Abstract
The impact of delays in blood pressure (BP) reduction is particularly relevant for patients with severe hypertension who are at risk for hypertensive emergencies. Combination therapies may hold promise as initial treatment for these patients. The safety and efficacy of initial irbesartan/hydrochlorothiazide (angiotensin receptor blocker/thiazide) treatment was compared with that of irbesartan as monotherapy in a multicenter, double‐blind, parallel‐group study in patients with severe hypertension. After 5 weeks, 47% of patients receiving combination therapy achieved a target diastolic BP <90 mm Hg compared with 33% with monotherapy (P=.0005). Similarly, systolic BP/diastolic BP <140/90 mm Hg targets were reached by more subjects treated with combination therapy than subjects receiving monotherapy (34.6% vs 19.2%, P<.0001). Initial use of combination therapy instead of monotherapy is estimated to prevent between 250 and 4500 cardiovascular events in every 100,000 severely hypertensive patients over 5 years, without significantly increasing serious adverse event rates.
In patients with severe hypertension, the short‐term risk of hypertensive emergencies, including hospitalization for extremely elevated levels of blood pressure (BP), chronic heart failure, intracranial hemorrhage, progression of hypertensive retinopathy and nephropathy, and rupture of aneurysms, is substantial. 1 , 2 , 3 , 4 Hypertensive crises are believed to occur at a rate of one in 5–10 patient‐years of exposure to severe hypertension. 1 , 2 The impact of delays in BP reduction is particularly relevant in this patient population.
Severe hypertension is still prevalent today and is associated with substantial morbidity and mortality. 3 , 4 , 5 One‐year event rates are higher in severely hypertensive patients compared with patients with mild to moderate hypertension and cumulative cardiovascular event rates, for this population, increase dramatically over time. 5
Recently, a body of clinical trial experience has revealed important advantages to more aggressive approaches to treatment. 6 , 7 Two or 3 therapies prescribed from the start of treatment, for example, were significantly more efficacious than gradual titration and add‐on strategies. 6 , 7
Recently the US Food and Drug Administration evaluated pivotal data for initial combination therapy with irbesartan/hydrochlorothiazide (HCTZ) for patients with severe hypertension and requested a quantitative benefit/risk analysis. This review presents the benefit/risk analysis in the context of current guidelines and the relevance to practicing physicians.
Efficacy of Initial Irbesartan/HCTZ Combination Therapy for the Treatment of Severe Hypertension: The Pivotal Trial
The safety and efficacy of irbesartan/HCTZ as initial treatment of severe hypertension were evaluated in a multicenter, double‐blind, active‐control, 7‐week, parallel‐group study in patients with severe hypertension who were uncontrolled on monotherapy (diastolic BP [DBP] ≥110 mm Hg). 8 Following a 1‐week placebo lead‐in period, patients were randomized in a 2:1 ratio to receive fixed‐dose irbesartan/HCTZ combination therapy (150 mg/12.5 mg forced titration to 300 mg/25 mg after 1 week) or irbesartan monotherapy (150 mg forced titration to 300 mg after 1 week). This 2‐step rapid titration scheme was developed to lower the risk of adverse events due to sudden BP lowering and at the same time to avoid unnecessary exposure to severe hypertension due to suboptimal drug doses. The primary objective was to compare the proportion of subjects whose DBP was controlled at week 5 (DBP >90 mm Hg). Calculation of sample size and power of the test was based on the Fisher exact test performed at a 2‐sided 5% level of significance. 8
Subjects were mostly middle aged (mean age of 52.5 years) and Caucasian (84%). 8 Subjects had a history of hyperlipidemia (34%), diabetes mellitus (12%), stable angina pectoris (3%), transient ischemic attack or stroke (2%), and myocardial infarction (1%). Baseline hypertension was severe with a baseline systolic BP (SBP)/DBP of 172/113 mm Hg. The mean duration of hypertension was 7 years.
Better, Faster Control With Combination Therapy: Efficacy Data
As expected, this study demonstrated that initial irbesartan/HCTZ combination therapy provided clinically relevant benefits over monotherapy in the treatment of patients with severe hypertension. 8 The results are similar to those noted in other studies where combination therapy is more effective than monotherapy.
More patients responded to combination therapy than to irbesartan alone (Figure 1). 8 Forty‐seven percent of patients receiving combination therapy achieved a target DBP <90 mm Hg at week 5 compared with 33% with monotherapy (P=.0005). Similarly, SBP/DBP <140/90 mm Hg targets were reached by more subjects treated with combination therapy than those who received monotherapy (34.6% vs. 19.2% at week 5, P<.0001).
Figure 1.
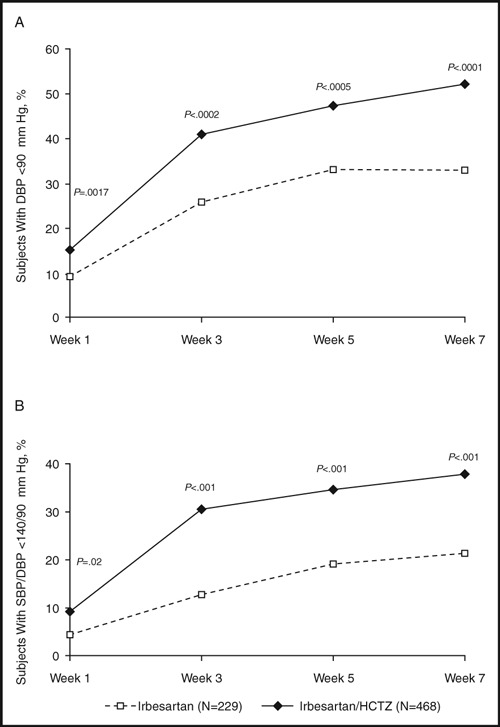
Blood pressure (BP) control in severely hypertensive patients after treatment with irbesartan or irbesartan/hydrochlorothiazide (HCTZ). Percentage of patients achieving (A) diastolic BP (DBP) <90 mm Hg; (B) systolic BP (SBP)/DBP <140/90 mm Hg after treatment with irbesartan or irbesartan/HCTZ. The primary efficacy endpoint was DBP <90 mm Hg at 5 weeks. Adapted with permission from Neutel et al. 8
Again, as expected, combination therapy reduced BP more than irbesartan therapy alone (Figure 2). 8 At week 5, the mean decreases from baseline in SBP/DBP were 31/24 mm Hg and 21/19 mm Hg for the combination and monotherapy arms, respectively (P<.0001). These data translate into a mean difference between groups of 10/5 mm Hg. For most subjects, these BP reductions meant a change from the severe to the moderate hypertension category (Figure 3). At week 5, only 1.7% of patients receiving combination therapy continued to have severe high BP compared with 6.1% of patients receiving monotherapy (P=.004). In addition, over the course of 7 weeks of treatment, the probability of having an episode of DBP ≥110 mm Hg at least once was 24.9% with monotherapy and 16.4% with combination therapy.
Figure 2.
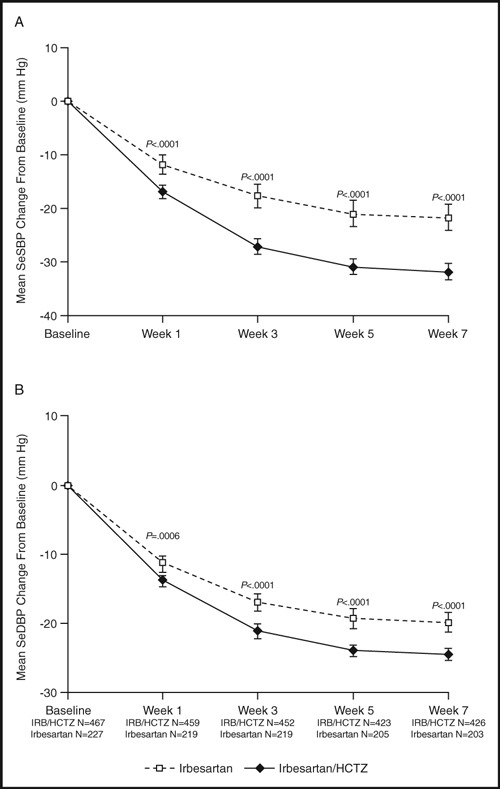
Changes in blood pressure over time in severely hypertensive patients after treatment with irbesartan or irbesartan (IRB)/hydrochlorothiazide (HCTZ). Reproduced with permission from Neutel et al. 8 SeSBP indicates seated systolic blood pressure; SeDBP seated diastolic blood pressure.
Figure 3.
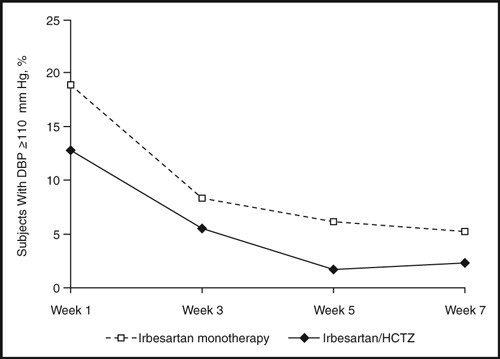
The proportion of patients with diastolic blood pressure (DBP) ≥110 mm Hg over time after treatment with irbesartan or irbesartan/hydrochlorothiazide (HCTZ).
Combination therapy also took effect rapidly. As early as 1 week after the start of treatment, significantly fewer patients taking irbesartan/HCTZ combination therapy (12.8%) continued to have severely high BP compared with patients taking irbesartan monotherapy (18.8%, P=.04; Figure 3).
In summary, in this study, for every 12 patients treated with combination therapy rather than monotherapy, one case of recurrent DBP ≥110 mm Hg was avoided. Furthermore, for every 100 patients treated with combination therapy, at least 26 fewer weeks of exposure to severe hypertension were experienced than with monotherapy (P=.004).
Clinical Relevance: Discussion of the Efficacy Data
Benefits of rapid BP reduction in severely hypertensive patients, as seen in this irbesartan/HCTZ program, are two‐fold. In the short term, hypertensive emergecies, such as encephalopathy, retinopathy, nephropathy, cardiomyopathy, and hemorrhages, are more likely to be avoided. In the intermediate to long term, cardiovascular events such as myocardial infarction, stroke, and cardiovascular death could be reduced.
The overall 10/5 mm Hg difference in BP between the irbesartan/HCTZ and irbesartan groups is clinically relevant. In both observational and clinical studies, 5 mm Hg decreases in DBP were associated with continuous and independent decreases in stroke rates of 34%–42%. 9 , 10 In patients with type 2 diabetes, each 10 mm Hg decrease in SBP was associated with reductions in risk of 12% for diabetes‐related complications, 15% for diabetes‐related deaths, 11% for myocardial infarctions, and 13% for microvascular complications. 11 Since physicians are slow to adjust medication, in an add‐on scheme patients may not benefit from the additional 10/5 mm Hg reduction in BP attributable to HCTZ for a year or more.
The more rapid BP‐lowering effects in the combination group compared with the monotherapy group are also clinically relevant. The importance of prompt reduction of severe hypertension has been known for many years. 1 , 2 In the 1967 Veterans Administration Cooperative 1 study, investigators reported 27% fewer cardiovascular events with initial combination therapy vs placebo when DBP was reduced from severe (115–129 mm Hg) to more moderate levels (reduction of 30 mm Hg) within 2 months of treatment. More recently, in less severe populations in the Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial, SBP normalization after 6 months of treatment was associated with reduced stroke, myocardial infarction, and cardiovascular mortality (45%, 14%, and 21%, respectively). 12 Other trials suggest that delays of as little as 1 month in starting antihypertensive therapy in high‐risk individuals can significantly increase the risk of certain cardiovascular events. 6 , 13
Longer term trials have shown that sustained reductions in BP are consistently associated with better long‐term outcomes. 1 , 2 , 14 , 15 , 16 , 17 In the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), 2 mm Hg differences in SBP over 5 years of treatment with chlorthalidone compared to lisinopril resulted in a 15% difference in stroke rates. 16 In the Study on Cognition and Prognosis in the Elderly (SCOPE), 3.5 years of candesartan treatment led to a 28% reduction in stroke rates compared with control treatment, 17 and in the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT), 5.5 years of the amlodipine‐based regimen reduced stroke rates by 23%. 15
Lastly, these results are consistent with other studies using combination antihypertensive medication. In previous trials, combination therapy reduced BP more than either of its constituents prescribed separately and the efficacy of the 2 components was approximately additive when they were given in combination. 18 , 19 Irbesartan/HCTZ combination therapy has also been used effectively in difficult to treat patients. 20 , 21 In the large Irbesartan/Hydrochlorothiazide Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) trial, 30% of patients had diabetes mellitus, 48% of patients had hyperlipidemia and baseline SBPs ranging from 130 to 180 mm Hg. 22 In this cohort of mixed severities, 77% of patients achieved their SBP goal; 83% achieved their DBP goal (<90 mm Hg; <80 mm Hg for type 2 diabetes mellitus); and 69% achieved their SBP/DBP goal after 18 weeks of sequential antihypertensive therapy (HCTZ 12.5 mg for 2 weeks, irbesartan/HCTZ 150/12.5 mg for 8 weeks, then irbesartan/HCTZ 300/25 mg for 8 weeks). 22
Safety of Initial Irbesartan/HCTZ Combination Therapy for the Treatment of Severe Hypertension
Safety Data
As discussed earlier, use of irbesartan/HCTZ as initial treatment in severely hypertensive patients depends on the benefit/risk ratio. Not only do the efficacy benefits have to be significant, but safety risks associated with the initial addition of HCTZ need to be minimal.
This study was prospectively designed to evaluate the 2 main safety concerns surrounding angiotensin receptor blocker (ARB)/HCTZ treatment. First, the initial BP response may be so great that orthostatic hypotension and syncope may ensue. Second, some patients may be unnecessarily exposed to rare adverse events due to 12.5 mg or 25 mg doses of HCTZ. In particular, prespecified adverse events (hypotension, dizziness, syncope, headaches, hypokalemia, and hyperkalemia) were carefully monitored.
The overall frequency of adverse events during the 7 weeks of treatment was lower in the irbesartan/HCTZ therapy group than in the irbesartan group (Table). 8 The majority of adverse events were mild to moderate and unrelated to treatment. Fewer patients in the combination therapy group experienced the prespecified adverse events than in the monotherapy group (8.8% vs. 11.5%, respectively). Headache was the most frequently reported prespecified adverse event (4.3% with combination therapy and 6.6% with monotherapy).
Table.
Incidence of Adverse Events (All Randomized Patients) Following Initial Treatment of Severely Hypertensive Patients for 7 Weeks
| Adverse Events (AEs), n (%) | Irbesartan/ HCTZ Combination Therapy ( n =468) | Irbesartan Monotherapy ( n =229) |
|---|---|---|
| Total | 140 (29.9) | 82 (36.1) |
| Prespecified | 41 (8.8) | 26 (11.5) |
| Dizziness | 17 (3.6) | 9 (4.0) |
| Headache | 20 (4.3) | 15 (6.6) |
| Hyperkalemia/increased potassium | 1 (0.2) | 0 |
| Hypokalemia/decreased potassium | 3 (0.6) | 1 (0.4) |
| Hypotension | 3 (0.6) | 0 |
| Serum potassium<3.0 mmol/L | 0 | 0 |
| Serum potassium>6.0 mmol/L | 3 (0.6) | 3 (1.3) |
| Serious | 1 (0.2) | 1 (0.4) |
| Deaths | 0 | 0 |
| Discontinuations due to AE | 9 (1.9) | 5 (2.2) |
Abbreviation: HCTZ, hydrochlorothiazide. Adapted with permission from Neutel et al.8
Hyperkalemia and hypokalemia (defined by the investigator according to clinical relevance) occurred slightly more frequently in the irbesartan/HCTZ group (0.2% and 0.6%, respectively) than in the irbesartan group (0% and 0.4%, respectively). Hypotension and dizziness occurred with similar frequency in both groups. Hypotension was rare with an incidence of 0.6% in the irbesartan/HCTZ group and 0% in the irbesartan group. No cases of syncope were observed. The minimum SBP for the entire study was 99 mm Hg at week 7. Study medication was well tolerated with very few discontinuations due to adverse events in either group (1.9% with combination therapy and 2.2% with monotherapy).
Clinical Context: Discussion of the Safety Data
In this 7‐week trial, no evidence of excess risk for irbesartan/HCTZ compared with irbesartan could be detected. The only common adverse event that seemed to be related to the addition of HCTZ was hypokalemia, which is a well documented and easy to manage effect of nonpotassium sparing diuretics. Though hypokalemia was observed in 0.6% of subjects, serum potassium levels <3.0 mmol/L were not observed. Data from other studies suggest that the hypokalemic effects of HCTZ were most likely attenuated by the hyperkalemic effects of irbesartan. In a matrix study of irbesartan (0, 37.5, 100, and 300 mg) and HCTZ (0, 6.25, 12.5, and 25 mg), hypokalemia associated with 25 mg of HCTZ was reduced with increasing doses of irbesartan. 18 Overall, irbesartan is believed to ameliorate the dose‐related biochemical abnormalities associated with HCTZ.
Despite the slight increase in risk of hypokalemia, the long‐term risk of renal complications with initial irbesartan/HCTZ treatment is believed to be low due to the positive effects of ARBs on renal function. ARBs, including irbesartan, have been associated with improved renal outcomes. For example, in patients with diabetes, hypertension, and microalbuminuria enrolled in the Irbesartan Microalbuminuria II trial (IRMA II), treatment with 300 mg of irbesartan significantly slowed the progression of microalbuminuria to overt nephropathy when compared with placebo (P<.001). 23 In addition, in long‐term studies, diuretics have been used safely as concomitant medications in patients treated with irbesartan or losartan for the prevention of renal disease. 24 , 25 Lastly, in another study, diuretics were shown to provide additional reductions in proteinuria and albuminuria when prescribed on top of ARBs. 26
Although high doses of diuretics carry a risk of insulin resistance, 27 in long‐term studies, ARBs delay the onset of diabetes in hypertensive patients. 28 , 29 , 30 In subjects with diabetes and hypertension, for example, irbesartan improved lipid, blood glucose, and hemoglobin A1c levels. 31 Furthermore, it has been suggested that the combination of an ARB, which downregulates the renin‐angiotensin system thereby reducing damage to the kidney, and a diuretic, which reduces sodium retention, could be particularly beneficial in hypertensive diabetic patients, which constitute a particularly difficult to treat population. In the INCLUSIVE trial, more than 50% of type 2 diabetes patients treated with combination irbesartan/HCTZ reached their SBP goals and 40% reached their SBP/DBP goals (<130/80 mm Hg). 32
Other safety concerns with HCTZ include extremely rare events like pancreatitis and severe allergic reactions (eg, interstitial pneumonitis). 33 Because this trial was short, such events were not observed. However, they have been evaluated through postmarketing surveillance of second‐line use of irbesartan/HCTZ. With the exception of hypokalemia, numbers of cases per 1 million patient‐years of exposure were lower for irbesartan/HCTZ than for irbesartan alone: 1.3 vs 2.1 for pancreatitis, 6.8 vs 21.3 for renal failure, 3.2 vs 7.4 for syncope, 7.8 vs 11.0 for allergic reactions, 0.1 vs 0.7 for interstitial pneumonitis, and 2.7 vs 2.0 for hypokalemia. Although these postmarketing surveillance event rates are based on second‐step use of irbesartan/HCTZ and on drug sales to wholesalers (17.7 million treatment years for irbesartan and 6.8 million patient treatment years for irbesartan/HCTZ) and therefore should be interpreted with caution, they are consistent with the safety profile in previous trials and in the original New Drug Application. 18 , 19 , 20 , 21
Poor compliance is an especially common problem with severe hypertension. Self‐reported poor compliance is associated with almost twice the likelihood of presentation to the emergency room for severe hypertension. 34 Since studies have also shown that a single combination pill is associated with better compliance than the simultaneous prescription of the 2 separate components, 35 a good tolerability profile associated with simple posology suggests better patient compliance.
Lastly, these data are consistent with safety data collected from other ARB/HCTZ combination therapies. In a metaanalysis of trials comparing combination products with monotherapy, ARBs showed no relationship between dose and the total proportion of patients reporting adverse events. 36 By contrast, doubling the diuretic dose (from half‐standard to standard) increased adverse events five‐fold (from approximately 2%–10% more than placebo).
Overall Benefit/Risk Ratio Assessment
For the purpose of the formal review of irbesartan/HCTZ for indication as initial therapy in patients with severe hypertension, a post hoc benefit risk analysis based on 100,000 patients and a 5‐year treatment period was performed. Estimates suggest that combination treatment prevented 50–100 hypertensive crises, 50–100 cardiovascular events during the initial 5‐week treatment period, and 250–4500 cardiovascular events during the remaining 5 years. By contrast, zero to a few serious complications would be expected due to diuretic therapy; the risk of pancreatitis and interstitial pneumonitis were both between zero and 5 cases. Although formal estimates of benefit/risk are limited by the fact that they are imprecise, in this program the benefits and risks are separated by orders of magnitude. Thus, overall, it was concluded that the benefits of initial treatment with irbesartan/HCTZ compared with irbesartan significantly outweighed risks associated with the addition of HCTZ.
Conclusion
Initial use of irbesartan/HCTZ instead of irbesartan has the potential to prevent between 250 and 4500 cardiovascular events in every 100,000 severely hypertensive patients over 5 years, without significantly increasing serious adverse event rates. Because of the urgency associated with lowering BP in severely hypertensive patients, this positive benefit/risk profile has led to expanded labeling that now incorporates initial irbesartan/HCTZ use for moderate to severe hypertensive patients.
Disclosures: Dr Pablo Lapuerta conducted this work as an employee of Bristol‐Myers Squibb. Dr Stanley Franklin is a member of the Speakers’ Bureaus for Boehringer Ingelheim, Bristol‐Myers Squibb, and Merck, and is a consultant for AtCor Medical and Bristol‐Myers Squibb.
Acknowledgments
Acknowledgments: Editorial support for this article was provided by Bristol‐Myers Squibb and Sanofi‐Aventis.
References
- 1. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 2. Wolff FW, Lindeman RD. Effects of treatment in hypertension. Results of a controlled study. J Chronic Dis. 1966;19:227–240. [DOI] [PubMed] [Google Scholar]
- 3. Preston RA, Baltodano NM, Cienki J, et al. Clinical presentation and management of patients with uncontrolled, severe hypertension: results from a public teaching hospital. J Hum Hypertens. 1999;13:249–255. [DOI] [PubMed] [Google Scholar]
- 4. Zampaglione B, Pascale C, Marchisio M, et al. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27:144–147. [DOI] [PubMed] [Google Scholar]
- 5. US Food and Drug Administration . Cardiovascular and Renal Drugs Advisory Committee Meeting. April 2007; http://www.fda.gov/ohrms/dockets/ac/07/transcripts/2007‐4287t1‐part1.pdf. Accessed April 14, 2009. [Google Scholar]
- 6. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 7. Mourad JJ, Waeber B, Zannad F, et al. Comparison of different therapeutic strategies in hypertension: a low‐dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped‐care approach. J Hypertens. 2004;22:2379–2386. [DOI] [PubMed] [Google Scholar]
- 8. Neutel JM, Franklin SS, Oparil S, et al. Efficacy and safety of irbesartan/HCTZ combination therapy as initial treatment for rapid control of severe hypertension. J Clin Hypertens (Greenwich). 2006;8:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. [DOI] [PubMed] [Google Scholar]
- 10. MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 11. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 13. Krakoff LR. Systems for care of hypertension in the United States. J Clin Hypertens (Greenwich). 2006;8:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 15. Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 16. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 17. Lithell H. The study of Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double‐blind intervention trial. J Hypertens. 2003;21:875–886. [DOI] [PubMed] [Google Scholar]
- 18. Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens. 1999;12:797–805. [DOI] [PubMed] [Google Scholar]
- 19. Neutel JM, Franklin SS, Lapuerta P, et al. A comparison of the efficacy and safety of irbesartan/HCTZ combination therapy with irbesartan and HCTZ monotherapy in the treatment of moderate hypertension. J Hum Hypertens. 2008;22:266–274. [DOI] [PubMed] [Google Scholar]
- 20. Coca A, Calvo C, Sobrino J, et al. Once‐daily fixed‐combination irbesartan 300 mg/hydrochlorothiazide 25 mg and circadian blood pressure profile in patients with essential hypertension. Clin Ther. 2003;25:2849–2864. [DOI] [PubMed] [Google Scholar]
- 21. Neutel JM, Saunders E, Bakris GL, et al. The efficacy and safety of low‐ and high‐dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trial. J Clin Hypertens (Greenwich). 2005;7:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franklin S, Lapeurta P, Bhaumik A, et al. Rapid blood pressure reduction with irbesartan/HCTZ as a first‐line treatment of severe hypertension – a timepoint analysis. J Hypertens. 2006;24:S284–S285. [Google Scholar]
- 23. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. [DOI] [PubMed] [Google Scholar]
- 24. Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 25. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. [DOI] [PubMed] [Google Scholar]
- 26. Esnault VL. Diuretic and enhanced sodium restriction result in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol. 2005;16:474–481. [DOI] [PubMed] [Google Scholar]
- 27. Prichard BNC. Adverse reactions to diuretics. Eur Heart J. 1992;13:96–103. [DOI] [PubMed] [Google Scholar]
- 28. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 29. Gillespie EL. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new‐onset type 2 diabetes. Diabetes Care. 2005;28:2261–2266. [DOI] [PubMed] [Google Scholar]
- 30. Kjeldsen JE. Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high‐risk hypertensive patients: the VALUE trial. J Hypertens. 2006;24:1405–1412. [DOI] [PubMed] [Google Scholar]
- 31. Bramlage P, Pittrow D, Kirch W. The effect of irbesartan in reducing cardiovascular risk in hypertensive type 2 diabetic patients: an observational study in 16,600 patients in primary care. Curr Med Res Opin. 2004;20:1625–1631. [DOI] [PubMed] [Google Scholar]
- 32. Sowers JR, Neutel JM, Saunders E, et al. Antihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes. J Clin Hypertens (Greenwich). 2006;8:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lankisch PG. Drug induced acute pancreatitis; incidence and severity. Gut. 1995;37:565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shea S. Predisposing factors for severe, uncontrolled hypertension in an inner‐city minority population. N Engl J Med. 1992;327:776–781. [DOI] [PubMed] [Google Scholar]
- 35. Dezii CM. A retrospective study of persistence with single‐pill combination therapy vs. concurrent two‐pill therapy in patients with hypertension. Manag Care. 2000;9:2–6. [PubMed] [Google Scholar]
- 36. Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]


