Abstract
This retrospective study of office and hospital electronic medical records from June 1991 to June 2007 examines the occurrence of severe blood pressure (BP) elevation (>180/110 mm Hg) and the subsequent risk of cardiovascular events in a diverse set of primary care practices. A total of 18,747 patients were categorized according to BP using 3 methodologies based on the highest historical value, the first recorded value, and time‐averaged antecedent values. During the follow‐up period (median 3.8 years) there were 949 cardiovascular events and 80 cardiovascular‐related deaths. Severe BP elevation occurred in 1566 (8.4%) patients. The age‐adjusted incidence of cardiovascular events per 1000 patient‐years was 5.9 in the normal BP group, 10.1 in the mild group, 15.1 in the moderate group, and 25.0 in the severe group. An episode of severe BP elevation is common in primary care practice and is associated with substantial excess cardiovascular morbidity.
Currently more than 72 million adults in the United States are hypertensive, 1 and it is estimated that 9 of 10 older adults will develop hypertension in their lifetime 2 in what is the most rapidly growing segment of the US population. 3 Blood pressure (BP) elevation is a well‐described risk factor for cardiovascular disease (CVD) including stroke, heart failure, coronary heart disease (CHD), atrial fibrillation, and peripheral vascular disease (PVD). Epidemiologic studies have established that this risk is continuous, graded, and independent of other risk factors. 4 , 5 , 6 , 7 , 8 Population studies and randomized clinical trials have, however, typically focused on single events and employed narrow definitions of CVD when identifying outcomes. Although this approach has served to elucidate the role of BP in CVD, it may also result in underestimation of the disease burden seen in the typical primary care practice. In addition, there is little information available to guide clinicians in estimating CVD risk when faced with a specific patient followed longitudinally for BP elevation. BP can vary substantially over the course of time and even within a single day. 9 The use of the current BP to assign risk ignores the potential cumulative impact implied by past measurements. In one study, it was found to be inferior to the averaging of past BPs. 10 As a result, in actual clinical practice, it remains unclear which BP measurement or set of measurements provides the best estimate of CVD risk in a given patient.
This study examines a large diverse population of primary care patients without known pre‐existing CVD followed for up to 15 years using an electronic medical record (EMR). Several approaches to BP classification, based on the historical measurements, are compared and the evaluation of clinical outcomes includes a more inclusive definition of CVD as well as multiple events per individual.
Methods
Study Setting
The Christiana Care Health System (CCHS) is a large health care system located in northern Delaware providing 80% of acute care services to this region. It encompasses 2 hospitals and a number of primary care practices serving a diverse patient population. Six practices providing adult care by internal medicine and family practice physicians were chosen for inclusion in this study based on their use of the office EMR over an extended period of time. One‐third of these practices were involved in resident training, and one‐third were located in an urban setting.
Study Design
This retrospective observational analysis was limited to the period from June 1991 through May 2007. Participants were 18 years or older at study onset and had at least 2 office visits. All demographics, BP measurements, problem list entries, and laboratory values were extracted from the office clinical record. The research use of this database was approved by the CCHS institutional review board and procedures were followed in accordance with institutional guidelines.
At each office visit, the initial BP was typically obtained in a seated position by a nursing assistant using an automated sphygmomanometer. Up to 2 BPs could be recorded on any single visit, the second measurement often being performed by a physician using a manual sphygmomanometer. If 2 BPs were recorded during a visit, they were averaged into a single systolic and diastolic value for the purposes of this analysis. These BP recordings were then categorized as normal if the systolic BP was <140 mm Hg and the diastolic BP was <90 mm Hg. If the BP was elevated, each measurement was categorized as mild (140–159/90–99 mm Hg), moderate (160–179/100–109 mm Hg), or severe (≥180/110 mm Hg). When systolic and diastolic BPs fell into different categories, the higher category was selected for the purposes of classification.
Patient Assignment to BP Category
Patients were assigned to the BP category equal to the highest BP classification, and the patient’s index or start date set as the date of the first occurrence of this maximum BP class. For purposes of comparison, patients were also categorized using their first recorded BP and their time‐averaged BP’s recorded antecedent to the index date. Because the focus of this study was on the impact of BP elevation rather than the effectiveness of a specific treatment, the use of antihypertensive agents was not considered when assigning patients to a BP group.
Serum creatinine and hemoglobin A1c (HbA1c) values prior to and up to 18 months after the index date were extracted. The estimated glomerular filtration rate was calculated using the modified diet for renal disease equation, 11 and patients with an estimated glomerular filtration rate <15 mL/min/1.73 m2 were excluded from further analysis. Patients were classified as nondiabetic or diabetic with HbA1c <6%, HbA1c 6% to 6.9%, HbA1c 7% to 8.9%, or HbA1c≥9%. Patients with hyperlipidemia recorded in the EMR on or before the index date were classified “with hyperlipidemia.”
Outcomes
CVD outcomes were identified using principle International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes associated with hospital and emergency department records. The primary outcome of interest, established a priori, was the time to the occurrence of any of the following: stroke, acute myocardial infarction (AMI), heart failure, CHD, cardiac dysrhythmia (PVD), and deaths related to these events. In addition, hypertensive urgencies were also evaluated but not included in the combined CVD outcome or its analysis. To avoid counting revisits as distinct clinical events, visits with the same principle diagnosis occurring within 30 days of each other were considered a single event. Patients with a history of CVD indicated on their office problem list, as well as those with CVD events occurring prior to their index date, were excluded from further analysis. Figure 1 describes the application of these inclusion and exclusion criteria. To validate the use of CCHS administrative data in identifying CVD outcomes, all AMI admissions and a random sample from within each remaining event type were selected for detailed chart review by a physician.
Figure 1.
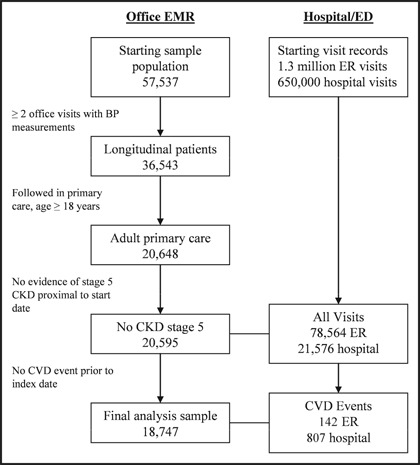
Criteria for sample selection. The final analysis sample comprises 32.5% of the patients with records in the office electronic medical record (EMR) and 90.8% of the primary care population. BP indicates blood pressure; CKD, chronic kidney disease; CVD, cardiovascular disease; ED, emergency department; ER, emergency room.
Statistical Analysis
The chi‐square statistic was used to compare proportions, the Kruskal‐Wallis test was conducted to compare means and medians, and the Bonferroni correction procedure was used for all pair‐wise comparisons. Poisson and negative binomial regression models were used to adjust observed incidence rates for age. The cumulative incidence of first and multiple cardiovascular events for each BP category was obtained by the Kaplan‐Meier method, 12 and differences among BP categories were evaluated using the log‐rank test. Time‐to‐event was measured in years from the date of index until the date of cardiovascular event or censoring. Hazards ratios (HRs) were estimated from the Cox proportional hazards models 13 and adjusted for age, sex, race, diabetes status and control, hyperlipidemia, and BP category at the index date. The proportional hazard assumption was assessed for each covariate and trend tests were performed by using the median value of each BP category as a continuous variable in a proportional hazards model.
A conditional multiple event technique was applied to account for multiple cardiovascular events and the Prentice–Williams–Peterson multiplicative hazards model 14 was used to estimate HRs. Stata version 9 (Stata Corporation, College Station, TX), SAS version 9.1 (SAS Institute, Cary, NC), and SPSS version 15.0 (SPSS Inc, Chicago, IL) were used for statistical analyses.
Results
Characteristics of Study Participants
Complete follow‐up data were obtained on 18,747 patients, with 197,036 office visits and 210,271 BP measurements collected over a median of 5.2 years (range, 15.9 years). Systolic and diastolic BPs were averaged for the 13,235 office visits with 2 recorded BPs. The mean age was 42.7 years (standard deviation, 16.4), with 40% male (n=7491), 28.1% African American (n=5261), 11.5% diabetic (n=2160), and 25.2% with a diagnosis of hyperlipidemia (n=4725). BP measurements were classified as normal in 72.7% (n=143,336), mild in 19.3% (n=37,979), moderate in 6.0% (n=11,772), and severe in 2.0% (n=3,949). Patients categorized with elevated BP (n=9446) reached their index level within a median of 4.9 months of their first visit. Nearly one‐third of these patients (32.8%, n=3098) reached their maximum BP class on their first visit and an additional 19.7% (n=1857) did so within 6 months. Patients were followed for a median of 3.8 years (range, 15.2 years) after index, contributing a total of 75,099 person‐years to the follow‐up period. Overall, severe hypertension was relatively common, with 8.4% of the population reaching BPs ≥180/110 mm Hg. Table I compares these population characteristics across BP categories.
Table I.
Population Characteristics by Maximum BP Category
| Patient BP Category | |||||
|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | ||
| (<140/90 mm Hg) | (140–159/90–99 mm Hg) | (160–179/100–109 mm Hg) | (≥180/110 mm Hg) | P Value | |
| Patients, No. | 9301 (49.6) | 5098 (27.2) | 2782 (14.8) | 1566 (8.4) | |
| Age (±SD), y | 35.9 (±13.6) | 45.6 (±15.7) | 52.5 (±15.4) | 56.8 (±15.7) | <.001 |
| Male sex, No. (%) | 3347 (36.0) | 2405 (47.2) | 1168 (42.0) | 571 (36.5) | <.001a |
| Black race, No. (%) | 2048 (22.0) | 1448 (28.4) | 990 (35.6) | 775 (49.5) | <.001 |
| Diabetic, No. (%) | 309 (4.4) | 678 (13.3) | 587 (21.1) | 486 (31.0) | <.001 |
| Diabetic control, No. (%) | |||||
| HbA1c <6.0% | 74 (18.1) | 63 (9.3) | 55 (9.4) | 45 (9.3) | <.001 |
| HbA1c 6.0–6.9% | 115 (28.1) | 236 (34.8) | 187 (31.9) | 156 (32.1) | NSb |
| HbA1c 7.0–8.9% | 97 (23.7) | 195 (28.8) | 202 (34.4) | 143 (29.4) | .004 |
| HbA1c≥9.0% | 123 (30.1) | 184 (27.1) | 143 (24.4) | 142 (29.2) | NS |
| Hyperlipidemia | 1178 (12.7) | 1534 (30.1) | 1240 (44.6) | 773 (49.4) | <.001 |
| Months to index BP, median (IQR) | 0 | 3.0 (0, 18.4) | 7.5 (0, 28.1) | 8.0 (0, 31.6) | <.001c |
| Years follow‐up, median (IQR) | 3.7 (1.5, 6.3) | 3.8 (1.6, 6.1) | 4.1 (1.8, 6.2) | 4.3 (2.0, 6.2) | <.001d |
| Cardiovascular disease events, No. (%) | 131 (1.4) | 221 (4.3) | 261 (9.4) | 336 (21.5) | <.001 |
Abbreviations: BP, blood pressure; HbA1c, hemoglobin A1c; IQR, interquartile range; SD, standard deviation. aNormal, severe<moderate<mild. bNot significant (NS) after Bonferroni correction. cMild<moderate, severe. dNormal, mild<moderate, severe.
Outcomes
Prior to the application of the exclusion criteria, 78,564 emergency visits, 21,576 hospital admissions, and 1230 potential CVD events were identified in this population. Chart review was performed on a random sample of 431 (35.0%) of these encounters and 408 (94.7%) were correctly identified (Table II). After removing the misclassified events and applying exclusions, there remained 949 CVD events (142 emergency department visits and 807 hospitalizations) in the follow‐up period, with 685 (72.2%) of these occurring as first events and 80 CVD‐related deaths. Stroke/transient ischemic attack contributed the greatest proportion of events (23.7%, n=225) followed by heart failure (20.2%, n=192), dysrhythmias (18.2%, n=173), CHD (16.9%, n=160), AMI (14.6%, n=139), and PVD (6.3%, n=60). The majority of dysrhythmias were atrial fibrillation (n=92, 53.2%); however, 41 fatal events (23.7%) within this class were coded as cardiac arrest and comprised more than one‐half of the CVD‐related deaths in this population. Only 9 (0.9%) CVD events occurred on the same day as the index office visit. There were also 199 hypertensive urgencies, with 35.2% (n=70) resulting in hospitalization.
Table II.
Validation of Cardiovascular Disease Events
| Validated, No. (% of Sample) | |||
| Event Type | False Positives | True Positives | Sample size a No. (%) |
| Stroke/TIA | 5 (6.5) | 72 (93.5) | 77 (30.1) |
| Heart failure | 5 (6.7) | 70 (93.3) | 75 (30.2) |
| AMI | 7 (4.5) | 149 (95.5) | 156 (100) |
| CHD | 2 (5.6) | 34 (94.4) | 36 (20.3) |
| Dysrhythmia | 3 (4.5) | 64 (95.5) | 67 (20.4) |
| PVD | 1 (5.0) | 19 (95.0) | 20 (30.7) |
| CVD events | 23 (5.3) | 408 (94.7) | 431 (35.0) |
Abbreviations: AMI, acute myocardial infarction; CHD, coronary heart disease; CVD, cardiovascular disease; PVD, peripheral vascular disease; TIA, transient ischemic attack.
aNumber and percent within event type.
Risk of First CVD Events
The median time from the index date to the first CVD event was 3.6 years (interquartile range, 1.5–6.2; range 15.6 years). These events became increasingly more common as BP category became more severe (P<.001). The cumulative incidence of CVD events (Figure 2) in the severe category was 25.3% at 7 years (95% confidence interval [CI], 22.4%–28.2%) while that for moderate was 10.2% (95% CI, 8.7%–11.7%), mild was 5.6% (95% CI, 4.7%–6.5%), and normal was 1.8% (95% CI, 1.4%–2.2%).
Figure 2.
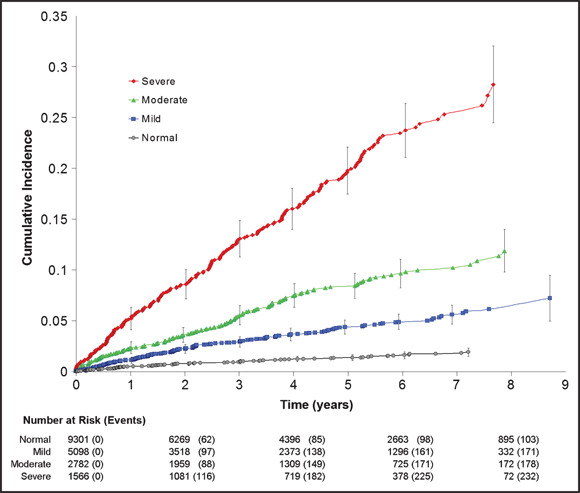
Cumulative incidence of first cardiovascular disease events according to blood pressure (BP) category at index visit. Vertical bars indicate the 95% confidence intervals. Normal BP is <140/90 mm Hg, mild elevation is 140–159/90–99 mm Hg, moderate elevation is 160–179/100–109 mm Hg, and severe elevation is ≥180/110 mm Hg. Markers indicate an event.
In multivariate analyses using Cox regression, adjusting for age, sex, race, diabetes and diabetic control, hyperlipidemia, and maximum BP categorization, there were significant differences in event‐free survival among the 4 BP categories, with, as expected, increasing severity of BP having progressively greater risk of CVD events (Table III). The trend tests exhibited a stepwise increase in the risk from one BP category to the next higher category (P<.001). When the time followed prior to index was included as an adjustment there was no increase in risk associated with this variable or significant change in the HRs of the BP categoric variables. When this BP was analyzed as a continuous variable there was a 15% increase in risk associated with every 10‐mm Hg increase in systolic BP (HR, 1.15; 95% CI, 1.10%–1.19%) and a 10% increase in risk associated with every 10‐mm Hg increase in diastolic BP (HR, 1.10; 95% CI, 1.05%–1.17%). In addition, there was no difference in c‐index when comparing models using index BP as a continuous variable vs a categoric variable (HR 0.7; 95% CI, 0.67%–0.71%).
Table III.
Risk of First CVD Event by Maximum BP Categorya
| Hazard Ratio | 95.0% CI (Lower, Upper) | P Value | |
|---|---|---|---|
| Age (10 y) | 1.57 | (1.50, 1.65) | <.001 |
| Male sex | 1.29 | (1.11, 1.51) | .001 |
| Black race | 1.30 | (1.11, 1.53) | .001 |
| Diabetes | |||
| HbA1c <6.0% | .81 | (0.44, 1.48) | .492 |
| HbA1c 6.0%–6.9% | 1.51 | (1.16, 1.96) | .002 |
| HbA1c 7.0%–8.9% | 1.88 | (1.46, 2.43) | <.001 |
| HbA1c≥9.0% | 3.03 | (2.37, 3.87) | <.001 |
| Hyperlipidemia | 1.17 | (1.01, 1.39) | .037 |
| BP category | |||
| Mild | 1.66 | (1.29, 2.14) | <.001 |
| Moderate | 2.13 | (1.64, 2.77) | <.001 |
| Severe | 3.89 | (2.99, 5.07) | <.001 |
Abbreviations: HbA1c, hemoglobin A1c; CI, confidence interval; CVD, cardiovascular disease. aAdjusted for age, sex, race, diabetic status, and hyperlipidemia. Reference category for diabetes is nondiabetic and reference for blood pressure (BP) category is normal.
The HRs obtained when substituting first BP and time‐averaged antecedent BP for the maximum BP classification in the Cox regression demonstrate qualitatively similar results for these models (Figure 3). The maximum BP classifier when compared with the other models, however, consistently assigned higher risk across BP categories, with the greatest difference occurring in the severe category. This figure also demonstrates the HR obtained in a fourth model using the maximum BP classification while adjusting for time‐averaged antecedent systolic and diastolic BPs. The maximum BP classifier remains consistent in its assignment of risk across BP categories and continues to contribute significantly to the model even when adjusting for prior BP measurements.
Figure 3.
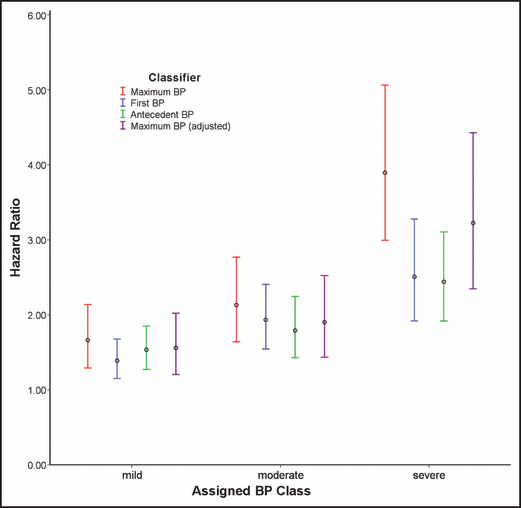
Blood pressure (BP) classification methodology and associated cardiovascular disease risk. Hazard ratios are compared across BP categories obtained using 3 approaches to classification while adjusting for age, sex, race, diabetes, and hyperlipidemia. Maximum BP assigns categories according to the highest BP achieved historically: First BP according to the first BP recorded on the initial office visit, and Antecedent BP according to the time‐averaged BPs recorded prior to the index date. The fourth model, Maximum BP (adjusted), adjusts for the time‐averaged systolic and diastolic BPs recorded prior to the index date. Normal BP serves as the reference category for all models. Vertical bars indicate 95% confidence intervals.
Multiple CVD Events
Given that nearly 30% of these events were recurrent, incidence rates were calculated and multiple‐event survival analyses were conducted to examine the risk of multiple CVD events in the study population. The observed and age‐adjusted incidence rates per 1000 patient‐years for each cardiovascular event type other than death and dysrhythmia were significantly greater (P<.001) in the moderate and severe BP categories (Table IV). The incidence rate of CVD for patients with severe BP elevation was two‐thirds greater than moderate and nearly 2.5 times that of mild BP elevation.
Table IV.
Incidence of Cardiovascular Disease Events per 1000 Patient‐Yearsa
| Blood Pressure Category | |||||
|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | P Value b | |
| All CVD | 3.6 (5.9) | 10.9 (10.1) | 22.8 (15.1) | 51.4 (25.0) | <.001 |
| Major CVDe | 1.6 (2.8) | 5.9 (5.5) | 13.4 (8.67) | 34.3 (15.5) | <.001 |
| Stroke | 0.8 (1.3) | 2.2 (2.0) | 4.4 (2.9) | 15.6 (7.2) | <.001 |
| Heart failure | 0.4 (0.7) | 1.8 (1.7) | 5.9 (3.7) | 11.3 (5.0) | <.001 |
| AMI | 0.4 (0.8) | 1.9 (1.8) | 3.2 (2.0) | 7.4 (3.3) | <.001 |
| CHD | 0.7 (1.0) | 2.2 (2.0) | 3.8 (2.7) | 7.2 (3.8) | <.001 |
| Dysrhythmia | 1.0 (1.7) | 2.4 (2.1) | 3.4 (2.3) | 7.2 (3.5) | .002c |
| PVD | 0.2 (0.4) | 0.4 (0.4) | 2.3 (1.5) | 2.8 (1.3) | <.001d |
| CVD death | 0.46 (0.78) | 0.84 (0.78) | 1.84 (1.2) | 3.83 (1.76) | NS |
| All deaths | 1.84 (3.25) | 3.47 (3.19) | 7.79 (5.02) | 16.38 (7.55) | <.001d |
| Urgent HTN | 0.2 (0.2) | 1.3 (1.3) | 3.4 (2.5) | 19.4 (11.0) | <.001 |
Abbreviations: CHD, congestive heart disease; HTN, hypertension; PVD, peripheral vascular disease. aRates observed (adjusted for age). bSignificance level adjusted for all pair‐wise comparisons within a row using the Bonferroni correction (NS, not significant). cNormal<severe. dNormal, mild<moderate, severe. eMajor cardiovascular disease (CVD) includes stroke, heart failure, acute myocardial infarction (AMI).
In time‐to‐event analysis, the cumulative incidence of multiple CVD events in the severe category was 30.2% at 7 years (95% CI, 27.0%–32.4%), while that for moderate was 13.4% (95% CI, 11.7%–14.7%), mild 6.7% (95% CI, 5.6%–7.4%), and normal 2.2% (95% CI, 1.7%–2.5%). These differences were significant across BP category (P<.001). After adjusting for the same factors as for the first cardiovascular event, there were again significant differences in event‐free survival among the 4 BP categories, with severe BP having the highest risk of CVD events. As compared with reference group of normal, the adjusted HR for mild, moderate, and severe categories were 1.66 (CI, 1.33–2.07; P<.001), 2.36 (CI, 1.88–2.96; P<.001), and 3.92 (CI, 3.12–4.93; P<.001), respectively.
Discussion
In these primary care practices, the risk of first and multiple CVD events increased significantly and incrementally as the BP category increased in severity. This risk, however, was most extreme in those patients with a history of severely elevated BP (>180/110 mm Hg on at least 1 occasion) with an adjusted incidence rate two‐thirds greater than moderate (160–179/100–109 mm Hg) and over 4 times that of normal BPs. Furthermore, these relationships remained consistent when adjusted for age, sex, race, diabetes and diabetic control, and hyperlipidemia in the Cox models for first and multiple events. A significant segment of our practice population was classified as severe. Although the overall probability of recording a severely elevated BP on any given visit was relatively small (2.0%), 8.4% of our population at some point in time reached this category, placing a significant portion of our population at the most severe risk for cardiovascular events. Fully 24% of our population were classified as having moderate to severe BP elevation in contrast to the 12% to 20% described in studies of the general population. 15 , 16
Approach to BP Classification
We hypothesized that categorization based on the historically most severe BP would function as a superior predictor of CVD risk than a single initial BP measurement, while still being relatively simple to apply in actual clinical practice. When considering barriers to adequate BP control, it is likely that an episode of severe BP elevation represents more than just a single physiologic measurement, but, instead, an extreme case of failure across a combination of factors including significant underlying disease, limited access to care, erratic adherence to therapy, or failure of the physicians to adequately monitor BP and therapy. Many of these factors are exceedingly difficult to measure in clinical practice; however, the net result, severe hypertension, is easily measured and may reflect a synergistic negative impact of multiple factors on CVD risk. Our analyses comparing risk estimates using these different approaches to classification demonstrate qualitatively similar results, regardless of the classification scheme employed. Quantitatively speaking, however, use of the first BP or time‐averaged antecedent BPs produces similar risk estimates in the mild and moderate categories, but underestimates risk in the severe category when compared with the maximum BP classification. In addition, the maximum BP‐based classifier continues to contribute significantly to risk estimates even when including prior BP measurements in the model. Furthermore, this risk status is established relatively quickly, within 1 year of follow‐up, making this categorization scheme both clinically meaningful and practical in its application to primary care practice.
Strengths and Limitations
This study provides a unique perspective into clinical practice using an EMR in place for over a decade and linked across office and hospital settings. The resulting large practice‐based sample drawn from a demographically diverse population strengthens this investigation. The overall 94.7% positive predictive value for identifying CVD events seen in our database compares well with that seen in other studies and reduces concerns over misidentification of CVD events. 17 , 18 , 19 The decision to base event identification solely on principle discharge diagnosis, however, likely results in conservative estimates of disease outcomes. In addition, the limitation of this study to a single health care provider, despite its regional dominance, almost certainly underestimates the rate of CVD events. This investigation also fails to account for the impact of subsequent BP control. It may be that patients categorized as severe who then become well controlled can reduce their CVD risk. This has been demonstrated repeatedly in clinical studies. This would then place the importance of this single measurement in the greater context of subsequent management and control and is an important area for future study.
Conclusions
This investigation supports previous findings demonstrating a continuous and graded increase in CVD risk associated with BP elevation. In addition, there appears to be considerable risk associated with even a single severe BP elevation (>180/110 mm Hg). The consequences in terms of excess CVD morbidity and mortality for a typical primary care practice are substantial given the relatively frequent appearance of these more severe BP elevations in this setting. These results highlight both the prognostic importance of a severely elevated BP measured in the office setting and the need for more aggressive management of hypertension in actual clinical practice.
Disclosures: This study was funded by a grant from Bristol‐Myers Squibb. Drs Ewen, Zhang, Kolm, Jurkovitz, and Weintraub report having received grant support from Sanofi‐Aventis, Bristol–Myers Squibb, and CV Therapeutics. Drs Zhang, Kolm, Jurkovitz, and Weintraub also report having received grant support from Otsuka. Dr Jackson is employed by Bristol‐Myers Squibb. Dr Fidan is employed by Sanofi‐Aventis.
References
- 1. Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle‐aged women and men: The Framingham Heart Study. JAMA. 2002;287(8):1003–1010. [DOI] [PubMed] [Google Scholar]
- 3. Meyer J. Age: 2000. Census 2000 Brief. In: US Department of Commerce EaSA, ed. Washington, DC: US Census Bureau, 2001. [Google Scholar]
- 4. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153(5):598–615. [DOI] [PubMed] [Google Scholar]
- 5. Van Den Hoogen PC, Feskens EJ, Nagelkerke NJ, et al. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven Countries Study Research Group. N Engl J Med. 2000;342(1):1–8. [DOI] [PubMed] [Google Scholar]
- 6. Blood pressure, cholesterol, and stroke in eastern Asia . Eastern Stroke and Coronary Heart Disease Collaborative Research Group. Lancet. 1998;352(9143):1801–1807. [PubMed] [Google Scholar]
- 7. Kannel WB, Wolf PA, Verter J, et al. Epidemiologic assessment of the role of blood pressure in stroke: the Framingham Study. 1970. JAMA. 1996;276(15):1269–1278. [PubMed] [Google Scholar]
- 8. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 9. Morgan TO, Anderson A. Different drug classes have variable effects on blood pressure depending on the time of day. Am J Hypertens. 2003;16(1):46–50. [DOI] [PubMed] [Google Scholar]
- 10. Vasan RS, Massaro JM, Wilson PW, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2002;105(1):48–53. [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 12. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13. Cox DR. Regression models and life‐tables (with discussion). J R Stat Soc Series B. 1972;34:187–222. [Google Scholar]
- 14. Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika. 1981;68(2):373–379. [Google Scholar]
- 15. Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. [DOI] [PubMed] [Google Scholar]
- 16. Lloyd‐Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294(4):466–472. [DOI] [PubMed] [Google Scholar]
- 17. Tirschwell DL, Longstreth WT Jr,. Validating administrative data in stroke research. Stroke. 2002;33(10):2465–2470. [DOI] [PubMed] [Google Scholar]
- 18. Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims‐based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. [DOI] [PubMed] [Google Scholar]
- 19. Goff DC Jr, Pandey DK, Chan FA, et al. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160(2):197–202. [DOI] [PubMed] [Google Scholar]


