Abstract
Diabetic nephropathy management should include the use of an angiotensin‐converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker with additional antihypertensive medications to reduce proteinuria and cardiovascular events. Some studies suggest that adding a nondihydropyridine rather than a dihydropyridine calcium channel blocker (CCB) may more effectively lower proteinuria. We hypothesized that a trandolapril/verapamil SR (T/V) fixed‐dose combination (FDC) was superior to a benazepril/amlodipine (B/A) FDC for reducing albuminuria in 304 hypertensive diabetic nephropathy patients when treated for 36 weeks. No statistically significant differences were observed between groups in the primary end point; adjusted percentage change in urinary albumin/creatinine ratio (UACR), which increased (mean T/V, 29.29%; mean B/A, 8.49%; difference, 20.80%; P=.34); or in change in absolute UACR, which decreased (mean [g/g] T/V, −0.11; mean [g/g] B/A, −0.08; difference −0.03; P=.78). There were significant reductions in log UACR (mean change in T/V, −0.28; P<.01; mean change in B/A, −0.31; P<.001) and diastolic blood pressure in both groups and in systolic blood pressure in the B/A group. T/V was not superior to B/A for reducing UACR. Both ACEI/CCB FDCs may reduce albuminuria; in the case of T/V, this appears to be independent of systolic blood pressure reduction in patients who had previously been treated and had baseline blood pressure levels of 142/77 mm Hg.
Diabetic nephropathy, the leading cause of end‐stage renal disease, is characterized by persistent albuminuria, hypertension, or decline in glomerular filtration rate and excessive cardiovascular morbidity and mortality. 1 , 2 , 3 Cumulative incidence of diabetic nephropathy is 25%–40% after 25 years in type 1 and 2 diabetics. Within 5 years of onset of overt proteinuria, end‐stage renal disease develops in up to 50% of patients. Clinical trials have demonstrated that hypertension and increased activity of the renin‐angiotensin‐aldosterone system (RAAS) are major factors responsible for kidney damage and cardiovascular events in diabetic nephropathy and that antihypertensive regimens that include RAAS‐blocking drugs, angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or β‐blockers confer long‐term renoprotection. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
Similar blood pressure (BP) reduction by other antihypertensive agents may not result in comparable renoprotection. In studies comparing calcium channel blockers (CCBs) to ACEIs or ARBs in patients with nephropathy, renoprotection by CCBs was inferior. 12 , 14 , 15 An analysis of studies evaluating the effect of CCBs in diabetic and nondiabetic nephropathy concluded that nondihydropyridine (NDHP) CCBs (verapamil, diltiazem) may confer greater reduction of proteinuria than dihydropyridine (DHP) CCBs (amlodipine, felodipine, nisoldipine, etc), 15 even with comparable BP reduction, suggesting that the renoprotective effect of NDHP CCBs may be due to actions other than BP effects. However, these results occurred in patients who did not receive an ARB or an ACEI as baseline therapy. In one study, an NDHP CCB was superior to a DHP CCB for reducing albuminuria in type 2 diabetics with nephropathy despite similar BP control. 16 Guidelines recommend RAAS inhibitors as first‐step therapy for diabetic nephropathy. 17 There are no prior studies directly comparing an NDHP CCB to a DHP CCB added to an RAAS inhibitor. We tested the hypothesis that the fixed‐dose combination (FDC) of an ACEI and an NDHP CCB is superior to the FDC of an ACEI and a DHP CCB for reducing albuminuria in hypertensive type 2 diabetics with nephropathy.
Methods
Study Population
We randomized 304 type 2 diabetics with hypertension and nephropathy from 65 US sites. Participants were at least 18 years of age, with type 2 diabetes, hypertension (requiring ≥2 medications, or uncontrolled on monotherapy [systolic blood pressure (SBP) ≥130 mm Hg and/or diastolic blood pressure (DBP) ≥80 mm Hg]), urinary albumin/creatinine ratio (UACR) ≥0.2 g/g on a screening spot urine collection, and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 based on the 6‐variable Modification of Diet in Renal Disease equation. Excluded at screening were persons with secondary, malignant, or poorly controlled hypertension (SBP ≥160 mm Hg and/or DBP ≥100 mm Hg); hyperkalemia (>5.5 mmol/L, or >6.0 mmol/L if taking an ACEI or ARB); type 1 diabetes, poorly controlled type 2 diabetes (hemoglobin A1c [HbA1c] >10%), nondiabetic renal disease, or stroke; myocardial infarction, coronary revascularization, or transient ischemic attack within 3 months prior to screening; unstable angina; heart failure (New York Heart Association class III or higher); or requiring treatment with medication that would influence urinary protein excretion. Patients were excluded with diagnosed human immunodeficiency virus, diagnosed hepatitis C with abnormal liver function, hematuria or pyuria, active drug or alcohol abuse, expected survival <1 year, poor response to randomized study drugs, allergy to ACEI or CCB, sick sinus syndrome or second‐ or third‐degree atrioventricular block (except with a pacemaker), SBP <90 mm Hg, or receiving any investigational drug within 4 weeks of screening. Exclusion criteria at randomization were sitting SBP ≥160 mm Hg or DBP ≥100 mm Hg or poor study drug compliance (<80% or >120% during last 2 weeks of the run‐in). The institutional review board at each site approved the study protocol, and participants provided written informed consent.
Study Design
This study, a multicenter, active‐control, prospective, randomized, open‐label, blinded–end point (PROBE) trial, was designed to determine whether the trandolapril and verapamil (T/V) FDC was superior to the benazepril and amlodipine (B/A) FDC in reduction of albuminuria in patients with hypertension and diabetic nephropathy. Investigators were blinded to the primary end point determined by the central laboratory. The study was open‐label to allow for dose titration and addition of antihypertensive drugs needed for BP control. The study had 3 phases: (1) screening, (2) a 4‐week run‐in, and (3) a 36‐week postrandomization efficacy assessment.
Screening Visit
Fasting blood and urine samples were obtained at screening to determine eligibility. A complete medical history, physical examination, 12‐lead electrocardiography, and urine pregnancy test were performed.
Run‐In Period
Eligible participants entered a 4‐week run‐in period at which time their antihypertensive medication was replaced with once‐daily lisinopril 20 mg and torsemide 10 mg. Visits were conducted at 2‐week intervals, or more often if needed for safety reasons, prior to study drug administration. The purpose of the run‐in was to standardize treatment with an ACEI and diuretic regimen, assess adherence to study protocol and visits, and wash out any previous antihypertensive agents. Two 8‐hour urine collections were obtained on consecutive nights beginning 3 days prior to randomization for measurement of UACR and sodium. One day prior to randomization, fasting blood and urine samples were obtained.
Randomization
Patients who completed the run‐in period and satisfied the additional exclusion criteria discontinued lisinopril and torsemide and were randomized to receive the FDC of T/V 2 mg/180 mg or B/A 10/5 mg once daily for 4 weeks. WedRando, a validated in‐house program, was used to generate randomization numbers and treatment assignments. Randomization was stratified by site.
Among the 342 persons screened, 330 entered the run‐in phase and 304 were randomized. Forty‐two T/V and 25 B/A patients discontinued prior to the final visit (Figure 1). The leading cause reported for discontinuation was adverse events (23 T/V and 12 B/A patients).
Figure 1.
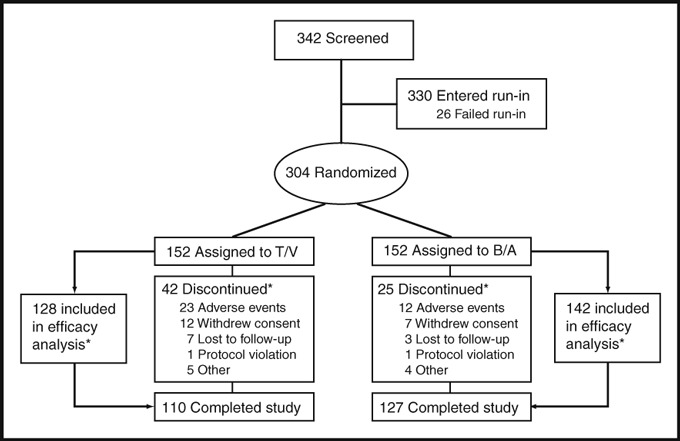
Patient flow from enrollment through study completion. Source: Study completion case report forms. T/V indicates trandolapril/verapamil SR; B/A, benazepril/amlodipine. *See text for details.
Postrandomization
Visits were conducted at weeks 4, 6, 8, 12, 24, and 36 for BP measurement, blood sampling, and collection of study drug adherence and adverse event data. Participants could have additional visits for antihypertensive medication adjustment. At week 4, the T/V dosage was increased to 4/240 mg once daily, and the B/A dosage was increased to 20/10 mg once daily. For those in whom target BP (<130/80 mm Hg) was not achieved after week 4, torsemide was added in 10‐mg increments to a maximum of 40 mg once daily. If needed for BP control, another non‐ACEI, non‐CCB, non‐ARB, non‐mineralocorticoid antagonist was then added.
Procedures and Measurements
BP Measurement
Sites used Omron HEM 907 BP or HME 907XL monitors (Omron IA, Santa Clara, CA) and dedicated cuffs. Three BP measurements were to be made in the nondominant arm at 2‐minute intervals after 10 minutes of rest in the sitting position, with the arm supported near chest level by an armrest. A large cuff was used for patients with an arm circumference of 33 to 42 cm. Heart rate was also measured.
Eight‐Hour Urine Collections
Patients collected two 8‐hour overnight urine samples on 2 consecutive nights prior to randomization (baseline), one at 12 and 24 weeks and two on 2 consecutive nights at 36 weeks/final visit from approximately 10 pm to 6 am for measurement of albumin, total protein, creatinine, and biomarkers.
Statistical Procedures and Analysis
Primary Efficacy Variable and Analyses
The primary outcome was percentage change in UACR from baseline to end point (week 36 or final visit). Baseline was the latest available result recorded prior to the first dose of the randomized study drug. The end point was the latest available result recorded after the first dose of the randomized study drug. UACR was calculated using the average value from two 8‐hour urine samples, except when only one sample was available. At baseline, 2 T/V and 4 B/A patients had only one urine sample. At the end point, 32 T/V and 24 B/A patients had only one urine sample, primarily because their end point value was from week 12 or 24, at which time only one collection was made. Efficacy analyses were performed on the full analysis set, containing all patients who received at least one dose of a randomized drug (T/V or B/A) and had baseline and end point efficacy assessments. This analysis included 128 T/V and 142 B/A patients (Figure 1, Table I). During run‐in, 42 T/V and 48 B/A patients had their UACR decline to <0.2 g/g based on baseline 8‐hour urine collections (eligibility was based on a screening spot urine UACR ≥0.2 g/g). We conducted a subgroup analysis excluding those with a baseline UACR <0.2 g/g.
Table I.
Baseline Characteristics
| Parameter | Treatment Group | Total (n=304) | P Valuea | |
|---|---|---|---|---|
| T/V (n=152) | B/A (n=152) | |||
| Male, No. (%) | 94 (61.84) | 102 (67.11) | 196 (64.47) | .402 |
| Race, No. (%) | ||||
| White | 113 (74.34) | 111 (73.03) | 224 (73.68) | .894 |
| Black | 31 (20.39) | 34 (22.37) | 65 (21.38) | |
| Asian | 7 (4.61) | 6 (3.95) | 13 (4.28) | |
| American Indian/Alaska Native/Other | 1 (0.66) | 1 (0.66) | 2 (0.66) | |
| Hispanic, No. (%) | 62 (40.79) | 68 (44.74) | 130 (42.76) | .562 |
| Age, yb | 61.2 (9.9) | 60.4 (11.0) | 60.8 (10.4) | .507 |
| SBP, mm Hgb | 141.4 (21.6) | 142.7 (19.2) | 142.0 (20.4) | .577 |
| DBP, mm Hgb | 77.2 (11.6) | 76.4 (12.4) | 76.8 (12.0) | .549 |
| Heart rate, beats/minb | 74.0 (11.7) | 75.6 (11.3) | 74.8 (11.5) | .232 |
| Weight, kgb,c | 94.6 (22.9) | 92.7 (19.4) | 93.7 (21.2) | .417 |
| Height, cmb | 168.5 (10.4) | 169.0 (10.5) | 168.8 (10.4) | .693 |
| UACR, g/gb,d | 0.94 (1.39) | 0.72 (0.92) | 0.83 (1.18) | .10 |
| UACR <0.2 g/g, No. (%)d | 47 (30.9) | 51 (33.6) | 98 (32.2) | .71 |
| eGFR, mL/min/1.73 m2 b,e | 58.6 (26.8) | 61.4 (28.6) | 60.0 (27.7) | .378 |
| HbA1c, %b,f | 8.0 (1.7) | 8.2 (1.7) | 8.1 (1.7) | .382 |
| Potassium, mmol/Lb | 4.6 (0.6) | 4.6 (0.5) | 4.6 (0.6) | .872 |
Abbreviations: B/A, benazepril/amlodpine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; SBP, systolic blood pressure; T/V, trandolapril/verapamil SR; UACR, urinary albumin/creatinine ratio. aFrom Fisher’s exact test for categorical measures, one‐way ANOVA for continuous measures, and Wilcoxon test for median comparisons. bMean (SD). cT/V, n=152; B/A, n=151; total, N=303. dT/V, n=149; B/A, n=152; total, N=299. eT/V, n=146; B/A, n=147; total, N=293.fT/V, n=151; B/A, n=152; total, N=303.
The sample size calculation assumed a baseline mean (SD) urinary albumin excretion rate of 600 (200) mg/d. Employing a type I error rate of 0.05 for a two‐tailed test, it was determined that 123 patients per group would provide 80% power to detect a difference of 72 mg/d (12%) in mean change from baseline to end point in albuminuria. Assuming a 20% withdrawal rate after randomization, 325 participants needed to be enrolled in order to randomize 300 patients.
Comparison analysis tests were two‐tailed. Unless otherwise specified, P values ≤.05 were considered statistically significant. Differences between randomized groups were evaluated using a two‐way analysis of covariance (ANCOVA) model with factors for treatment group and site and with baseline albuminuria as a covariate. To assess differences in treatment group effects between sites, a factor for treatment group by site interaction was added to the ANCOVA model, with 2 groupings of sites (US mainland and Puerto Rico). If the interaction term was significant at the 0.10 level, site differences were investigated further. Estimates of treatment effect were derived from the model without the interaction. To assess robustness of the primary end point analysis and account for early participant withdrawal and missing data, a mixed model was formed, assigning available data to the closest scheduled visit (baseline, week 12, week 24, week 36) and including factors for treatment group, site, and week, with a random subject effect.
Secondary Analyses
Prespecified secondary outcomes included within‐group paired t‐test analyses of absolute change in UACR and urinary protein/creatinine ratio (UPCR) from baseline to end point and absolute change in SBP, DBP, logarithm of UACR, and eGFR from baseline to weeks 12, 24, 36, and end point. Analyses of treatment differences in absolute change in UACR from baseline to end point; absolute and percentage change in UPCR, SBP, DBP, and eGFR from baseline to end point; and absolute change in SBP, DBP, logarithm of UACR, and eGFR from baseline to weeks 12, 24, and 36 were assessed using methods described for the primary efficacy variable with the baseline value of the variable as the covariate in the model.
Adverse Events
Safety analyses were performed in all patients who received at least one dose of T/V or B/A. Adverse events, laboratory data, weight, office vital signs, and 12‐lead electrocardiographic recordings were assessed throughout the study. Laboratory variables included percentage change from baseline to week 36/final visit in lipids (total cholesterol, triglycerides, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol), change in HbA1c, potassium, and fasting blood glucose levels.
Results
The study population had a mean age of 61 years, was approximately two‐thirds male, and was ethnically diverse (43% Hispanic, 22% African American; Table II). Participants were obese (mean body mass index, 33 kg/m2), with a mean HbA1c concentration of 8.1%. At baseline, mean BP was 142/77 mm Hg, mean UACR was 0.83, approximately a third of patients had a UACR <0.2 g/g, mean eGFR was 60 mL/min/1.73 m2, and 14% of patients had an eGFR <30 mL/min/1.73 m2. Baseline mean (SD) eGFR declined with age (30 [31.8] mL/min/1.73 m2; ≤50 years, 73.5 [31.8]; >50–60 years, 62.0 [28.8]; >60–70 years, 58.3 [24.6]; >70 years, 46.9 [20.2]), with significant differences between the 2 youngest groups and the 2 oldest groups (P=.01 for both comparisons). There were no significant differences between treatment groups with respect to baseline parameters.
Table II.
Absolute and Percentage Change From Baseline to End Point in UACR, UPCR, Blood Pressure, and eGFR by Treatment Groupa
| No. | Measurement | Absolute Change | Percentage Change (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | End Point Mean (SD) | Change (SE)b | Difference in the Means (SE)c | P Value | Change (SE)b | Difference in Means (SE)c | P Value | ||
| UACR, g/g | |||||||||
| T/V | 128 | 0.86 (1.27) | 0.77 (1.30) | −0.11 (0.08) | −0.028 (0.098) | .779 | 29.29 (16.96) | 20.795 (21.955) | .344 |
| B/A | 142 | 0.71 (0.91) | 0.66 (1.16) | −0.08 (0.07) | 8.49 (15.96) | ||||
| UPCR, g/g | |||||||||
| T/V | 128 | 1.31 (1.83) | 1.22 (2.14) | −0.10 (0.12) | −0.055 (0.158) | .728 | 14.31 (10.47) | 1.201 (13.556) | .929 |
| B/A | 142 | 1.09 (1.34) | 1.07 (1.82) | 0.04 (0.12) | 13.11 (9.85) | ||||
| SBP, mm Hg | |||||||||
| T/V | 149 | 141.22 (21.431) | 142.41 (20.499) | 1.50 (1.565) | 6.435 (2.059) | .002 | 2.64 (1.129) | 4.917 (1.485) | .001 |
| B/A | 151 | 142.62 (19.215) | 136.67 (18.646) | −4.93 (1.521) | −2.27 (1.097) | ||||
| DBP, mm Hg | |||||||||
| T/V | 149 | 77.22 (11.727) | 75.12 (12.511) | −1.35 (0.903) | 3.332 (1.189) | .005 | −0.48 (1.239) | 4.411 (1.632) | .007 |
| B/A | 151 | 76.32 (12.386) | 71.39 (11.838) | −4.69 (0.879) | −4.90 (1.207) | ||||
| eGFR, mL/min/1.73 m2 | |||||||||
| T/V | 143 | 59.14 (26.85) | 54.67 (26.41) | −4.76 (1.35) | −2.684 (1.751) | .126 | −4.97 (2.36) | −2.153 (3.073) | .484 |
| B/A | 149 | 61.72 (28.67) | 59.72 (32.88) | −2.08 (1.29) | −2.81 (2.26) | ||||
Abbreviations: B/A, benazepril/amlodipine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; T/V, trandolapril/verapamil SR; UACR, urinary albumin/creatinine ratio; UPCR, urinary protein/creatinine ratio. a152 participants were randomized to each study arm. The number of patients in each row above represents the number of participants in each group who received at least one dose of randomized study drug (T/V or B/A) and for whom both baseline and end point efficacy assessments were available. bAdjusted for center and baseline. cTreatment difference in means calculated as T/V minus B/A. End point = week 36/final visit.
Primary End Point
The primary end point, adjusted mean (SE) percentage change in UACR from baseline to end point, increased in both groups (T/V, 29.29% [16.96%]; B/A, 8.49% [15.96%]); the 20.80% difference between means was not statistically significant (P=.344; Table I). There were no significant differences between treatment groups using mixed models (baseline UACR as a covariate and treatment‐by‐week interaction and baseline UACR as a covariate) or the ANCOVA model with baseline eGFR, site, and baseline albuminuria value as a covariate (data not shown). A small number of patients in both groups had large percentage increases in UACR and low baseline UACR values. In a subgroup analysis excluding patients with baseline UACR <0.2 g/g, adjusted mean percentage change in UACR from baseline to end point declined in both groups (T/V, −18.88% [n=86]; B/A, −19.92% [n=94]; P=.91).
Secondary Analyses
In absolute terms, UACR decreased in both treatment groups (T/V, −0.11; B/A, −0.08) and UPCR decreased in the T/V group (−0.10); these changes were not statistically significant. Log UACR declined significantly in both groups (mean change [SD] in T/V, −0.28 [0.97]; P<.01; in B/A, −0.31 [0.92]; P<.001) beginning at week 24 for T/V and at week 12 for B/A, with no difference between treatment groups at the end point (mean [SD] in T/V −1.21 [1.42] vs in B/A −1.40 [1.45]; P=.67; Figure 2b).
Figure 2.
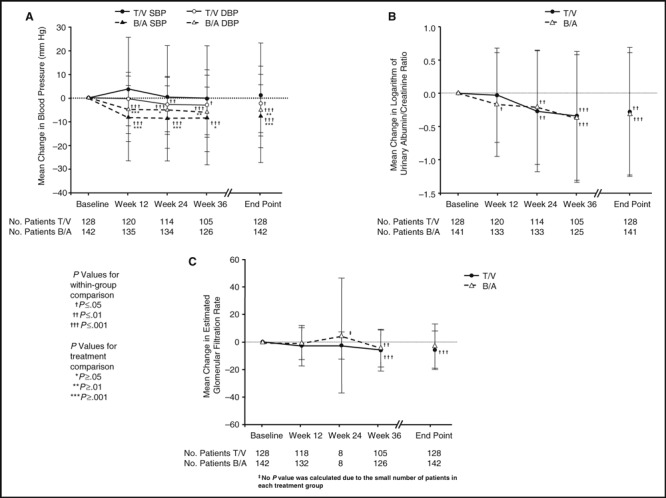
Blood pressure, log urinary albumin/creatinine ratio and estimated glomerular filtration rate by treatment group and visit (baseline; weeks 12, 24, and 36; and end point) for patients with both blood pressure and urinary albumin/creatinine ratio data. T/V indicates trandolapril/verapamil SR; B/A, benazepril/amlodipine; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Mean eGFR declined in both groups (T/V, −4.76 mL/min/1.73 m2; B/A, −2.08 mL/min/1.73 m2); the end point difference between groups was not statistically significant (Table I). There were significant decreases in eGFR for T/V at week 36 and at the end point and for B/A at week 36 (Figure 2c).
BP changes were statistically significantly different between groups, with significant reductions in DBP beginning at week 24 for T/V and in SBP and DBP for B/A beginning at week 12 (Table I, Figure 2a). End‐of‐study mean BP was 142.4/75.1 mm Hg for T/V and 136.7/71.4 mm Hg for B/A. There was no significant correlation between change from baseline to end point in UACR and change from baseline to end point in BP (r<0.2 for both SBP and DBP).
Laboratory Values
There were no significant differences in lipid parameters, fasting blood glucose levels, serum potassium concentration, or HbA1c values within or between groups at end point (data not shown), except for significant increases in high‐density lipoprotein cholesterol in both groups (T/V from 1.26 to 1.32 mmol/L; P<.001; B/A from 1.20 to 1.24 mmol/L; P<.05) and in fasting plasma glucose in the B/A group (from 8.58 to 9.34 mmol/L; P<.05). Both groups had similar rates of hypoglycemia reported as an adverse event (Table III).
Table III.
Treatment‐Emergent Adverse Events Occurring in ≥5% of Patients or in Patients Who Discontinued Due to Adverse Event(s) by Treatment Group
| No. and Percentage of Total Patients | ||
|---|---|---|
| System Organ Class Preferred Term | Occurring in ≥5% of Patients | |
| T/V (n=152) | B/A (n=152) | |
| Total any adverse event | 104 (68) | 116 (76) |
| Cardiovascular disorders | ||
| Bradycardiaa | 6 (4) | 0b |
| Peripheral edema | 9 (6) | 21 (14)c |
| Infections and infestations | ||
| Bronchitis | 7 (5) | 3 (2) |
| Upper respiratory tract infection | 8 (5) | 8 (5) |
| Urinary tract infection | 13 (9) | 11 (7) |
| Investigations | ||
| Hypoglycemia | 8 (5) | 5 (3) |
| Nervous system disorders | ||
| Dizziness | 9 (6) | 4 (3) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Cough | 8 (5) | 7 (5) |
| Vascular disorders | ||
| Hypotension | 9 (6) | 1 (1)d |
Abbreviations: B/A, benazepril/amlodipine; T/V, trandolapril/verapamil SR. Source: Adverse event case report forms. Values are expressed as No. (%). aData included due to statistically significant difference between treatment groups. b P=.03. c P=.033. d P=.019.
Adverse Events
Table III reports treatment‐emergent adverse events occurring in ≥5% of patients in a treatment group. Bradycardia and hypotension were more frequent in the T/V group, while peripheral edema was more common in the B/A group. Five (3%) T/V patients and 1 (1%) B/A patient reported constipation. The most frequently reported adverse events leading to discontinuation were bradycardia (4/152, 3%) and hypotension (4/152, 3%) in the T/V group and hyperkalemia (2/152, 1%) in the B/A group.
Medication Use
Most patients (T/V, 138/152 [91%]; B/A, 143/152 [94%]) received the maximum study drug dose. Mean (SD) treatment duration was 206.1 (89.6) days for T/V and 228.9 (67.5) days for B/A. The proportion of patients taking study medication at each visit was similar between groups (data not shown). Protocol‐allowed antihypertensive medications were used by 121/152 T/V (80%) and 111/152 B/A (73%) patients. Mean number of protocol‐allowed antihypertensive drugs per patient in addition to the randomized study drug was similar between treatment groups (mean [SE]: T/V, 1.6 [0.087]; B/A, 1.5 [0.070]). Five patients (3.3%) in each group used hydrochlorothiazide. More patients used clonidine hydrochloride in the T/V group (17 [11.2%]) than in B/A group [7 [4.6%]).
Discussion
The study hypothesis, that T/V was superior to B/A for reducing albuminuria, was not supported by the primary end point (adjusted percentage change from baseline in UACR). However, other end points provide internally consistent evidence that both treatments reduced albuminuria, with a significant reduction in log UACR in both groups (Figure 2B) and significant reductions in UACR in both groups when patients with a low baseline UACR are excluded. These results suggest that FDCs of an ACEI and a DHP or NDHP CCB can effectively and safely reduce albuminuria and control BP in a multiethnic cohort of hypertensive type 2 diabetics with persistent albuminuria. Despite better BP control in individuals taking the ACEI benazepril and the DHP CCB amlodipine, there was no significant difference in reduction in albuminuria. On average, the BP goal of 130/80 mm Hg, recommended for renal protection, was not reached in either group. 17 Average DBP, which was below goal at baseline (76.8 mm Hg), was reduced to a similar extent in both groups. However, SBP was only reduced in the B/A group, and in that group it was still above goal at the end point (136.7 mm Hg). These results may indicate the difficulty of achieving target SBP in patients who had controlled DBP following an active, as opposed to a placebo, run‐in period.
We found no significant association between change in BP and change in UACR. This, along with the finding that albuminuria was lowered with T/V, although there was no significant reduction in SBP, suggests that some mechanism other than BP reduction may have been involved in the T/V group. This is consistent with a previous study in type 2 diabetics with nephropathy in which diltiazem was superior to nifedipine in reducing albuminuria despite similar BP lowering. 16 We did not perform ambulatory BP measurements, so we cannot exclude the possibility that time‐averaged BP in the T/V group was lower than could be detected at clinic visits. Our findings suggest that adding an NDHP CCB to an ACEI‐based regimen in type 2 diabetic patients can be effective for lowering residual albuminuria with or without a significant reduction in SBP.
This is the first multicenter trial in type 2 diabetics with nephropathy to directly assess the differential effects of DHP and NDHP CCBs when given with an RAAS inhibitor. Unique features of this study design include standardized doses of lisinopril and torsemide during the run‐in (agents recommended by guidelines as first and second steps for diabetics with nephropathy and hypertension) and subsequent randomization to an ACEI/CCB regimen that provides a direct head‐to‐head comparison of different classes of CCBs on the background of an ACEI and a loop diuretic. The study population was a multiethnic cohort, nearly half of whom were Hispanic. Due to the relatively small size of the study, we could not detect differences in outcomes by ethnicity. Studies in type 2 diabetics have shown that ARB effects on albuminuria and outcome are similar among racial and ethnic groups. 18 Our findings have broad application to the population of type 2 diabetics with hypertension and persistent albuminuria despite ACEI and diuretic treatment, inasmuch as a high percentage of such patients require ≥3 antihypertensive agents to achieve adequate BP control as supported by clinical trials and suggested by guidelines. 6 , 7 , 12 , 17
Previous studies demonstrating differential effects of these classes of CCBs were not conducted on the background of ACEI therapy. 15 , 16 , 19 , 20 , 21 In a small study in 37 patients with diabetic nephropathy, reduction of proteinuria was greater with T/V (62%) compared with either agent alone (33% and 27%, respectively). 22 The PROCOPA study found a greater reduction in proteinuria with T/V (49%) compared with verapamil (1%) or trandolapril (41%) alone in 119 patients with nephropathy of various etiologies. 23 However, in 69 patients with nondiabetic nephropathy, adding verapamil or amlodipine to trandolapril therapy did not confer additional proteinuria reduction. 24
Studies with amlodipine have generally not demonstrated renoprotective effects in patients with chronic kidney disease, including diabetic nephropathy. In the African American Study of Kidney Disease and Hypertension (AASK) trial in 1094 African Americans with hypertensive renal disease, proteinuria increased by 58% in the amlodipine group, compared with a 20% reduction in the ramipril group. 25 In the Irbesartan in Diabetic Nephropathy Trial (IDNT) in 1715 patients with hypertension and diabetic nephropathy, mean reduction of proteinuria was 6% in the amlodipine group compared with 10% with placebo and 33% with irbesartan. 12
Our study has several limitations. First, similar levels of BP control were not achieved as planned; thus, differences between groups with respect to change in UACR could have resulted from differences in BP lowering. Still, the fact that there was not a greater decline in albuminuria in the B/A group suggests that BP lowering does not explain our findings or that the degree of BP lowering for the levels tested (ie, 142/77 mm Hg) do not make a difference. This might not have been noted if initial BP levels had been higher and the degree of BP lowering was greater. Second, the discontinuation rate was higher in the T/V compared with the B/A group; this combined with the variability in UACR at the time of randomization and the potential for increased variability in UACR among patients with only one 8‐hour urine collection limited our ability to identify significant differences between treatment arms, even though the results were adjusted for baseline and site. Third, approximately one‐third of patients had a baseline UACR below the 0.2 g/g screening criterion (measured by 8‐hour urine collection) at the end of the run‐in. Some of these patients had relatively large percentage changes in UACR during the study; this influenced the direction of the primary end point. Our subgroup analysis indicated that after excluding these patients, both FDC arms were associated with percentage and absolute value decreases in UACR. Fourth, when designing this trial we may not have fully understood the treatment effect size in such an ethnically and racially diverse population with large variations in kidney function; consequently, the study may have been underpowered. Fifth, our study design employed lisinopril and torsemide during the run‐in, with the intent that a common ACEI would minimize any confounding effect introduced by different ACEI strategies. It is possible that differences in response to trandolapril and benazepril in this population could have influenced subsequent changes in UACR and may have masked differences in outcomes between the CCBs. Finally, as noted, the relatively low baseline BP may have blunted the impact of treatment on albuminuria, which might be more pronounced in patients with higher BP levels.
Conclusions
We did not demonstrate that the FDC of an ACEI and an NDHP CCB was superior to the FDC of an ACEI and a DHP CCB for reducing albuminuria in hypertensive type 2 diabetics with nephropathy. However, secondary analyses provide evidence that both treatments reduced albuminuria. For the FDC of T/V, this effect appears to be independent of BP reduction in patients with only slightly elevated BP at baseline. There was no difference in the magnitude of reduction in albuminuria between the treatment regimens, and both regimens were relatively well tolerated. This study suggests that administration of FDCs of an ACEI with either an NDHP CCB or a DHP CCB can effectively reduce albuminuria in type 2 diabetics with hypertension and nephropathy.
Investigators for the TANDEM Study: R. Toto (Coordinating Investigator), H. Abboud, R. Albery, M. Allende‐Vigo, M. W. Anderson, J. S. Aponte, G. M. Argoud, A. Awad, D. Bahri‐Jovet, G. L. Bakris, M. O. Belladonne, J. E. Benabe, G. M. Benchimol, J. Blondin, J. M. Bloom, H. M. Brodsky, R. Broughton, C. L. Brown, E. Camilo Vazquez, J. L. Cangiano, A. A. Carr, H. K. Cathcart, L. Catoni, H. Chandna, F. Charles, R. S. Cherlin, D. G. Cheung, G. Colon Vega, D. J. Connito, W. C. Cushman, J. M. Dimen, F. Eelani, R. O. Estacio, S. Z. Fadem, T. C. Fagan, D. B. Fischer, D. Fitz‐Patrick, J. M. Flack, T. W. B. Gehr, E. Gonzalez‐Ortiz, J. E. Greenwald, K. V. Hackshaw, J. Hone, M. U. Jesrani, T. J. Jones, K. S. Kant, W. A. Kaye, H. J. Kerstein, G. C. Kim, M. S. Kipnes, M. J. Kozinn, L. M. Lehrner, A. J. Lewin, M. L. Lynn, A. T. Maddux, J. Martinez Mendez, J. B. McGill, R. H. Miles, K. B. Mills, R. E. Mills, C. S. Monder, F. Montilla Rivera, J. M. Neutel, S. T. Ong, J. Otero‐Martinez, F. Ovalle, E. Perez‐Bailon, J. Prasad, L. M. Prisant, H. A. Punzi, L. Quesada‐Suarez, R. M. Raja, E. Reisin, D. G. Robertson, J. B. Rosen, J. G. Rothman, L. R. Ruiz Rivera, M. E. Rush, G. T. Serfer, D. Shah, E. M. Skobeloff, J. M. Soba Nouel, M. Sosa‐Padilla, C. M. Sotolongo, D. H. Sugimoto, Z. Talor, A. A. Taylor, I. Teitelbaum, K. E. Tse, N. M. Unger, J. Uribarri, F. M. Vazquez‐Roura, J. B. Vazquez‐Tanus, G. D. Yeoman, E. T. Zawada Jr, and S. Zeig.
Acknowledgments
Acknowledgments: This study was supported by grants from Abbott and the National Institute of Digestive Diabetes and Kidney Disease (grant 5K24DK002818‐07).
References
- 1. Rossing P. Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history. Curr Diab Rep. 2006;6:479–483. [DOI] [PubMed] [Google Scholar]
- 2. Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16(suppl 1):S30–S33. [DOI] [PubMed] [Google Scholar]
- 3. Adler S. Diabetic nephropathy: linking histology, cell biology, and genetics. Kidney Int. 2004;66:2095–2106. [DOI] [PubMed] [Google Scholar]
- 4. Andersen S, Tarnow L, Rossing P, et al. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;57:601–606. [DOI] [PubMed] [Google Scholar]
- 5. Anderson S. Renal effects of converting enzyme inhibitors in hypertension and diabetes. J Cardiovasc Pharmacol. 1990;15(suppl 3):S11–S15. [DOI] [PubMed] [Google Scholar]
- 6. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. [DOI] [PubMed] [Google Scholar]
- 7. Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 8. Epstein M. Hypertension as a risk factor for progression of chronic renal disease. Blood Press Suppl. 1994;1:23–28. [PubMed] [Google Scholar]
- 9. Estacio RO, Jeffers BW, Gifford N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(suppl 2):B54–B64. [PubMed] [Google Scholar]
- 10. Garg J, Bakris GL. Angiotensin converting enzyme inhibitors or angiotensin receptor blockers in nephropathy from type 2 diabetes. Curr Hypertens Rep. 2002;4:185–190. [DOI] [PubMed] [Google Scholar]
- 11. Lewis EJ, Hunsicker L, Bain R, et al. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. [DOI] [PubMed] [Google Scholar]
- 12. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. [DOI] [PubMed] [Google Scholar]
- 13. UKPDS group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 14. Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. Erratum in: JAMA. 2006;295:2726. [DOI] [PubMed] [Google Scholar]
- 15. Bakris GL, Weir MR, Secic M, et al. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004;65:1991–2002. [DOI] [PubMed] [Google Scholar]
- 16. Smith AC, Toto R, Bakris GL. Differential effects of calcium channel blockers on size selectivity of proteinuria in diabetic glomerulopathy. Kidney Int. 1998;54:889–896. [DOI] [PubMed] [Google Scholar]
- 17. Kidney Disease Outcomes Quality Initiative clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;42(2, suppl 2):S1–S179. [Google Scholar]
- 18. De Zeeuw D, Ramjit D, Zhang Z, et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int. 2006;69:1675–1682. [DOI] [PubMed] [Google Scholar]
- 19. DeMarie BK, Bakris G. Effects of different calcium antagonists on proteinuria associated with diabetes mellitus. Ann Int Med. 1990;113:987–988. [DOI] [PubMed] [Google Scholar]
- 20. Bakris GL. Effects of diltiazem or lisinopril on massive proteinuria associated with diabetes mellitus. Ann Intern Med. 1990;112:707–708. [DOI] [PubMed] [Google Scholar]
- 21. Gashti CN, Bakris GL. The role of calcium antagonists in chronic kidney disease. Curr Opin Nephrol Hypertens. 2004;13:155–161. [DOI] [PubMed] [Google Scholar]
- 22. Bakris GL, Weir MR, DeQuattro V, et al. Effects of an ACE inhibitor/calcium antagonist combination on proteinuria in diabetic nephropathy. Kidney Int. 1998;54:1283–1289. [DOI] [PubMed] [Google Scholar]
- 23. PROCOPA Study Group . Dissociation between blood pressure reduction and fall in proteinuria in primary renal disease: a randomized double‐blind trial. J Hypertens. 2002;20:729–737. [DOI] [PubMed] [Google Scholar]
- 24. Boero R, Rollino C, Massara C, et al. The verapamil versus amlodipine in nondiabetic nephropathies treated with trandolapril (VVANNTT) study. Am J Kidney Dis. 2003;42:67–75. [DOI] [PubMed] [Google Scholar]
- 25. Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril versus amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. [DOI] [PubMed] [Google Scholar]


