Abstract
Distal embolic protection (DEP) may prevent embolization of atherosclerotic debris during renal artery stenting. The authors retrospectively identified 48 hypertensive patients with chronic kidney disease (CKD) who underwent renal artery stenting between 2002 and 2005 and compared stenting alone (n=17) to stenting/DEP (n=31). Blood pressure (BP) and estimated glomerular filtration rate (eGFR) (mL/min/1.73m2) at baseline at 6 and 12 months were compared. Overall, eGFR improved by 4.7 (P=.005) at 6 months and 3.8 (P=.003) at 12 months compared with baseline. Comparing stent to stent/DEP patients, eGFR improvement did not differ at 6 months (7.6 vs 2.9; P=.15) or at 12 months (4.4 vs 3.5; P=.74). Systolic BP reduction was similar between stent and stent/DEP patients at 6 months (−9 vs −14 mm Hg; P=.59) and at 12 months (−18 vs −16 mm Hg; P=.89). Renal artery stenting improved eGFR and systolic BP in patients with hypertension and CKD; however, DEP did not enhance these effects.
Renal artery stenosis may represent a cause as well as a consequence of hypertension and renal insufficiency. Associated risk factors of increasing age, diabetes, dyslipidemia, and other vascular disease contribute to the progressive nature of atherosclerotic renal vascular disease. Prevalence of renal artery disease is estimated at 4% and increases to 10% in individuals with hypertension and diabetes. 1 In patients with peripheral arterial disease or cardiovascular disease, prevalence may be as high as 30% to 50%. 2 , 3
Not all patients who have anatomic disease may demonstrate clinically or physiologically significant renal artery stenosis. In selected patients, percutaneous treatment of renal artery stenosis may improve BP control and slow progression of ischemic nephropathy. 4 , 5 , 6 , 7 , 8 , 9 The potential for embolization of atherothrombotic debris during renal stenting has raised concern that procedure‐related renal damage could offset the benefits of improved blood flow. 10 Embolic protection devices used during saphenous vein graft angioplasty has been reported to reduce periprocedural complications, translating to reduced cardiac events. 11 The use of distal embolic protection (DEP) devices in renal artery interventions has been advocated as a means of potentially preserving renal function. However, there has been limited evaluation of the effectiveness of stenting on renal function and hypertension. 12 , 13 , 14 , 15 With the emergence of several DEP devices, even fewer studies have assessed selection criteria or the efficacy of specific instruments. 16 Our study sought to assess the degree of renal preservation in hypertensive patients with chronic kidney disease (CKD) who underwent renal artery stenting and to retrospectively compare long‐term results of patients who received a stent alone compared to a stent with DEP.
Methods
We analyzed a retrospective cohort of consecutive hypertensive patients with CKD undergoing primary renal artery stenting with and without DEP at Yale‐New Haven Hospital and the West Haven Veterans Affairs Medical Center between 2002 and 2005. CKD was defined in terms of estimated glomerular filtration rate (eGFR) derived from the abbreviated Modification of Diet in Renal Disease equation incorporating serum creatinine, age, sex, and ethnicity. 17 Impaired renal function was designated by an eGFR <60 mL/min/1.73 m2. 18 Patients with an eGFR >60 mL/min/1.73 m2 were excluded; patients who have preserved renal function are at very low risk for clinically evident embolic complications. 5
Following diagnostic angiography, patients received unilateral or bilateral stents when indicated. Interventions for in‐stent restenosis (n=2) were excluded. All patients had a minimum of 6 months’ follow‐up. One of 3 devices was utilized in stent/DEP patients: (1) the GuardWire Temporary Occlusion and Aspiration System (Medtronic, Inc., Minneapolis, MN); 16 (2) the RX ACCUNET Embolic Protection System (Abbott Laboratories, Abbott Park, IL); 19 and (3) the FilterWire EZ Embolic Protection System (Boston Scientific, Natick, MA). 20
At time of the procedure, operators evaluated findings of diagnostic angiography for feasibility of DEP deployment, and if significant renal function was believed to be jeopardized by potential distal embolization, DEP was employed. Elevated baseline creatinine level, bilateral obstructive disease, single kidney, and visibly atrophic contralateral kidney were clinical features that suggested limited renal reserve and therefore potential benefit of DEP. To define an appropriate control group in this retrospective evaluation, patients who underwent renal stenting without DEP were evaluated for DEP eligibility on the basis of the following angiographic criteria: (1) location of the lesion, either ostial or proximal (<1 cm from the ostium); (2) existence of an adequate landing zone for the DEP device placement distal to the lesion; (3) absence of proximal arterial branching between the lesion and the DEP device; and (4) adequate distal vessel caliber to accommodate the DEP device (Figure ). Mid or distal renal artery lesions would be eligible provided that the criteria of adequate landing zone, absence of proximal arterial branching, and appropriate vessel caliber were fulfilled. Based on the angiographic criteria and the clinical situation, operators decided whether DEP would have been appropriate. Inclusion of non‐DEP (stent alone) patients was determined by majority agreement of 3 operators blinded to intervention type.
Figure.
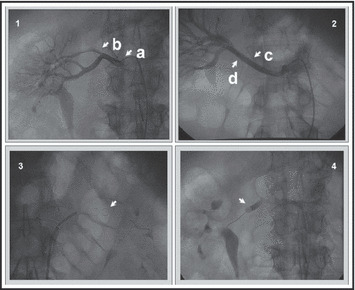
Angiographic considerations for distal protection. (1) Renal angiogram demonstrating (a) significant ostial stenosis with (b) proximal branching artery. (2) Renal angiogram demonstrating (c) available landing zone for embolic protection device and (d) appropriate caliber of vessel to allow complete protection. (3) Example of FilterWire EZ Embolic Protection System deployment prior to stent expansion. (4) Example of GuardWire Temporary Occlusion and Aspiration System deployment distal to pre‐stent balloon dilation.
Baseline characteristics included demographics, BP levels, serum creatinine value, and use of antihypertensive medications. We acquired follow‐up data from outpatient records at 6 and 12 months after the procedure. The minimum time interval was selected to allow adequate time for periprocedural fluctuations of renal function to equilibrate. The maximum time interval was chosen to distinguish the periprocedural effects of DEP from long‐term consequences of the natural progression of renal disease. Prior trends in serum creatinine levels were unavailable; serum creatinine values at baseline and follow‐up were obtained to calculate eGFR. The most recent serum creatinine level obtained preprocedure was used as a baseline level. Follow‐up values were obtained from routine outpatient laboratory assessments within 3 months of the 6‐ and 12‐month appointments, respectively. The lowest recorded baseline and office BP values were noted, as well as current medications at the time of the visit. Treatment failures included patients who required dialysis or died of cardiac or renal causes.
Discrete variables were expressed as counts and analyzed by chi‐square testing. Probability values of P<.05 were considered significant. Statistical evaluation of continuous variables was determined by independent Student’s t‐test, and paired Student’s t‐test was used when comparing baseline and follow‐up values. The study protocol was reviewed and approved by the Yale University Human Investigational Committee and the Veterans Affairs Investigational Review Board.
Results
From 2002 to 2005, 78 patients underwent renal artery stenting. Excluding patients with normal renal function, 65 patients had CKD as defined by eGFR <60 mL/min/1.73 m2. There were 42 patients (23 DEP/19 stent only) older than 70 years. Only 4 (1 DEP/3 stent only) of these patients had an eGFR >50 mL/min/1.73 m2 but <60 mL/min/1.73 m2. Therefore, 90% of elderly individuals had concomitant renal impairment not entirely attributed to age, by eGFR calculation. Follow‐up of at least 6 months was unavailable for 9 patients (3 stent, 6 stent/DEP). All patients who were unavailable for follow‐up had similar baseline characteristics compared to the group that was evaluated. The remaining 56 patients underwent adjudication for DEP eligibility; 17 of the 25 patients who received stents could have been DEP candidates. The dominant factor for exclusion of these 8 non‐DEP patients was the presence of a proximal branching artery in the setting of unilateral disease. All remaining 48 patients had 6 months’ follow‐up data available; 12 months’ follow‐up was, however, limited to 38 patients. Six patients were lost to follow‐up (1 stent, 5 stent/DEP), 3 patients required dialysis (1 stent, 2 stent/DEP), and 1 stent patient died at month 7 with exacerbation of underlying pulmonary disease and pneumonia.
Of the 48 stented patients included in this analysis, 31 received DEP (stent/DEP); these were compared to 17 patients deemed suitable for but not receiving DEP (stent alone). A total of 62 vessels were stented with 34 unilateral (14 stent alone, 20 stent/DEP) and 14 bilateral (3 stent alone, 11 stent/DEP). A majority of patients received the FilterWire EZ distal protection device (n=20), with fewer receiving GuardWire (n=8) or RX ACCUNET (n=3).
Predominantly male (71%) and white (94%), patients who received DEP were similar to patients only receiving stenting, except for a small but significant difference in mean age. Baseline renal function was not different despite incorporating age in calculating eGFR. All patients were hypertensive; 79% of patients had resistant hypertension defined as uncontrolled BP despite use of 3 medications including a diuretic. 21 Although the stent/DEP group had lower diastolic BP, there were no other differences in baseline characteristics between patients receiving stents with or without DEP (Table I).
Table I.
Baseline Characteristics
| Stenting (n=17) | Stenting/DEP (n=31) | P Valuea | |
|---|---|---|---|
| Age, y | 69±7 | 75±6 | .005 |
| Male | 12 (71) | 22 (71) | NS |
| White | 17 (100) | 28 (90) | NS |
| Hypertension | 17 (100) | 31 (100) | NS |
| Diabetes | 8 (47) | 9 (29) | NS |
| CAD | 11 (65) | 21 (67) | NS |
| CVA | 4 (24) | 4 (13) | NS |
| PAD | 9 (53) | 14 (45) | NS |
| Hyperlipidemia | 13 (76) | 20 (65) | NS |
| CHF (EF <50%) | 2 (12) | 7 (23) | NS |
| CHF (EF >50%) | 1 (6) | 0 (0) | NS |
| eGFR | 37±15 | 34±13 | .47 |
| SBP | 161±26 | 152±21 | .18 |
| DBP | 82±12 | 73±13 | .02 |
| No. of meds | 3.1±0.8 | 3.2±1.0 | .87 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; DEP, distal embolic protection; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; PAD, peripheral arterial disease; SBP, systolic blood pressure. Values are expressed as No. (%) or mean ± SD. a P<.05, significant.
Overall, renal artery stenting was associated with increased eGFR by 4.7 mL/min/1.73 m2 (P=.005) and reduced systolic BP (SBP) by 12 mm Hg (P=.009) at 6 months (n=48) and by 3.8 mL/min/1.73 m2 (P=.003) and 17 mm Hg (P=.0004) at 12 months (n=38) compared with baseline, respectively. At 6 months (n=48), 30 patients who received stenting had stable or improving renal function, as did 29 patients at 12 months (n=38). Stability or improvement of eGFR in patients only receiving stenting was found in 13 patients at 6 months (n=17) and 10 patients at 12 months (n=14). At 6 months, 17 patients receiving stenting/DEP (n=31) showed stability or improvement of renal function, as did 19 patients at 12 months (n=24). There were no significant differences in strength or number of BP medications between baseline and follow‐up. There was no difference in eGFR or BP between stent alone compared to stent/DEP patients (Table II). Among patients with unilateral disease (n=34), 20 received DEP, and of the 14 patients with bilateral disease all but 3 received DEP. There was no difference in characteristics or outcomes between stenting and stenting/DEP in patients receiving unilateral or bilateral interventions (Table III).
Table II.
Change in BP and eGFR vs Baseline
| eGFR, mL/ min/1.73 m2 | P Value | Systolic BP, mm Hg | P Value | Diastolic BP, mm Hg | P Value | No. of Meds | P Value | |
|---|---|---|---|---|---|---|---|---|
| All | ||||||||
| Baseline | 35 (±14) | 155 (±23) | 76 (±13) | 3.1 (±0.9) | ||||
| 6 months (n=48) | +4.7 (±10.6) | .005 | −12 (±30) | .009 | −0.2 (±16) | .934 | −0.3 (±1.3) | .113 |
| 12 months (n=38) | +3.8 (±7.5) | .003 | −17 (±27) | .0004 | −6 (±15) | .02 | −0.1 (±1.2) | .507 |
| Stenting alone | ||||||||
| Baseline | 37 (±15) | 161 (±26) | 82 (±12) | 3.1 (±0.8) | ||||
| 6 months (n=17) | +7.6a (±9.0) | .003 | −9a (±28) | .239 | −3a (±12) | .357 | −0.5a (±1.1) | .058 |
| 12 months (n=14) | +4.4b (±9.4) | .107 | −18b (±36) | .092 | −9b (±15) | .038 | −0.2b (±1.1) | .292 |
| Stenting/DEP | ||||||||
| Baseline | 34 (±13) | 152 (±21) | 73 (±13) | 3.2 (±1.0) | ||||
| 6 months (n=31) | +2.9a (±11) | .177 | −14a (±31) | .020 | +2a (±18) | .589 | −0.2a (±1.3) | .509 |
| 12 months (n=24) | +3.5b (±6.4) | .013 | −16b (±20) | .0007 | −4b (±14) | .212 | 0.0b (±1.3) | 1 |
Abbreviations: BP, blood pressure; DEP, distal embolic protection; eGFR, estimated glomerular filtration rate. aNot significantly different between stenting alone vs stenting/DEP. bNot significantly different between stenting alone vs stenting/DEP. P<.05, significant.
Table III.
Unilateral and Bilateral Interventions: Change in Blood Pressure and eGFR vs Baseline
| eGFR, mL/ min/1.73 m2 | P Value | Systolic BP, mm Hg | P Value | Diastolic BP, mm Hg | P Value | |
|---|---|---|---|---|---|---|
| Unilateral (n=34) | ||||||
| Baseline | 35 (±14) | 154 (±23) | 76 (±14) | |||
| 6 months (n=34) | +4.4 (±9.8) | .017 | −10 (±30) | .058 | +2 (±16) | .457 |
| 12 months (n=28) | +3.2 (±7.9) | .037 | −17 (±28) | .004 | −7 (±15) | .024 |
| Stenting alone | ||||||
| Baseline | 36 (±17) | 165 (±25) | 82 (±12) | |||
| 6 months (n=14) | +8a (±9.4) | .007 | −11a (±30) | .227 | −3a (±13) | .382 |
| 12 months (n=11) | +4.7b (±9.9) | .147 | −19b (±38) | .139 | −11b (±15) | .046 |
| Stenting/DEP | ||||||
| Baseline | 34 (±12) | 147 (±17) | 71 (±14) | |||
| 6 months (n=20) | +1.6a (±9.4) | .492 | −10a (±31) | .159 | +5a (±17) | .166 |
| 12 months (n=17) | +2.2b (±5.9) | .146 | −16b (±21) | .006 | −4b (±14) | .241 |
| Bilateral (n=14) | ||||||
| Baseline | 37 (±13) | 156 (±24) | 77 (±10) | |||
| 6 months (n=14) | +5.3 (±12.7) | .145 | −16 (±29) | .068 | −4 (±16) | .353 |
| 12 months (n=10) | +5.6 (±7.2) | .036 | −16 (±23) | .058 | −3 (±14) | .529 |
| Stenting alone | ||||||
| Baseline | 43 (±7) | 140 (±20) | 80 (±10) | |||
| 6 months (n=3) | +6.0a (±9.0) | .348 | 0a (±19) | 1 | −1a (±10) | .843 |
| 12 months (n=3) | +3.0b (±8.7) | .612 | −14b (±34) | .552 | −3b (±12) | .667 |
| Stenting/DEP | ||||||
| Baseline | 35 (±14) | 160 (±24) | 76 (±10) | |||
| 6 months (n=11) | +5.1a (±14) | .256 | −20a (±31) | .059 | −5a (±18) | .379 |
| 12 months (n=7) | +6.7b (±6.9) | .043 | −17b (±20) | .072 | −3b (±16) | .665 |
Abbreviations: BP, blood pressure; DEP, distal embolic protection; eGFR, estimated glomerular filtration rate. aNot significantly different between stenting alone vs stenting/DEP. bNot significantly different between stenting alone vs stenting/DEP. P<.05, significant.
Hemodialysis was required in 1 patient in the stent alone group at month 8 postintervention and in 2 patients in the stenting/DEP group at months 8 and 10, respectively. Patients who were dialyzed had a baseline eGFR <21 mL/min/1.73 m2; however, 7 other patients with a similar baseline eGFR (3 stent alone, 4 stent/DEP) did not require dialysis at 12 months.
Discussion
Our study demonstrated that renal artery stenting improved SBP without deterioration of baseline renal function regardless of whether DEP was used. These results suggest that the use of DEP may not provide clinically important protection of renal function during renal artery interventions. Accordingly, the use of DEP should be studied in a prospective and longitudinal fashion before it is adopted as standard practice in renal artery stenting.
The subtypes of patients with renal artery stenting, if any, who would best respond to DEP have not been clarified. Prior investigators have observed that patients with preserved renal function have fewer complications and decreased mortality following renal artery stenting than patients with impaired renal function. 5 Advocates of DEP in patients undergoing renal interventions have hypothesized that the observed differences in outcome may, in part, be due to procedurally related kidney damage. However, the absence of DEP benefit to renal function observed in this study suggests that differences in mortality outcomes are more likely explained by advanced vasculopathy in patients with CKD. Patients with CKD generally have greater cardiovascular risk with concomitant uncontrolled BP, have diabetes, and are more prone to potential atheroemboli from existing aortorenal plaques. 22 , 23 , 24 Modification of cardiovascular risk factors with optimum medical therapy combined with stent revascularization may result in better outcomes. 25
Data from the Randomized Multicenter Study Comparing the Safety and Efficacy of Renal Artery Stenting With/Without Distal Protection Device (AngioGuard; Cordis, Warren, NJ) and With/Without the Use of a Platelet Aggregator Inhibitor (abciximab) (RESIST) indicate a nonsignificant change in eGFR regardless of whether DEP was employed in patients with preserved renal function. However, a significant improvement in eGFR was observed in a subset of patients in whom glycoprotein IIb/IIIa inhibitors were used in combination with DEP. 26 There was no benefit when DEP was utilized in patients with preserved renal function: our study found similar results in patients with impaired renal function. Because glycoprotein IIb/IIIa inhibitors were not routinely employed in our series, we cannot exclude the possibility that DEP may be beneficial if combined with routine use of anticoagulants.
In another prospective observational study, Holden and colleagues 27 , 28 examined patients with abnormal renal function receiving stents with DEP. This series showed stable renal function after stenting with DEP in patients with impaired renal function. Our study, however, compared patients with depressed renal function who received stenting with DEP to those without DEP. Further studies to examine the value of DEP in this high‐risk group should prospectively compare patients with CKD receiving stents with or without DEP. Currently in progress, the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) study will examine medical therapy with the option of combined stenting/DEP compared with medical management alone. Treating with or without DEP will not be evaluated. 25
We propose anatomic factors that address the feasibility and utility of incorporating DEP (Table IV). To avoid embolization during angioplasty and stenting, effective placement of the device is necessary for the intended result. 29 Anatomic characteristics that would favor use of DEP in stent patients who did not receive DEP were evaluated to compare these patients with those who received stents with DEP. Patients who received DEP predominantly had ostial lesions with an adequate landing zone for the device. However, the presence of a proximal arterial branch influenced exclusion in a majority of patients who received stents without DEP. Utility of DEP would be decreased as debris might be shunted to the proximal arterial branch rather than undergo capture distally by the device. Development of branch occlusion devices may address this issue, yet anatomic complexity makes this method technically challenging.
Table IV.
Proposed Criteria for Clinical Decision Favoring the Use of Distal Embolic Protection
| Angiographic Criteria |
|---|
| Ostial (≤1 cm from ostium) or proximal vs distal lesions |
| Absence of major proximal branching artery |
| Available landing zone for embolic protection device |
| Appropriate caliber of vessel to allow complete protection |
Consideration of unilateral or bilateral disease further influenced selection for DEP usage. Hypothetically, the benefit of DEP may be greater in patients with either bilateral disease or a single functioning kidney, where the entire renovascular bed is at risk from procedurally related atheroemboli. Patients with unilateral disease had significant benefit from baseline; however, use of DEP did not further improve eGFR. Changes in eGFR with stenting compared with stenting/DEP in patients with bilateral disease in this study were not significantly different, although these results may be explained by the limited sample size. Further study of this high‐risk group is warranted.
Renal artery stenting remains a promising intervention for resistant hypertension and possibly for ischemic nephropathy, as well. However, a systematic review of published studies could not determine whether there are clinical benefits to renal artery stenting because of lack of prospective trials comparing medical therapy to current available technology. 30 Advances in protective technology adapted from coronary vein grafting intervention suggest potential benefit, yet our experience did not detect improved eGFR with addition of DEP to stenting beyond stenting alone.
This small dual‐center experience was limited by lack of randomization, incomplete follow‐up information, and the fact that it was retrospective. Findings in patients who were seen at 12‐month follow‐up suggest a beneficial result of renal artery stenting with or without DEP. Patients unavailable for follow‐up at 12 months (n=10) included those who either died (n=1) or were put on hemodialysis (n=3). Patients with an initial decline of renal function at 6 months in the stenting/DEP group were less likely to be included in the 12‐month follow‐up analysis, either due to dialysis (n=2) or lack of follow‐up data (n=5). This may be due to an increased disease burden in the patients selected for DEP.
This study complements existing studies evaluating renal artery stenting with DEP, especially among patients with abnormal renal function. 27 , 28 Angiographic criteria and clinical considerations proposed in this study could form the basis of a prospective trial examining the utility of DEP with renal artery stenting. Other studies that have shown promising clinical predictors for renal artery intervention success, such as angiographic blush and frame count, have not assessed utility when using DEP. 31 Ongoing trials, as presently constructed, do not include a long‐term examination of the effectiveness of stenting/DEP compared with stenting alone. 9 A longitudinal analysis of stenting alone compared with stenting/DEP, in the context of risk factor management, is needed to define the role of DEP technology and its use in renovascular disease, particularly in hypertensive patients with CKD.
Acknowledgments
Acknowledgments: The authors would like to thank Ms. Brenda Reig for her important assistance in patient identification and information gathering for this work
References
- 1. Sawicki PT, Kaiser S, Heinemann L, et al. Prevalence of renal artery stenosis in diabetes mellitus‐an autopsy study. J Intern Med. 1991;229:489–492. [DOI] [PubMed] [Google Scholar]
- 2. Olin JW, Melia M, Young JR, et al. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am J Med. 1990;88:46N–51N. [PubMed] [Google Scholar]
- 3. Crowley JJ, Santos RM, Peter RH, et al. Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J. 1998;136:913–918. [DOI] [PubMed] [Google Scholar]
- 4. Moser M, Setaro JF. Clinical practice. Resistant or difficult‐to‐control hypertension. N Engl J Med. 2006;355:385–392. [DOI] [PubMed] [Google Scholar]
- 5. Dorros G, Jaff M, Mathiak L, et al. Multicenter Palmaz stent renal artery stenosis revascularization registry report: four‐year follow‐up of 1,058 successful patients. Catheter Cardiovasc Interv. 2002;55:182–188. [DOI] [PubMed] [Google Scholar]
- 6. Blum U, Krumme B, Flugel P, et al. Treatment of ostial renal‐artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med. 1997;336:459–465. [DOI] [PubMed] [Google Scholar]
- 7. Kennedy DJ, Colyer WR, Brewster PS, et al. Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis. 2003;42:926–935. [DOI] [PubMed] [Google Scholar]
- 8. Van de Ven PJ, Kaatee R, Beutler JJ, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999;353:282–286. [DOI] [PubMed] [Google Scholar]
- 9. Murphy TP, Cooper CJ, Dworkin LD, et al. The cardiovascular outcomes with renal atherosclerotic lesions (CORAL) study: rationale and methods. J Vasc Interv Radiol. 2005;16:1295–1300. [DOI] [PubMed] [Google Scholar]
- 10. Hiramoto J, Hansen KJ, Pan XM, et al. Atheroemboli during renal artery angioplasty: an ex vivo study. J Vasc Surg. 2005;41:1026–1030. [DOI] [PubMed] [Google Scholar]
- 11. Baim DS, Wahr D, George B, et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto‐coronary bypass grafts. Circulation. 2002;105:1285–1290. [PubMed] [Google Scholar]
- 12. Gill KS, Fowler RC. Atherosclerotic renal arterial stenosis: clinical outcomes of stent placement for hypertension and renal failure. Radiology. 2003;226:821–826. [DOI] [PubMed] [Google Scholar]
- 13. Tuttle KR, Chouinard RF, Webber JT, et al. Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis. 1998;32:611–622. [DOI] [PubMed] [Google Scholar]
- 14. Henry M, Amor M, Henry I, et al. Stents in the treatment of renal artery stenosis: long‐term follow‐up. J Endovasc Surg. 1999;6:42–51. [DOI] [PubMed] [Google Scholar]
- 15. Rocha‐Singh KJ, Mishkel GJ, Katholi RE, et al. Clinical predictors of improved long‐term blood pressure control after successful stenting of hypertensive patients with obstructive renal artery atherosclerosis. Catheter Cardiovasc Interv. 1999;47:167–172. [DOI] [PubMed] [Google Scholar]
- 16. Henry M, Klonaris C, Henry I, et al. Protected renal stenting with the PercuSurge GuardWire device: a pilot study. J Endovasc Ther. 2001;8:227–237. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 19. Grube E, Gerckens U, Yeung AC, et al. Prevention of distal embolization during coronary angioplasty in saphenous vein grafts and native vessels using porous filter protection. Circulation. 2001;104:2436–2441. [DOI] [PubMed] [Google Scholar]
- 20. Stone GW, Rogers C, Ramee S, et al. Distal filter protection during saphenous vein graft stenting: technical and clinical correlates of efficacy. JACC. 2002;40:1882–1888. [DOI] [PubMed] [Google Scholar]
- 21. The seventh report of the Joint National Committee on the prevention, detection, evaluation, and treatment of high BP. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 22. Conlon PJ, Little MA, Pieper K, et al. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60:1490–1497. [DOI] [PubMed] [Google Scholar]
- 23. Johansson M, Herlitz H, Jensen G, et al. Increased cardiovascular mortality in hypertensive patients with renal artery stenosis. Relation to sympathetic activation, renal function and treatment regimens. J Hypertens. 1999;17:1743–1750. [DOI] [PubMed] [Google Scholar]
- 24. Dorros G, Jaff M, Mathiak L, et al. Renal function and survival after renal artery stent revascularization may be influenced by embolic debris. J Invasive Cardiol. 2004;16:189–195. [PubMed] [Google Scholar]
- 25. Cooper CJ, Murphy TP, Matsumoto A, et al. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006;152:59–66. [DOI] [PubMed] [Google Scholar]
- 26. Cooper CJ, Haller ST, Colyer W, et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117:2752–2760. [DOI] [PubMed] [Google Scholar]
- 27. Holden A, Hill A. Renal angioplasty and stenting with distal protection of the main renal artery in ischemic nephropathy: early experience. J Vasc Surg. 2003;38:962–968. [DOI] [PubMed] [Google Scholar]
- 28. Holden A, Hill A, Jaff MR, et al. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int. 2006;70:948–955. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Mubarak N, Roubin GS, Vitek JJ, et al. Effect of the distal‐balloon protection system on microembolization during carotid stenting. Circulation. 2001;104:1999–2002. [DOI] [PubMed] [Google Scholar]
- 30. Balk E, Raman G, Chung M, et al. Effectiveness of management strategies for renal artery stenosis: a systematic review. Ann Intern Med. 2006;145:901–912. [DOI] [PubMed] [Google Scholar]
- 31. Mahmud E, Smith TW, Palakodeti V, et al. Renal frame count and renal blush grade: quantitative measures that predict the success of renal stenting in hypertensive patients with renal artery stenosis. J Am Coll Cardiol Intv. 2008;1:286–292. [DOI] [PubMed] [Google Scholar]


