank Maggie McIlwaine and Leyla Osman for kindly providing an additional unpublished paper and additional raw data respectively at the time of the original review, as well as Nir Helper and Gil Sokol for answering our queries regarding their publications and, finally, Aurélie Crabbé for kindly providing us with additional unpublished data in time for the publication of this update.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Electronic search strategies
| Search terms | Date last searched | |
| ClincalTrials.gov (www.clinicaltrials.gov/) |
'autogenic drainage' and 'forced expiratory techniques', as well as 'autogenic drainage' and 'cystic fibrosis' | 2 February 2021 |
| WHO ICTRP (apps.who.int/trialsearch/) |
"autogenic drainage AND forced expiratory techniques" as well as "autogenic drainage AND cystic fibrosis" | 2 February 2021 |
Data and analyses
Comparison 1. AD versus PD&P.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 FEV₁ (change in % predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Hospital admissions | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 FVC (change in % predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 FEF25-75% (change in % predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. AD versus spontaneous cough.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 FEV₁ (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 30 minutes following physiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 30 minutes following physiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 Sputum weight (g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 30 minutes following physiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. AD versus ACBT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 FEV₁ (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. AD versus PEP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 FEV₁ (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 FEV₁ (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.1 30 minutes following physiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3 FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3.1 30 minutes following physiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4 Sputum weight (g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4.1 Following physiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. AD versus Cornet®.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 FEV₁ (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. AD versus Flutter®.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 FEV1 (L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.2 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.2.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3 Sputum volume wet (g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. AD versus IPV .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 AD versus IPV 200 FEV1 % predicted (change from baseline) | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐0.57, 8.17] |
| 7.2 AD versus IPV 400 FEV1 % predicted (change from baseline) | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐7.67, 1.87] |
| 7.3 AD versus IPV 200 FVC % predicted (change from baseline) | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [1.19, 10.01] |
| 7.4 AD versus IPV 400 with AD FVC % predicted (change from baseline) | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐8.15, ‐0.65] |
7.2. Analysis.

Comparison 7: AD versus IPV , Outcome 2: AD versus IPV 400 FEV1 % predicted (change from baseline)
7.3. Analysis.

Comparison 7: AD versus IPV , Outcome 3: AD versus IPV 200 FVC % predicted (change from baseline)
7.4. Analysis.

Comparison 7: AD versus IPV , Outcome 4: AD versus IPV 400 with AD FVC % predicted (change from baseline)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
App 1998.
| Study characteristics | ||
| Methods | RCT. Cross‐over design: AD or Flutter® therapy used for 4 weeks each with an additional one‐week “washout period” prior to starting each arm, without any kind of physiotherapy administered. Multicentre. Location: Germany. |
|
| Participants | 17 participants with CF diagnosed by clinical history and a positive sweat test. 17 initially randomised, 3 dropouts reported (1 for time‐related reasons and the other 2 for acute bronchopulmonary exacerbation), therefore 14 analysed (7 in each treatment group). Age: range 7 to 41 years; mean (SD) 19.6 (10.3) years. Gender split: 6 male, 8 female. |
|
| Interventions |
Treatment 1: 2x daily AD for 30 minutes. Treatment 2: 2x daily Flutter® therapy for 30 minutes. |
|
| Outcomes | Respiratory function (FEV1, FVC) measured at the beginning and end of each 4‐week therapy cycle. Measurements were taken before and after 30 minutes physiotherapy. Sputum volume (wet) was collected, weighed and stored at the end of each physiotherapy session. Blood oxygen saturation levels measured by pulse oximetry technique. This paper also considered the implications of the Flutter® on sputum viscoelasticity but this was not an outcome measured in this review. |
|
| Notes | Only first‐arm data used for analysis as it was felt a 1‐week washout was insufficient to exclude a carry‐over effect into the second arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper only states that patients were “randomly assigned to one of the two treatment arms”. Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Insufficient information provided about the concealment of allocation process. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the self‐administered physiotherapy techniques under study. As it is not possible to blind by design, the risk of bias is deemed to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants 'dropped out' with reasons stated: 1 for business‐related time constraints after the first examination; and the other 2 for acute bronchopulmonary exacerbations during the course of the study (1 from each arm). |
| Selective reporting (reporting bias) | Unclear risk | Oxygen saturation levels were taken but only reported at baseline. It is unknown whether this parameter changed over the course of the study or as a result of any intervention received. FEV1 and FVC baseline characteristics given as % predicted values. However, the values recorded during the study are not presented as % predicted but as absolute figures (L). |
| Other bias | Low risk | None identified. |
Dingemans 2018.
| Study characteristics | ||
| Methods | RCT. Pilot study. Cross‐over design. Three treatments arms: AD alone, AD in combination with IPV at medium (200bpm) frequency or AD in combination with IPV at high (400bpm) frequency. Patients were randomised to receive a different treatment during each of three consecutive hospital admissions for IV antibiotics. Length of hospital stay reflected time in each treatment arm, which ranged from 5‐10 days. Each treatment technique was separated by a minimum washout period of 3 months, during which each participant reverted to using AD as their standard ACT. The first treatment was preceded by at least 3 months of daily AD routines. The participants acted as their own control group. Single centre. Location: Belgium. |
|
| Participants | 4 CF adults, chronically colonised with P aeruginosa and receiving inpatient IV antibiotics for respiratory infection. Age range: 20‐34yrs. Gender split: 1 male, 3 female. FEV1 range: 24‐60%. FVC range: 49‐75%. |
|
| Interventions |
Treatment 1: 2x daily AD treatment sessions (30 minutes each). Treatment 2: 2x daily AD combined with IPV at medium (200 bpm) frequency (30 minutes each). Treatment 3: 2x daily AD combined with IPV at high (400 bpm) frequency (30 minutes each). |
|
| Outcomes | FEV1 percentage change from baseline FVC percentage change from baseline This paper also considered the effect of IPV treatments on bacterial load in CF sputum and P aeruginosa gene expression analysis, but these outcome measures were beyond the scope of this review. |
|
| Notes | FEV1 and FVC outcomes were measured at three different time points during inpatient treatments (day 1, day 3 ‐ 5 and day 5 ‐ 10). Time points varied both between participants and hospital admissions. The study authors were contacted and kindly provided us with additional unpublished data for each of the four participants: all FEV1 and FVC values (% predicted) and the specific time points at which they were measured, together with the number of days spent in each of the 3 treatment arms. No raw data on bacterial load and gene expression given ‐ only what was displayed in graphs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The paper states that "A randomized cross‐over study was conducted." The authors were contacted and kindly provided additional unpublished information, stating that "AD, IPV 200, and IPV 400 were assigned a treatment number (1, 2 or 3) and each patient was given a random order of treatments. Ten index cards with a random order of treatments were enclosed in sealed envelopes. For patient 1 we took the first envelope, for patient 2 we took the second, etc." |
| Allocation concealment (selection bias) | Low risk | The authors were contacted and kindly provided additional unpublished information, stating that "AD, IPV 200, and IPV 400 were assigned a treatment number (1, 2 or 3) and each patient was given a random order of treatments. Ten index cards with a random order of treatments were enclosed in sealed envelopes. For patient 1 we took the first envelope, for patient 2 we took the second, etc." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the physiotherapy techniques under study. As it is not possible to blind by design, the risk of bias is deemed to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The paper states that lab staff analysing the sputum samples were blinded. The study authors were contacted and provided this review with additional unpublished information, stating that: "Spirometry technicians were blinded. Statistics were not blindly performed, but performed when all data were obtained." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data missing for some participants; some of the outcomes stated in the protocol were not reported in the published pilot study. Withdrawals from the study were not reported as withdrawals. The study authors were contacted for clarification on this point and stated that "we actually performed the clinical trial including 8 patients originally (4 chronically colonized with P. aeruginosa vs 4 not colonized with P. aeruginosa). However, some of the patients that were not colonized with P. aeruginosa dropped out of the trial after only receiving 1 treatment, making it difficult to statistically compare the 3 treatments. Because of this, we focused on the 4 patients chronically colonized with P. aeruginosa, for which sufficient data could be collected to draw conclusions supported by statistical analysis." |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes in the protocol were reported in the pilot study and some of the participants' outcomes were missing. Out of a small sample size of four patients, lung function data relating to one of the treatment arms were not available for two of these and, together with the bacterial count for one patient, were not reported in the published paper. Following correspondence with the study authors for additional information, they clarified that "some participants did not complete all 3 treatments, due to their participation in other clinical trials" and that for the latter patient "bacterial loads... were not determined because of technical issues." |
| Other bias | High risk | Two out of the four participants only took part in 2 of the 3 treatment arms. Another participant received IPV high (400 bpm) frequency treatment twice and only one data set was presented. Treaments under review were administered during inpatient hospital admissions, with different IV antibiotic combinations and varying lengths of stay. As such, data collection points varied between patients and hospital admissions. |
McIlwaine 1991.
| Study characteristics | ||
| Methods | RCT. Cross‐over design: participants randomised into 3 groups (PD&P, AD and PEP) and used this technique for the first "treatment period" of 2 months, they sequentially performed the other techniques. Each treatment technique was separated by an interval of 1 month "off period" when the pre‐study regimen of PD was reinstated. Single centre. Location: Canada. |
|
| Participants | 18 participants with CF diagnosed by sweat test > 60 mEq/L. Age: mean (range) 17.3 (11 to 27) years. FVC: range 38% predicted to 117% predicted. Shwachman score: range 50 ‐ 94. |
|
| Interventions | Technique 1: 2x daily PEP mask treatment in sitting using cycles of 15 tidal volume breaths against a resistor creating a PEP of between 10 ‐ 20 cms H₂0 followed by FET and cough. Sequence repeated 6 times or for a minimum of 20 minutes (whichever was longer). Technique 2: 2x PD&P (PD&P, vibrations, deep breathing and FET) performed in 11 different PD positions, draining 6 positions in the morning and the other 5 in the afternoon. Treatment time of 30 minutes each session. Technique 3: 2x AD performed in sitting until all mucus was evacuated (maximum treatment session length no more than 45 minutes). | |
| Outcomes | FEV1, FVC, and FEF25−75% clinical assessment and Shwachman score were measured at the start and end of each 2‐month treatment period.
Sputum expectorated during the weekly physiotherapist‐supervised physiotherapy session was collected and weighed. Other measures included reported treatment duration, treatment comfort, requirement for assistance with treatment, flexibility of treatment times, control in performing own treatment, and how interruptive treatment was to daily living. Physical activity and compliance with treatment were monitored using a weekly questionnaire. |
|
| Notes | The authors were contacted and an unpublished paper was kindly provided by the study investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. "In order to avoid seasonal variations which may have affected the outcome of the study, the patients were randomized into three groups. Each group was assigned by a different physiotherapy regiment for the first treatment period, then sequentially performed the other techniques". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the self‐administered physiotherapy techniques under study. As it is not possible to blind by design, the risk of bias is deemed to be low overall. The extent to which the lack of blinding may have had an effect on the reporting of subjective outcomes such as patient preference and QoL measures is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physician was not told what type of physiotherapy was being performed by the participant at the time of assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The results are reported from 14/18 participants who completed the study, 4 withdrawals discussed. 1 participant required hospitalisation during the first period of the study (treatment regimen was PD&P) due to exacerbation of her pulmonary disease and was found to have ABPA. She was then considered too unstable to continue in the study. A second participant was dropped at the end of the first period, after requiring Prednisone to control an allergic reaction to an antibiotic. 2 other participants (treatment regimen AD) refused to complete the cross‐over study, instead they insisted on continuing AD. These participants were excluded from the analysis of sputum production. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None identified. |
McIlwaine 2010.
| Study characteristics | ||
| Methods | RCT. Cross‐over design. Single paediatric centre. Location: Canada. |
|
| Participants | 36 participants with "proven diagnosis" of CF. Age: 12 ‐ 18 years. Shwachman score 65 ‐ 98. Compliant performing daily chest physiotherapy using PD&P technique for at least 1 year prior to the study. |
|
| Interventions |
Treatment 1: 2 sessions of AD 30 min daily in sitting. The length of time to complete this technique varied with each participant but on average required 30 minutes. Treatment 2: 2 sessions of PD&P approximately 30 min daily, 6 positions drained in morning and 5 in evening using percussion, deep breathing exercises combined with vibrations on expiration. This was followed by huffs. Each treatment regimen was performed for 1 year before crossing over to the other treatment regimen for a further year. |
|
| Outcomes | FEV1, FVC, FEF25-75% sputum weight (partial and subjective), number of hospital admissions, participant preference, and need for extra treatment. A change in Shwachman and Huang scores were also measured. | |
| Notes | The study was powered as a 2‐year cross‐over study. Only data from the first year were reported due to 10/17 participants from Group B (AD‐PD&P) withdrawing from the study before starting PD&P arm; this completely biased the results. “No formal matched cross‐over analysis of the data could be performed.” Also, “...as the study was not powered to detect single group differences, these results may not truly reflect treatment differences.” Sputum weight was not measured by the investigators, but it was the participants who "reported an increased expectoration with AD". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were matched as pairs, using FEV1 (within 15%) as the primary match, Shwachman scores (within 15 points), age (within 3 years) and same sex as secondary matches. Members of each pair were randomly assigned by computer to 1 of the 2 groups (A or B). |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the self‐administered physiotherapy techniques under study. As it is not possible to blind by design, the risk of of bias is deemed to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...full clinical assessment, including Shwachman and Huang scores, performed at the CF clinic by physician blinded as to the method of physiotherapy the patient was performing in the study...” and “The pulmonary function technician was blinded as to the patient’s physiotherapy technique.” Not stated if statistician was blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36 participants entered the study. Data on 33 available at 12 months. 3 withdrew from the study in the first year: 2 in Group A (pregnancy, ABPA), 1 in Group B (non‐compliant). In the first year of the study, 33 stayed in the group to which they were randomised. In the 2nd year, 10/17 participants from Group B (AD/PD) did not return for PD&P arm of study, due to preference to continue with AD (completely biased 2nd arm of study). Strong cross‐over effect in 7 participants who continued with the study as they incorporated AD breathing technique into PD&P; therefore only year 1 data reported. The results from the 2nd year could not be analysed as single‐group differences could not be studied. Secondary analysis of PFTs in Group A (PD&P, then AD) comparing years 1 and 2 was performed but no significant differences were found. |
| Selective reporting (reporting bias) | High risk | “Full clinical assessment” was undertaken and would include weight and height, but these are not reported. Adherence measured by monthly phone calls, but not reported in paper. Likewise, duration of hospitalisations and sputum bacteriology recorded but not reported. Antibiotic use was partially reported (none received home IV antibiotics). FEV1, FEF25-75% and Shwachman scores are fully reported though P values not given and only described as non‐significant. Huang score was significantly improved (P = 0.04) in the AD group versus PD&P group. Baseline FVC and Huang score recorded but unreported. |
| Other bias | Low risk | None identified. |
Miller 1995.
| Study characteristics | ||
| Methods | RCT. Cross‐over design: each participant used 2 treatment regimens: AD alone or ACBT with PD in randomised order over 2 days 1 week apart. Single centre. Location: UK. |
|
| Participants | 18 participants with CF, all clinically stable at the time of the study and were not receiving IV antibiotics. Age: 11 to 32 years. Gender split: 10 male, 8 female. Shwachman‐Kulczycki scores modified with the Chrispin‐Norman scores: range 34 ‐ 87. |
|
| Interventions |
Treatment 1: AD alone for 2 days, each day consisting of 2 identical treatment sessions (morning and afternoon) with each session lasting 30 minutes. Treatment 2: ACBT with PD for 2 days, each day consisting of 2 identical treatment sessions (morning and afternoon) with each session lasting 30 minutes. Treatment preceded either by nebulised salbutamol (2.5 mL salbutamol and 1.5 mL saline) or saline (4 mL), based on reversibility response to bronchodilator. Approximate nebulisation time of 8 minutes. Participants were asked to be regular with their home physiotherapy in the week leading up to the study and in the intervening period. |
|
| Outcomes | The same measurements were taken on day 1 and day 2. Lung function tests (FEV1, FVC, FEF25-75% and PEF) recorded at the beginning of the day and before and after each physiotherapy treatment. Oxygen saturation levels measured before, during and after each physiotherapy session. Sputum collected and weighed during treatment and for a further hour after it. Participant preference. Additional outcome: Xenon‐133 gas ventilation study at the start and end of each day. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eighteen patients with cystic fibrosis took part in a randomized two‐day crossover trial". Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the self‐administered physiotherapy techniques under study. As it is not possible to blind by design, the risk of bias is deemed to be low overall. The extent to which the lack of blinding may have had an effect on the reporting of subjective outcomes such as patient preference is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions, all participants analysed in the groups to which they were assigned. |
| Selective reporting (reporting bias) | Low risk | Taken overall, lung function tests were reported, but only FVC and FEF25-75% in any detail. Xenon‐133 gas ventilation study was reported, as were oxygen saturation levels, sputum weights and preference of technique. No baseline or raw data provided. Conclusions based on the statistical analysis were summarised. |
| Other bias | High risk | Those on their ACBT day were asked to breathe normally during their pre‐treatment nebuliser. Those on AD, however, performed AD breathing exercises during inhalation, adding 8 minutes of "extra" treatment time. No statistically significant differences were found between the 2 treatment groups for any of the outcomes measured. Despite this, the risk of bias was deemed to be high as the stated treatment time for the 2 groups was unequal, favouring the AD group. |
Osman 2010.
| Study characteristics | ||
| Methods | RCT. Cross‐over design: 4 consecutive study days where participants received either HFCWO on days 1 and 3 and their "usual" ACT on days 2 and 4 or vice versa. Single centre. Location: UK. |
|
| Participants | 30 participants with a diagnosis of CF based on genotype or sweat test who were admitted to hospital with an acute infective pulmonary exacerbation. Age: mean (SD) 29.4 (8.4) years. Gender split: 22 male, 8 female. FEV1 % predicted: mean (SD) 38% (16.7). Inclusion criteria: FEV1 ≥ 20% predicted, age ≥ 16 years and have an acute infective pulmonary exacerbation. |
|
| Interventions | 4 consecutive study days where participants received either HFCWO on days 1 and 3 and their "usual" ACT on days 2 and 4 or vice versa. Treatment 1: 2x daily HFCWO sessions (am and pm) of 30 min each where participants remained in an upright position throughout the session; 8 minutes at each of the frequencies in sequence (10, 13 and 15 Hz), with each frequency followed by a 2‐minute rest period. Pulse pressure set according to the individual’s reported comfort. Participants advised to huff or cough as they felt necessary to expectorate secretions. Treatment 2: 2x daily "usual" ACT sessions (am and pm) of 30 min each. For those practicing an assisted ACT, the physiotherapist provided percussion (i.e. ACBT with PD&P), participants were allowed to perform combined ACTs where this was their usual practice. |
|
| Outcomes | Wet weight of expectorated sputum, FEV1, oxygen saturation levels, perceived efficacy and comfort of each ACT as well as the incidence of urinary leakage during treatment was measured using a Visual Analogue Scale. ACT preference was documented for each participant. | |
| Notes | "Usual" ACT incorporated: ACBT with PD&P (41%, n = 12), ACBT with modified PD alone (7%, n = 2), AD in sitting (28%, n = 8), AD with modified PD (7%, n = 2), PEP (7%, n = 2), Flutter® (10%, n = 3). ACTs in the published paper were analysed together and results were not separated out for the individual techniques. The study authors were contacted and kindly provided us with the raw data for each participant, including what their usual therapies were and all first‐arm data before the first cross‐over on day 1. Only 10 out of the 30 participants in the study performed AD as their usual ACT. It was felt that analysing these AD participants in a subset would not add relevance due to the very small numbers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation to HFCWO or usual ACT on day 1 was determined using a computer‐generated randomisation table." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the self‐administered physiotherapy techniques under study. As it is not possible to blind by design, the risk of bias is deemed to be low overall. The extent to which the lack of blinding may have had an effect on the reporting of subjective outcomes such as QoL measures is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Paper states that "An independent observer, blind to the daily method of airway clearance used, performed the spirometry, weighed the sputum samples and collected the 10 cm VAS throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Single withdrawal discussed; participant excluded due to a hypoglycaemic episode. Results based on the remaining 29 participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported; 2 out of 116 24‐hour sputum samples were discarded as they were incomplete. Powered to detect a 4 g difference in expectorated sputum. |
| Other bias | Unclear risk | Supported by the Robert Luff Foundation and Hill Rom Inc. Although Hill Rom provided some equipment for the study, they did not participate in the design, collection, analysis, interpretation of data or in the writing of the manuscript. |
Pfleger 1992.
| Study characteristics | ||
| Methods | RCT. Cross‐over design: in a random order, participants performed a different regimen of physiotherapy over 5 consecutive outpatient visits. Single centre. Location: Austria. |
|
| Participants | 15 participants with CF, diagnosis confirmed by "repeatedly positive sweat tests". All participants in a "stable clinical situation". All participants trained to cooperate with pulmonary function testing (6 months prior to the study, each participant trained in 2 self‐administered techniques (Hi‐PEP mask (PEP) and AD) and encouraged to use these 2 techniques daily until the onset of the study), able to perform chest physiotherapy 1 to 3 times daily and produce > 20 mL sputum per day. One participant excluded due to an acute respiratory viral infection. The remaining 14 participants were analysed. Age: > 6 years. Mean (range) age 16.0 (9.8 ‐ 22.4) years. Gender split: 5 male, 9 female. Shwachmann score mean (range): 62.2 (26 ‐ 90). Chest X‐ray score mean (range): 13.8 (6 ‐ 20). |
|
| Interventions | Treatment time individualised and performed 1x daily. Each treatment session was equal to the time taken for the individual to clear the lungs using AD, as judged from pre‐study experience. Regimen 1: Hi‐PEP mask alone (PEP). Regimen 2: AD alone (AD). Regimen 3: Hi‐PEP mask for the first half of the session, followed by AD (PEP‐AD). Regimen 4: AD for the first half of the session, followed by Hi‐PEP mask (AD‐PEP). Regimen 5: control (spontaneous coughing only). |
|
| Outcomes | FEV1 and FVC measured at all PFT measurement points. Total sputum weight (not stated whether wet or dry) during the complete treatment session also measured. | |
| Notes | One participant excluded from the study due to an acute respiratory viral infection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly selected from the patients of the local CF clinic". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the self‐administered physiotherapy techniques under study. As it is not possible to blind by design, the risk of bias is deemed to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sputum was collected by the participants and weighed by an investigator blinded to the method of physiotherapy used. Does not state whether the statisticians or those carrying out the PFTs were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Single withdrawal discussed; participant excluded due to an acute respiratory viral infection. Results based on remaining 14 participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | Treatment time individualised and the authors state that each treatment session was equal to the time taken for the individual to clear the lungs using AD, as judged from pre‐study experience. This would imply that duration of each of the 5 treatment sessions performed by an individual should be the same. Additionally, its duration would have been decided in advance and ought to remain unchanged over the course of the study. Nonetheless, the authors report that the "time needed to clear the lungs...for PEP, however, was shorter than for the other forms of physiotherapy and this difference reached statistical significance for AD (P < 0.05), PEP‐AD (P < 0.02), and AD‐PEP (P < 0.05)". In this case, the results reported are not consistent with the methods described. |
Pryor 2010.
| Study characteristics | ||
| Methods | RCT. Parallel design. Single centre. Location: UK. |
|
| Participants | 75 participants with "proven diagnosis" of CF (genotype and positive sweat test); 15 participants randomised to each of 5 intervention groups. Age: 16 years or older; range 17 ‐ 63 years. Gender split: 47 males, 28 females. FEV1: ≥ 25% predicted. Exclusion criteria: evidence of a current respiratory exacerbation, past history of pneumothorax, current severe haemoptysis, awaiting lung and heart or lung transplantation, pregnancy and recent (within 3 months) acquisition of Burkholderia cepacia. |
|
| Interventions | The number of sessions per day and the length of time for treatment was individualised in agreement with each participant, written instructions of the regimens agreed were given to each participant. Regimen 1: AD. Regimen 2: ACBT. Regimen 3: Cornet®. Regimen 4: Flutter®. Regimen 5: PEP. |
|
| Outcomes | Primary outcome: FEV1. Secondary outcomes: FVC, BMI, the modified shuttle test, number of courses of IV antibiotics and the Short Form‐36 and Chronic Respiratory Questionnaires. MEF25 and residual volume as a percent of total lung capacity were reported in the study, but are not included in our analysis as they were not outcomes relevant to our review. Participants requested to attend monthly for 12 months, for a review of their ACT and to record the outcome measurements. The measurements of lung function and BMI were undertaken at 0, 6 and 12 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computerised and used a random number sequence stratified by FEV1 % predicted (FEV1 < 50%; FEV1 ≥ 50%) and sputum expectorated (< 1 cupful per day; ≥ 1 cupful per day). Participants randomized to 1 of the 5 regimens of ACBT, AD, Cornet®, Flutter® or PEP. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither participants nor physiotherapy personnel were blinded to the self‐administered physiotherapy techniques under study. As it is not possible to blind by design, the risk of bias is deemed to be low overall. The extent to which the lack of blinding may have had an effect on the reporting of subjective outcomes such as QoL measures is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The measurements of lung function and BMI and the statistical analysis were undertaken by observers (physiologists and statistician) blind to the regimen to which the participants had been randomised. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75 entered the study, but only data on 65 available at 12 months (13.3 % excluded) ‐ "Intention to treat was used for the primary outcome of FEV1". 53 stayed in the group to which they were randomised. 22 did not complete the study – reasons provided but not according to specific group allocation. |
| Selective reporting (reporting bias) | High risk | FEV1 is the only outcome reported in detail. However, there is no report of the 6‐month data taken for lung function or BMI. FVC, BMI and exercise capacity report no significant difference and P values at 12 months. Some participants in each of the regimens required IV antibiotics, median number of courses per group 1.0 to 1.5, but these data were not analysed in the study due to small numbers and scattered nature of the data. QoL data reported. |
| Other bias | Unclear risk | Treatment used prior to baseline was not reported, which will have had an impact on the capacity of the individual to engage with a new technique. |
ABPA: allergic bronchopulmonary aspergillosis ACT: airway clearance technique ACBT: active cycle of breathing technique AD: autogenic drainage BMI: body mass index bpm: bursts per minute CF: cystic fibrosis FEF25-75%: forced mid‐expiratory flow between 25% and 75% of forced vital capacity FET: forced expiration technique FEV1: forced expiratory volume at one second FVC: forced vital capacity HFCWO: high frequency chest wall oscillation IPV: intrapulmonary percussive ventilation IV: intravenous MEF25%: maximal expiratory flow at 25% of forced vital capacity P aeruginosa: Pseudomonas aeruginosa PD: postural drainage PD&P: postural drainage and percussion PEP: positive expiratory pressure PFT: pulmonary function test QoL: quality of life RCT: randomised controlled trial VAS: visual analogue score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12611000160932 | Outpatient clinic seeking to optimise individual ACTs, increase treatment adherence and physical activity. AD is one of many interventions employed. Study looking at individual lifestyle changes. |
| Corten 2020 | Study describes assisted AD in children, which is distinctly different from AD and not the subject of this review. |
| CTRI/2020/10/028509 | Study population not appropriate; no CF patients included in study. |
| Davies 2012 | HFCWO vs usual ACTs. Investigators contacted and unable to retrieve the specific AD data required for this Review. |
| Giles 1995 | Single treatment session with AD. |
| Helper 2020 | Single intervention study comparing AD to Cough Assist device. This was confirmed by correspondence with the authors. |
| Herrero 2016 | Single treatment session with AD. |
| Lindemann 1992 | Single treatment session with AD. |
| NCT01854788 | Study involves non‐CF related bronchiectasis; population not pertinent to this review. |
| NCT02840136 | Trial suspended by investigators. Research subject unrelated to AD. |
| NCT03522480 | Trial withdrawn by investigators. |
| NCT03655249 | Single treatment session with AD. |
| NCT04187924 | Single treatment session with AD. |
| NCT04527796 | Multidisciplinary rehabilitation programme with physiotherapy, occupational therapy, social work and psychology input. Numerous variables preclude any inferences related to AD. |
| Poncin 2017 | Non‐CF related bronchiectasis; population not appropriate for this review. |
| Prusak 2020 | Methodology unclear; it was thought the study compared four single treatment sessions using AD through a PEP or an OPEP device. These combined techniques are not comparable to AD. Correspondence with authors did not yield further clarification. |
| Reix 2012 | After careful appraisal of the methodology of the paper it was considered that exercise and expiratory manoeuvres were being compared to a modified ACBT, and not an AD technique. |
| Roos 1987 | Study was not completed when abstract was published. Further information was unattainable from the authors after this length of time. |
| San Miguel‐Pagola 2020 | AD used in both treatment groups; study comparing hypertonic saline inhalation with and without PEP. |
| Skopnik 1986 | No evidence of randomisation in this study. Ventilation scintigraphy was the only outcome measure and this is not an outcome under evaluation in this review. |
| Sokol 2012 | Study related to the use of the Cough Assist for airway clearance in CF. No AD involved. |
| Sokol 2012a | Single treatment session with AD. This was confirmed by correspondence with the authors. |
| Stanford 2019 | Single treatment session. AD one of several ACTs used in both treatment groups; study comparing addition of NIV to normal ACT. |
| van Ginderdeuren 2001 | This study describes assisted AD in infants, which is distinct from AD and not the subject of this review. |
| van Ginderdeuren 2008 | The intervention under review in this study was not AD but a comparison of two different inhalation regimes prior to AD (i.e. saline alone or saline accompanied by IPV). Single intervention study. |
| van Ginderdeuren 2011 | AD is not compared to any other ACT. The variable is the time of administration of the hypertonic saline. |
| Vendrusculo 2019 | Single intervention study. The paper describes performing AD breathing with a PEP mask in place. This combined technique is not comparable to AD for the purposes of this Review. |
| Warwick 1990 | Intervention not appropriate for this review. Manual chest physiotherapy was compared to the Thairapy® bronchial drainage vest. |
ACBT; active cycle of breathing technique ACT: airway clearance technique AD: autogenic drainage IPV: intrapulmonary percussive ventilation NIV: non‐invasive ventilation PEP: positive expiratory pressure
Characteristics of ongoing studies [ordered by study ID]
NCT04010253.
| Study name | Impact of bronchial drainage by the medical device Simeox® on function and respiratory symptoms compared to manual autogenous drainage physiotherapy in adult cystic fibrosis patients |
| Methods | Randomised crossover trial |
| Participants | Recruitment: 42 participants Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Group 1: Simeox® device (Physio Assist, France) Group 2: AD |
| Outcomes | Primary outcomes: Low frequency resistance R5. Comparison of variations V4 and V8 sessions (pre and post airway clearance session) with forced oscillation technique measured by TremoFlo™ C‐100 Airwave Oscillometry System™ (THORASYS Thoracic Medical Systems Inc. Montreal, Quebec, Canada). Secondary outcomes: Comparison of the impact of Simeox® bronchial drainage compared to AD on respiratory symptoms: dyspnea, congestion and fatigue. Evaluation of the distal and / or proximal decluttering by the two techniques, according to the improvement profile of the forced oscillations. Evaluation of the impact of Simeox® bronchial drainage vs autogenous drainage by spirometry and plethysmography (distension and resistance). Evaluation of correlations between clinical benefit and changes in functional respiratory investigations (forced oscillations, spirometry, plethysmography). |
| Starting date | 30 September 2019 |
| Contact information | Jacqueline DELRIEU, PhD 0156814060 delrieu@antadir.com |
| Notes | Estimated study completion date: 31 January 2022 |
AD: autogenic drainage CF: cystic fibrosis FEF25-75%: forced mid‐expiratory flow between 25% and 75% of forced vital capacity FEV1: forced expiratory volume in one second
Differences between protocol and review
Update 2021
The Aerobika®, Metaneb® and VibraLung® devices have been added to the list of possible oscillatory devices in 'Types of interventions', although currently there are no relevant studies evaluating its use in CF when compared to autogenic drainage.
Post hoc changes for initial review version
Outcome measures
1. In the protocol, sputum weight was included as a primary outcome measure. For the review we downgraded sputum weight to a secondary measure and we promoted quality of life (QoL) assessment to a primary outcome. Reasons for this change were:
to better reflect the improving condition of people with cystic fibrosis (CF);
to reflect concerns over the validity and reliability of sputum weight collection as a primary outcome; and
to implement advice following discussion with other members of the Cochrane Review Group, including editors of physiotherapy reviews.
By making this change we feel the review better reflects outcomes that are meaningful to people with CF, although we appreciate that for more severely affected individuals sputum weight may be relevant and we keep this as an important secondary outcome.
2. We have included lung clearance index into the secondary outcomes as a post hoc change. It is an emerging outcome measure with increasing validity, which may provide a more sensitive assessment of change in respiratory function.
3. The secondary outcomes have also been re‐ordered so that they are listed in order of importance in the view of the new author team.
Inclusion criteria
The new authors also did not accept that single intervention episodes were appropriate for this technique and therefore excluded any studies that lasted for only a single episode.
Reporting data
When reporting short‐term studies (up to one month), the new authors reported outcomes of up to seven days, and from one to four weeks. Likewise, the outcome data for longer‐term studies were reported as those measured at one month, three months, six months, 12 months and annually thereafter.
Summary of findings table
A summary of findings table for each comparison of the review was added as a post hoc change. Outcomes presented in these tables were presented based on clinical relevance rather than those which contributed the most data.
Contributions of authors
At protocol stage: Narasimman Swaminathan prepared the protocol with feedback throughout the editorial process from Amita Ray, Karen Robinson and Nikki Jahnke.
At full review stage (first published version): Pamela McCormack, Paul Burnham and Kevin Southern revised the protocol, drafted and contributed to the review. Pamela McCormack and Paul Burnham independently selected the studies for inclusion in the review and extracted data. Paul Burnham contacted authors for additional information. Pamela McCormack acted as guarantor of the review.
For the 2021 update: The previous lead author of the original review Pam McCormack and fellow co‐author Kevin W Southern have stepped down from the review team. Paul Burnham took on the role of lead author. Two new co‐authors, Gemma Stanford and Ruth Stewart joined the review team. All three authors (PB, GS and RS) independently selected studies for inclusion in the update of this review. All authors contributed to data extraction and updated the text and analyses in this review.
Paul Burnham acts as guarantor of the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Paul Burnham declares that he has no interest in any of the papers or references within this document and has received no funding in whole or in part for any of this work.
Gemma Stanford is the Principal Investigator in one of the studies assessed for inclusion in the review update. This study was independently assessed by the other review authors (PB and RS) and, following this, was added to the list of excluded studies.
Ruth Stewart declares no potential conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
App 1998 {published data only}
- App EM, Danzl G, Schweiger K, Kieselmann R, Reinhardt D, Lindemann H, et al. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy - VRP1 (flutter) versus autogenic drainage. American Journal of Respiratory and Critical Care Medicine 1995;151(4 Suppl):A737. [CFGD REGISTER: PE29a] [Google Scholar]
- App EM, Kieselmann R, Reinhardt D, Lindemann H, Dasgupta B, King M, et al. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy: flutter vs autogenic drainage. Chest 1998;114(1):171-7. [CFGD REGISTER: PE29b] [DOI] [PubMed] [Google Scholar]
Dingemans 2018 {published data only}
- Dingemans J, Eyns H, Willekens J, Monsieurs P, Van Houdt R, Cornelis P, et al. Intrapulmonary percussive ventilation improves lung function in cystic fibrosis patients chronically colonized with Pseudomonas aeruginosa: a pilot cross-over study. European Journal of Clinical Microbiology & Infectious Diseases 2018;37(6):1143-51. [CENTRAL: CN-01981650] [CFGD REGISTER: PE271b] [EMBASE: 621915666] [PMID: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN75391385. Analysis of the Pseudomonas aeruginosa biofilm in the respiratory samples of cystic fibrosis patients [Effect of intrapulmonary percussive ventilation on Pseudomonas aeruginosa biofilm formation and virulence]. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN75391385 2013. [CENTRAL: CN-01879891] [CFGD REGISTER: PE271a]
McIlwaine 1991 {published and unpublished data}
- Davidson AG, McIlwaine PM, Wong TK, Nakielna EM, Pirie GE. Physiotherapy in cystic fibrosis: A comparative trial of positive expiratory pressure, autogenic drainage and conventional percussion and drainage techniques. Pediatric Pulmonology 1988;5(Suppl 2):137. [CFGD REGISTER: PE44a] [Google Scholar]
- McIlwaine PM, Davidson AG, Wong LT, Pirie GE, Nakielna EM. Comparison of positive expiratory pressure and autogenic drainage with conventional percussion and drainage therapy in the treatment of CF. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(d)3. [CFGD REGISTER: PE44b] [Google Scholar]
- McIlwaine PM, Davidson AG. Comparison of positive expiratory pressure and autogenic drainage with conventional percussion and drainage therapy in the treatment of CF. In: 17th European Cystic Fibrosis Conference; 1991 Jun 18-21; Copenhagen, Denmark. 1991:S8.4. [CFGD REGISTER: PE44c]
- McIlwaine PM, Wong LT, Pirie GE, Davidson AG. Comparison of positive expiratory pressure and autogenic drainage therapy in the treatment of cystic fibrosis. Unpublished Article 2014. [CENTRAL: 999917]
- McIlwaine PM, Wong LT, Pirie GE, Davidson AG. Comparison of positive expiratory pressure and autogenic drainage therapy in the treatment of cystic fibrosis (as supplied 21 July 2003). Data on file 2003. [CFGD REGISTER: PE44f]
McIlwaine 2010 {published data only}
- Davidson AG, Wong LT, Pirie GE, McIlwaine PM. Long-term comparative trial of conventional percussion and drainage physiotherapy versus autogenic drainage in cystic fibrosis. Pediatric Pulmonology 1992;14(Suppl 8):235. [CFGD REGISTER: PE47a] [Google Scholar]
- McIlwaine M, Wong LT, Chilvers M, Davidson GF. Long-term comparative trial of two different physiotherapy techniques; postural drainage with percussion and autogenic drainage, in the treatment of cystic fibrosis. Pediatric Pulmonology 2010;45(11):1064-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McIlwaine PM, Wong LTK, Pirie GE, Davidson AGF. Long-term comparative trial of conventional percussion and drainage physiotherapy versus autogenic drainage in cystic fibrosis. In: 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:AHP32. [CFGD REGISTER: PE47b] 5500100000004465
Miller 1995 {published data only}
- Hall DO, Miller S, Clayton CB, Nelson R. Chest physiotherapy in cystic fibrosis: A comparative study of autogenic drainage and the active cycle of breathing technique. In: 19th European Cystic Fibrosis Conference; 1994 May 29- Jun 3; Paris, France. 1994:O64. [CFGD REGISTER: PE28a] [DOI] [PMC free article] [PubMed]
- Miller S, Hall DO, Clayton CB, Nelson R. Chest physiotherapy in cystic fibrosis: A comparative study of autogenic drainage and the active cycle of breathing techniques with postural drainage. Thorax 1995;50(2):165-9. [CFGD REGISTER: PE28c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Hall DO, Clayton CB, Nelson R. Chest physiotherapy in cystic fibrosis (CF) a comparative study of autogenic drainage (AD) and active cycle of breathing technique (ACBT) (formerly FET). Pediatric Pulmonology 1993;16(Suppl 9):267. [CFGD REGISTER: PE28b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osman 2010 {published data only}
- NCT00817947. Airway clearance using high frequency chest wall oscillation [An assessment of the short-term effects of the airway clearance technique of high frequency chest wall oscillation in people with cystic fibrosis]. clinicaltrials.gov/show/NCT00817947 (first received 07 January 2009). [CENTRAL: CN-01523471] [CFGD REGISTER: PE171c]
- Osman LP, Roughton M, Hodson ME, Pryor JA. High frequency chest wall oscillation in cystic fibrosis. Journal of Cystic Fibrosis 2008;7(Suppl 2):S73. [CFGD REGISTER: PE171a] [Google Scholar]
- Osman LP, Roughton M, Hodson ME, Pryor JA. Short-term comparative study of high frequency chest wall oscillation and European airway clearance techniques in patients with cystic fibrosis. Thorax 2010;65(3):196-200. [CFGD REGISTER: PE171b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfleger 1992 {published data only}
- Pfleger A, Theißl B, Oberwaldner B, Zach MS. Self-administered chest physiotherapy in cystic fibrosis: a comparative study of high-pressure PEP and autogenic drainage. Lung 1992;170(6):323-30. [CFGD REGISTER: PE16a] [DOI] [PubMed] [Google Scholar]
- Theißl B, Pfleger A, Oberwaldner B, Zach M. Chest physiotherapy (PT) in cystic fibrosis (CF) - a comparative study of high-pressure PEP and autogenic drainage. Pediatric Pulmonology 1990;9(Suppl 5):259. [CFGD REGISTER: PE16b] [DOI] [PubMed] [Google Scholar]
Pryor 2010 {published data only}
- NCT00890370. Should any one airway clearance technique be recommended for people with cystic fibrosis? [A comparison of five airway clearance techniques in the treatment of adults with cystic fibrosis]. clinicaltrials.gov/show/NCT00890370 (first received 29 April 2009). [CENTRAL: CN-01500662] [CFGD REGISTER: PE164c]
- Pryor JA, Tannenbaum E, Cramer D, Scott SF, Burgess J, Gyi K, et al. A comparison of five airway clearance techniques in the treatment of people with Cystic Fibrosis. Journal of Cystic Fibrosis 2006;5(Suppl):S76. [CFGD REGISTER: PE164a] [DOI] [PubMed] [Google Scholar]
- Pryor JA, Tannenbaum E, Scott SF, Burgess J, Cramer D, Gyi K, et al. Beyond postural drainage and percussion: Airway clearance in people with cystic fibrosis. Journal of Cystic Fibrosis 2010;9(3):187-92. [CENTRAL: 759356] [CFGD REGISTER: PE164b] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12611000160932 {published data only}
- ACTRN12611000160932. Quality of life, days in hospital, lung function and fitness before and after attending the outpatient cystic fibrosis (CF) physiotherapy service [Children and adolescents with cystic fibrosis attending the Women's and Children's Hospital Ambulatory Cystic Fibrosis Physiotherapy Clinic - effect on quality of life, number of occupied bed days, pulmonary function and exercise capacity.]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000160932 (first posted 21 January 2011).
Corten 2020 {published data only}
- Corten L, Morrow BM. The use of assisted autogenic drainage in children with cystic fibrosis; a pilot randomized controlled study. Physiotherapy Practice and Research 2020;41(1):79-89. [CFGD REGISTER: PE297b] [DOI: 10.3233/PPR-190148] [DOI] [Google Scholar]
- PACTR201501001016415. Assisted autogenic drainage (chest physiotherapy) in South African children with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201501001016415 (first approved 26 January 2013). [CFGD REGISTER: PE297a]
CTRI/2020/10/028509 {published data only}
- Comparison of two different chest physiotherapy techniques in participants undergone upper abdominal surgery [Effectiveness of incentive spirometry and breathing exercise with active cycle of breathing technique vs autogenic drainage on peak expiratory flow rate in participants following upper abdominal surgery ]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/10/028509 30 November 2020;(11). [CENTRAL: CN-02186346]
Davies 2012 {published data only}
- Banks A, Davies G, Agent P, Osman L, Bilton D, Hodson M. The use of high frequency chest wall oscillation during an acute infective pulmonary exacerbation of cystic fibrosis. European Respiratory Journal (abstract) 2012;40(Suppl 56):P3374. [CENTRAL: 1100493] [CFGD REGISTER: PE197b] [EMBASE: 71925445] [Google Scholar]
- Davies GA, Banks AE, Agent P, Osman LP, Bilton D, Hodson ME. The use of high frequency chest wall oscillation during an infective pulmonary exacerbation of cystic fibrosis. Pediatric Pulmonology 2012;47 (S35):366. [ABSTRACT NO.: 396] [CFGD REGISTER: PE197a] [Google Scholar]
- NCT01057524. High frequency chest wall oscillation and cystic fibrosis [The use of high frequency chest wall oscillation during an acute infective pulmonary exacerbation of cystic fibrosis]. clinicaltrials.gov/show/NCT01057524 (first received 27 January 2010). [CENTRAL: CN-01527868] [CFGD REGISTER: PE197c]
Giles 1995 {published data only}
- Giles DR, Wagener JS, Accurso FJ, Butler Simon N. Short-term effects of postural drainage with clapping vs autogenic drainage on oxygen saturation and sputum recovery in patients with cystic fibrosis. Chest 1995;108(4):952-4. [CFGD REGISTER: PE66] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Helper 2020 {published data only}
- Helper N, Kodesh E, Sokol G, Hakimi R, Vilozni D, Efrati O. The benefits of mechanical insufflator-exsufflator compared to autogenic drainage in adults with cystic fibrosis. Pediatric Pulmonology 2020;55(11):3046-52. [CENTRAL: CN-02183101] [CFGD REGISTER: PE300b] [DOI: 10.1002/ppul.25020] [DOI] [PubMed] [Google Scholar]
- Helper N, Kodesh E, Vilozni D, Sokol G, Hakimi R, Efrati O. Efficiency of the mechanical insufflator - exsufllator for airway clearance in cystic fibrosis. Journal of Cystic Fibrosis 2019;18 Suppl:S34. [CENTRAL: CN-01987203] [CFGD REGISTER: PE300a] [Google Scholar]
Herrero 2016 {published data only}
- Cortina BH, Pagola MSM, Cadenas AF, Cortes ATR, Del Corral T, Del Mar Sanchez Palenzuela M, et al. Resistive inspiratory manoeuvre as airway clearance technique in stable patients with cystic fibrosis: a randomised crossover trial. European Respiratory Journal 2016;48 Suppl 60:PA1364. [CFGD REGISTER: PE249b] [Google Scholar]
- Herrero Cortina B, San Miguel Pagola M, Fernandez Cadenas A, Rios Cortes AT, Del Corral T, Sanchez Palenzuela MDM, et al. Resistive inspiratory manoeuvre as airway clearance technique in patients with cystic fibrosis: a randomised crossover trial. Journal of Cystic Fibrosis 2016;15 Suppl:S33-S34. [ABSTRACT NO.: WS21.4] [CFGD REGISTER: PE249a] [Google Scholar]
- NCT02261987. Resistive inspiratory manoeuvre as airway clearance technique in cystic fibrosis. https://clinicaltrials.gov/ct2/show/NCT02261987 (date first registered 10 Oct 2014). [CFGD REGISTER: PE249c]
Lindemann 1992 {published data only}
- Lindemann H. The value of physical therapy with VRP 1-Desitin ("Flutter") [Zum Stellenwert der Physiotherapie mit dem VRP 1-Desitin ("Flutter").]. Pneumologie 1992;46(12):626-30. [CFGD REGISTER: PE17] [PubMed] [Google Scholar]
NCT01854788 {published data only}
- NCT01854788. A comparative study of three airway clearance techniques with different autonomy degrees in non cystic fibrosis bronchiectasis: randomized cross-over trial. clinicaltrials.gov/ct2/show/NCT01854788 (first posted 16 May 2013).
NCT02840136 {published data only}
- NCT02840136. Optimisation, valorisation and application of LC-MS/MS based monitoring of antibiotic concentrations in sputum of cystic fibrosis patients - Part 3: non-blank sputum samples for method optimisation and validation. clinicaltrials.gov/ct2/show/NCT02840136 (first posted 21 July 2016).
NCT03522480 {published data only}
- The effectiveness of the Jamboxx respiratory therapy device: study 2 [The effectiveness of the Jamboxx respiratory therapy device in treatment of patients with decreased respiratory function. Study 2: children with cystic fibrosis for long term evaluation]. clinicaltrials.gov/show/NCT03522480 (first posted 11 May 2018). [CENTRAL: CN-01599501] [CFGD REGISTER: PE301]
NCT03655249 {published data only}
- NCT03655249. Effects of AD on VI in patients with CF [Effects of autogenic drainage on ventilation inhomogeneity in patients with cystic fibrosis]. clinicaltrials.gov/show/NCT03655249 (first posted 31 August 2018). [CENTRAL: CN-01662870] [CFGD REGISTER: PE298]
NCT04187924 {published data only}
- NCT04187924. Effects of the addition of the SIMEOX device on autogenic drainage in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT04187924 (first posted 05 December 2019).
NCT04527796 {published data only}
- NCT04527796. Influence of a residential rehabilitation program on body composition in patients with cystic fibrosis (children and adults). clinicaltrials.gov/ct2/show/NCT04527796 (first posted 27 August 2020).
Poncin 2017 {published data only}
- NCT02411981. Short-term effects of chest physiotherapy on ventilation inhomogeneity in non-CF bronchiectasis. clinicaltrials.gov/ct2/show/NCT02411981 (first posted 08 April 2016).
- Poncin W, Reychler G, Leeuwerck N, Bauwens N, Aubriot AS, Nader C, et al. Short-term effect of autogenic drainage on ventilation inhomogeneity in adult subjects with stable non-cystic fibrosis bronchiectasis. Respiratory Care 2017;62(5):524-531. [DOI: 10.4187/respcare.05194] [DOI] [PubMed] [Google Scholar]
Prusak 2020 {published data only}
- Prusak J, Warzeszak K, Slaby K, Kurzeja P, Pogorzelski A. Assessment of the short-term effectiveness of the use of positive and oscillating positive expiratory pressure in bronchial drainage in patients with cystic fibrosis. Journal of Cystic Fibrosis 2020;19 Suppl:S145. [CENTRAL: CN-02217808] [CFGD REGISTER: PE296] [Google Scholar]
Reix 2012 {published data only}
- NCT01509235. Self drainage in pediatric cystic fibrosis patients. clinicaltrials.gov/show/NCT01509235 (date first posted 12 January 2012). [CFGD REGISTER: PE183c]
- Reix P, Aubert F, Kassai B, Bige V, Bellon G. Better satisfaction of cystic fibrosis paediatric patients with autogenic drainage associated to exercise compared to conventional chest physiotherapy. Journal of Cystic Fibrosis 2009;8(Suppl 2):S73. [ABSTRACT NO: 293] [CFGD REGISTER: PE183a] [Google Scholar]
- Reix P, Aubert F, Werck-Gallois MC, Toutain A, Mazzocchi C, Moreux N, et al. Exercise with incorporated expiratory manoeuvres was as effective as breathing techniques for airway clearance in children with cystic fibrosis: a randomised crossover trial. Journal of Physiotherapy 2012;58(4):241-7. [CENTRAL: 841658] [CFGD REGISTER: PE183b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roos 1987 {published data only}
- Roos S, Birrer P, Rüdeberg A, Kraemer R. First experience with intrapulmonary percussive ventilation (IPV) in the treatment of patients with cystic fibrosis. In: 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987. [CFGD REGISTER: PE34]
San Miguel‐Pagola 2020 {published data only}
- NCT02303808. Positive expiratory pressure during inhalation of hypertonic saline in patients with cystic fibrosis [Effect of introducing a positive expiratory pressure device during inhalation of hypertonic saline in patients with cysticfibrosis: a randomized crossover trial]. clinicaltrials.gov/ct2/show/NCT02303808 (first posted 25 November 2014).
- San Miguel-Pagola M, Reychler G, Cebrià i Iranzo MA, Gómez-Romero M, Díaz-Gutiérrez F, Herrero-Cortina B. Impact of hypertonic saline nebulisation combined with oscillatory positive expiratory pressure on sputum expectoration and related symptoms in cystic fibrosis: a randomised crossover trial. Physiotherapy June 2020;107:243-251. [DOI: 10.1016/j.physio.2019.11.001] [DOI] [PubMed] [Google Scholar]
Skopnik 1986 {published data only}
- Skopnik H, Waters W, Horwitz AE, Roth B, Genz-Bohm G. Evaluation of knock and vibration therapy and autogenic drainage in cystic fibrosis using a ventilation zintigraphical method [Beurteilung der Klopf- und Vibrations-Therpie und autogene Drainage bei cystischer Fibrose mittels eines ventilationsszintigraphischen Verfahrens]. Monatsschrift fur Kinderheilkunde 1986;134:583. [CFGD REGISTER: PE131] [Google Scholar]
Sokol 2012 {published and unpublished data}
- Sokol G, Hakimi R, Better R, Efrati O. The short term effect of airway clearance using the “Cough Assist” on lung function in patients with cystic fibrosis [Abstract]. In: Journal of Cystic Fibrosis. Conference: 35th European Cystic Fibrosis Conference; Dublin, Ireland. Vol. 11 . June 2012:S104, [ABSTRACT NO: 188]. [CENTRAL: CN-00848818] [CFGD REGISTER: PE196a]
Sokol 2012a {published and unpublished data}
- Sokol G, Hakimi R, Vilozni D, Beter R, Rubinstein E, Larea Yona O, et al. The short term effect of the “cough assist”and “autogenic drainage” physiotherapy on lung function in patients with cystic fibrosis [Abstract]. 26th Annual North American Cystic Fibrosis Conference (October 11th-13th 2012); Orlando, Florida. In: Pediatric Pulmonology. Vol. 47 . 2012:Suppl 35, 365-366 [ABSTRACT NO: 394]. [CENTRAL: CN-01030594] [CFGD REGISTER: PE196b]
Stanford 2019 {published data only}
- NCT01885650. The addition of non-invasive ventilation to airway clearance techniques in adults with cystic fibrosis (NIV) [Non-invasive ventilation (NIV) for positive pressure support. A randomised cross-over trial to evaluate the short-term effects of NIV as an adjunct to airway clearance techniques in adults with cystic fibrosis.]. clinicaltrials.gov/ct2/show/NCT01885650 Date first received: 17 June 2013.
- Stanford G, Parrott H, Bilton D, Agent P, Banya W, Simmonds N. Randomised cross-over trial evaluating the short-term effects of non-invasive ventilation as an adjunct to airway clearance techniques in adults with cystic fibrosis. BMJ Open Respiratory Research 14 April 2019;6(1):e000399. [DOI: 10.1136/bmjresp-2018-000399] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Ginderdeuren 2001 {published data only}
- Ginderdeuren F, Malfroot A, Dab I. Influence of "assisted autogenic drainage (AAD)" "bouncing", and "AAD combined with bouncing" on gastro-oesophageal reflux (GOR) in infants. In: 24th European Cystic Fibrosis Conference; 2001 Jun 6-9; Vienna, Austria. 2001:P112. [CFGD REGISTER: PE124]
van Ginderdeuren 2008 {published data only}
- Ginderdeuren F, Verbanck S, Van Cauwelaert K, Vanlaethem S, Schuermans D, Vincken W, et al. Chest physiotherapy in cystic fibrosis: short-term effects of autogenic drainage preceded by wet inhalation of saline versus autogenic drainage preceded by intrapulmonary percussive ventilation with saline. Respiration; International Review of Thoracic Diseases 2008;76(2):175-80. [CFGD REGISTER: BD178] 5500100000004752 [DOI] [PubMed] [Google Scholar]
van Ginderdeuren 2011 {published data only}
- Ginderdeuren F, Vanlaethem S, Eyns H, De Schutter I, Dewachter E, Malfroot A. Influence of inhaled hypertonic saline (NaCI 6%) before or during autogenic drainage on sputum weight, oxygen saturation, heart frequency and dyspnoea in cystic fibrosis. Journal of Cystic Fibrosis 2011;10 Suppl 1:S62. [CFGD REGISTER: BD164] 5500100000010552 [Google Scholar]
Vendrusculo 2019 {published data only}
- Vendrusculo FM, Johnstone Z, Dhouieb E, Donadio MVF, Cunningham S, Urquhart DS. Airway clearance physiotherapy can reduce dead space and lead to improvements in ventilatory inhomogeniety during exercise in patients with cystic fibrosis: a pilot study. Journal of Cystic fibrosis 2017;16 Suppl 1:S8. [ABSTRACT NO.: WS04.6] [CFGD REGISTER: PE250a] [Google Scholar]
- Vendrusculo FM, Johnstone Z, Dhouieb E, Donadio MVF, Cunningham S, Urquhart DS. Airway clearance physiotherapy improves ventilatory dynamics during exercise in patients with cystic fibrosis: a pilot study. Archives of Disease in Childhood 2019;104(1):37-42. [CENTRAL: CN-01610807] [CFGD REGISTER: PE250b] [EMBASE: 622462575] [PMID: ] [DOI] [PubMed] [Google Scholar]
Warwick 1990 {published data only}
- Warwick WJ, Wielnski CI. Matched pair comparison of manual chest physical therapy (CPT) and the thairapy bronchial drainage vest (ThBVD) system. Pediatric Pulmonology 1990;9(Suppl 5):177. [CFGD REGISTER: PE43] [Google Scholar]
References to ongoing studies
NCT04010253 {published data only}
- NCT04010253. Impact of bronchial drainage technique by the medical device Simeox® on respiratory function and symptoms in adult patients with cystic fibrosis [Impact of bronchial drainage by the medical device Simeox® on function and respiratory symptoms compared to manual autogenous drainage physiotherapy in adult cystic fibrosis patients]. clinicaltrials.gov/show/NCT04010253 first posted 08 July 2019. [CENTRAL: CN-01953083] [CFGD REGISTER: PE302]
Additional references
ACPCF 2020
- Association of Physiotherapists in Cystic Fibrosis (ACPCF). Standards of Care and Good Clinical Practice for the Physiotherapy Management of Cystic Fibrosis - 4th edition. Cystic Fibrosis Trust website November 2020.
Agostini 2007
- Agostini P, Knowles N. Autogenic drainage: the technique, physiological basis and evidence. Physiotherapy 2007;93(2):157-63. [Google Scholar]
Andersen 1979
- Andersen JB, Qvist H, Kann T. Recruiting collapsed lung through collateral channels with positive end-expiratory pressure. Scandinavian Journal of Respiratory Disease 1979;60(5):260–6. [PubMed] [Google Scholar]
Boucher 2004
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. European Respiratory Journal 2004;23:146-58. [DOI] [PubMed] [Google Scholar]
Button 2016
- Button BM, Wilson C, Dentice R, Cox N, Middleton A, Tannenbaum E, et al. Physiotherapy for Cystic Fibrosis in Australia and New Zealand: A Clinical Practice Guideline. Respirology 2016;21(4):656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantin 2015
- Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. Journal of Cystic Fibrosis 2015;14(4):419-30. [DOI] [PubMed] [Google Scholar]
CFF 2020
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry. 2019 Annual Data Report. www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.pdf.
Chevaillier 1984
- Chevaillier J. Autogenic drainage. In: Lawson D, editors(s). CysticFibrosis: Horizons. London: Churchill Livingstone, 1984:65–78. [Google Scholar]
Corten 2017
- Corten L, Morrow BM. Autogenic Drainage in Children With Cystic Fibrosis. Pediatric Physical Therapy 2017;29:106-117. [DOI: 10.1097/PEP.0000000000000355] [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. III: The issue of carryover. Statistics in Medicine 2002;21(15):2161-73. [DOI] [PubMed] [Google Scholar]
Cystic Fibrosis NZ 2018
- Cystic Fibrosis NZ . Port CF Data Registry, 2017 Registry Report. www.cfnz.org.nz/assets/Uploads/cf3fb19c08/Port-CF-2017-NZ-CF-Data-Registry-1-v2.1.pdf.
David 1991
- David A. Autogenic Drainage; The German approach. In: Pryor J, editors(s). Respiratory Care. Edinburgh: Churchill Livingstone, 1991:143. [Google Scholar]
Dwyer 2011
- Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PT. Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest 2011;139:870–7. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Flume 2009
- Flume PA, Robinson KA, O´Sullivan BP, Finder JD, Vender RL, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respiratory Care 2009;54(4):522-37. [PubMed] [Google Scholar]
Gee 2000
- Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groth 1985
- Groth S, Stafanger G, Dirksen H, Andersen JB, Falk M, Kelstrup M. Positive expiratory pressure (PEP-Mask) physiotherapy improves ventilation and reduces volume of trapped gas in cystic fibrosis. Bulletin Europeen de Physiopathologie Respiratoire 1985;21(4):339-43. [PubMed] [Google Scholar]
Guyatt 1987
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42(10):773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Homnick 1995
- Homnick DN, White F, Castro C. Comparison of effects of an intrapulmonary percussive ventilator to standard aerosol and chest physiotherapy in treatment of cystic fibrosis. Pediatric Pulmonology 1995;20(1):50-5. [DOI] [PubMed] [Google Scholar]
Huang 1981
- Huang NN, Keith HH, Palmer J, Hsuan F. A scoring system for short-term evaluation of patients with cystic fibrosis: a possible means for assessment of antibiotic efficacy. In: Warwick WJ, editors(s). 1,000 years of cystic fibrosis collected papers. Minessota: University Dept of Pediatrics Medical School in cooperation with International Cystic Fibrosis Association, National Heart, Lung and Blood Institute and Fogarty International Center, 1981:207-16. [Google Scholar]
Hunt 1985
- Hunt SM, McEwen J, McKenna SP. Measuring health status: a new tool for clinicians and epidemiologists. Journal of the Royal College of General Practitioners 1985;35:185-188. [PMC free article] [PubMed] [Google Scholar]
IPG/CF 2019
- International Physiotherapy Group for CF (IPG/CF). Blue booklet on physiotherapy techniques. Physiotherapy for people with cystic fibrosis: from infant to adult - 7th edition. https://www.ecfs.eu/sites/default/files/general-content-files/working-groups/IPG%20CF_Blue%20Booklet_7th%20edition%202019.pdf 2019.
Kaplan 1989
- Kaplan RM, Anderson JP, Wu AW, Mathews WC, Kozin F, Orenstein D. The Quality of Well‐being Scale. Applications in AIDS, cystic fibrosis, and arthritis. Medical Care 1989;27 (3 Suppl):S27-43. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Altman DG, Williamson PR. Bias due to changes in specified outcomes during the systematic review process. PLoS ONE 2010;5(2):e9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konstan 1994
- Konstan MW, Stern RC, Doershuk CF. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. Journal of Pediatrics 1994;124(5 Pt 1):689-93. [DOI] [PubMed] [Google Scholar]
Konstan 1997
- Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: Onset and etiology. Pediatric Pulmonology 1997;24:137-42. [DOI] [PubMed] [Google Scholar]
Konstan 2007
- Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. Journal of Pediatrics 2007;151(2):134-9. [DOI: 10.1016/j.jpeds.2007.03.006] [DOI] [PubMed] [Google Scholar]
Main 2005
- Main E, Prasad A, Schans CP. Conventional chest physiotherapy compared to other airway clearance technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD002011. [DOI: 10.1002/14651858.CD002011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIllwaine 2014
- McIllwaine MP, Lee Son MN, Richmond ML. Physiotherapy and cystic fibrosis: what is the evidence base? Current Opinion in Pulmonary Medicine 2014;20(6):613-7. [DOI] [PubMed] [Google Scholar]
McIlwaine 2019
- McIlwaine, M, Button, B, Nevitt, S J. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD003147. [DOI: 10.1002/14651858.CD003147.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mckoy 2016
- Mckoy NA, Wilson LM, Saldanha IJ, Odelola OA, Robinson KA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD007862. [DOI: 10.1002/14651858.CD007862.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2015
- Morgan K, Osterling K, Gilbert R, Dechman G. Effects of autogenic drainage on sputum recovery and pulmonary function in people with cystic fibrosis: A systematic review. Physiotherapy Canada 2015;67(4):319-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2020
- Morrison L, Milroy S. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD006842. [DOI: 10.1002/14651858.CD006842.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nissenbaum 2020
- Nissenbaum C, Davies G, Horsley A, Davies JC. Monitoring early stage lung disease in cystic fibrosis. Current Opinion in Pulmonary Medicine 2020;26(6):671-678. [DOI] [PubMed] [Google Scholar]
Nolan 2016
- Nolan SJ, Hambleton I, Dwan K. The use and reporting of the cross-over study design in clinical trials and systematic reviews: a systematic assessment. PLoS One 2016;11(7):e0159014. [DOI: 10.1371/journal.pone.0159014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oberwaldner 1986
- Oberwaldner B, Evans JC, Zach MS. Forced expirations against a variable resistance:a new chest physiotherapy method in cystic fibrosis. Pediatric Pulmonology 1986;2(6):358–67. [DOI] [PubMed] [Google Scholar]
Okeson 2007
- Okeson C, McGowen P. The percussive characteristics of the acapella, flutter and quake during low-volume tidal breathing. Chest 2007;132(4 Meeting abstracts):608a. [DOI: 10.1378/chest.132.4_MeetingAbstracts.608a ] [Google Scholar]
Patel 2013
- Patel P, Fukushima L, Balekian A, Chou W, Lu A, Gali V, et al. Is Metaneb comparable to high frequency chest compression in the setting of a severe pulmonary exacerbation in adults with cystic fibrosis. Pediatric Pulmonology 2013;48 Suppl 36:359. [Google Scholar]
Patterson 2019
- Patterson KD, Walsh A, McCormack P, Southern KW. Exercise versus airway clearance techniques for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013285. [DOI: 10.1002/14651858.CD013285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poncin 2020
- Poncin W, Reychler G, Liistro M, Liistro G. Comparison of 6 Oscillatory Positive Expiratory Pressure Devices During Active Expiratory Flow. Respiratory Care 2020;65(4):492-499. [DOI: 10.4187/respcare.07271] [DOI] [PubMed] [Google Scholar]
Pryor 1999
- Pryor JA. Physiotherapy for airway clearance in adults. European Respiratory Journal 1999;14(6):1418-24. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2017
- Radtke, T, Nevitt, S J, Hebestreit, H, Kriemler, S. Physical exercise training for cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 2013
- Rand S, Hill L, Prasad A. Physiotherapy in cystic fibrosis: optimising techniques to improve outcomes. Peadiatric Respiratory Reviews 2013;14(4):263-9. [DOI] [PubMed] [Google Scholar]
Rogers 2005
- Rogers D, Doull IJM. Physiological principles of airway clearance techniques used in the physiotherapy management of cystic fibrosis. Current Paediatrics 2005;15(3):233-8. [DOI: 10.1016/j.cupe.2005.02.007] [DOI] [Google Scholar]
Sawicki 2009
- Sawicki JS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. Journal of Cystic Fibrosis. March 2009;8(2):91-96. [DOI: 10.1016/j.jcf.2008.09.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). In: Available from www.training.cochrane.org/handbook. The Cochrane Collaboration, 2021. [Google Scholar]
Scotet 2020
- Scotet V, L'Hostis C, Férec C. The changing epidemiology of cystic fibrosis: incidence, survival and impact of the CFTR gene discovery. Genes 2020;11(6):589-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Southern 2003
- Southern KW, Smyth RL. Design of clinical trials in cystic fibrosis. Lancet 2003;361(9354):349-50. [DOI] [PubMed] [Google Scholar]
Tiddens 2010
- Tiddens HA, Donaldson SH, Rosenfeld M, Paré PD. Cystic fibrosis lung disease starts in the small airways: Can we treat it more effectively? Pediatric Pulmonology 2010;45(2):107-17. [DOI: 10.1002/ppul.21154] [DOI] [PubMed] [Google Scholar]
UK CF Trust 2020
- UK Cystic Fibrosis Trust. UK Cystic Fibrosis Registry 2019 Annual Data Report. www.cysticfibrosis.org.uk/sites/default/files/2020-12/2019 Registry Annual Data report_Sep 2020.pdf.
VanDevanter 2015
- VanDevanter DR, Pasta DJ, Konstan MW. Treatment and demographic factors affecting time to next pulmonary exacerbation in cystic fibrosis. Journal of Cystic Fibrosis 2015;14(6):763-769. [DOI: 10.1016/j.jcf.2015.02.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volsko 2003
- Volsko TA, DiFiore JM, Chatburn RL. Performance comparison of two oscillatory positive pressure devices: Acapella versus Flutter. Respiratory Care 2003;48(2):124-30. [PubMed] [Google Scholar]
Wallaert 2018
- Wallaert E, Perez T, Prevotat A, Reychler G, Wallaert B, Le Rouzic O. The immediate effects of a single autogenic drainage session on ventilatory mechanics in adult subjects with cystic fibrosis. PLoS ONE 2018;13(3):e0195154. [DOI: 10.1371/journal.pone.0195154] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2019
- Ward N, Stiller K, Holland AE, Australian Cystic Fibrosis Exercise Survey group. Exercise is commonly used as a substitute for traditional airway clearance techniques by adults with cystic fibrosis in Australia: a survey. Journal of Physiotherapy. January 2019;65(1):43-50. [DOI] [PubMed] [Google Scholar]
Warnock 2015
- Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD001401. [DOI: 10.1002/14651858.CD001401.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warwick 1991
- Warwick WJ, Hansen LG. The long-term effect of high frequency chest compression therapy on pulmonary complications of cystic fibrosis. Pediatric Pulmonology 1991;11(3):265-71. [DOI] [PubMed] [Google Scholar]
Wheatley 2018
- Wheatley CM, Baker SE, Daines CM, Phan H, Martinez MG, Morgan WJ, et al. Influence of the Vibralung acoustical percussor on pulmonary function and sputum expectoration in individuals with cystic fibrosis. Therapeutic Advances in Respiratory Disease 2018;12:1-15. [CENTRAL: CN-01652453] [CFGD REGISTER: PE208b] [EMBASE: 624227485] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2019
- Wilson, L M, Morrison, L, Robinson, K A. Airway clearance techniques for cystic fibrosis: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD011231. [DOI: 10.1002/14651858.CD011231.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
McCormack 2017
- McCormack P, Burnham P, Southern KW. Autogenic drainage for airway clearance in cystic fibrosis.. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD009595. [DOI: 10.1002/14651858.CD009595.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swaminathan 2012
- Swaminathan N, Robinson KA, Ray A. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD009595. [DOI: 10.1002/14651858.CD009595] [DOI] [Google Scholar]


