Abstract
β‐blockers are prescribed for a variety of cardiovascular conditions including hypertension, heart failure, primary treatment of myocardial infarction (MI), and secondary prevention of ischemic cardiac events. Yet they remain underprescribed in populations at increased risk for cardiovascular disease because of tolerability and safety concerns. β‐Blockers are heterogeneous with respect to pharmacokinetic and pharmacodynamic effects. “Original” agents were nonselective, blocking both β1‐adrenoceptors and β2‐adrenoceptors. Later, new agents were developed with selectivity for β1‐adrenoceptors, and were subsequently followed by β‐blockers, which exhibit additional effects, such as vasodilation. Among newer agents, labetalol, carvedilol, and nebivolol have been approved for use in the United States. Nebivolol possesses both β1‐selectivity and nitric oxide–mediated vasodilatory effects, while carvedilol has attractive effects on insulin resistance and exhibits antioxidant effects. Newer β‐blockers may overcome concerns about efficacy, adverse effects, and tolerability, while delivering cardiovascular protection.
β‐Blockers are widely used for the treatment of ischemic heart disease, hypertension, and heart failure, as well as for a number of noncardiovascular diseases. They are recommended by the American Heart Association/American College of Cardiology and the European Society of Cardiology as standard therapy for patients who have acute myocardial infarction (MI), as secondary prevention in those with a history of MI, and for patients with heart failure. 1 , 2 As antihypertensive agents, β‐blockers are recommended as initial agents in patients with ischemic heart disease or heart failure and are suggested as an add‐on treatment with other antihypertensive agents in a variety of clinical situations. 3
Nevertheless, β‐blockers remain underutilized, even in situations in which they have proven mortality benefits. This is due in part to concerns about potential adverse effects or efficacy in certain subpopulations. The present article reviews the history of β‐blockers, the major indications for which these drugs are prescribed, and recent pharmacologic innovations that have occurred as this medication class has evolved.
History of β‐Blockers
The classification of the adrenoreceptors by Raymond Ahlquist in 1948 was not only a major scientific event but also provided a basis for the development of a new class of medications. 4 , 5 Later, Nobel Prize winner James Black developed the first β‐blocker called pronethalol. This compound showed attractive clinical effects of cardiac β‐blockade without peripheral effects on α‐receptors, but was withdrawn from the market because of its association with tumors in rodents. In 1964, propranolol became the successor, and ultimately was used for the treatment of a wide variety of cardiovascular diseases. 5 During his research, Black modified Ahlquist’s original dual α‐ and β‐receptor theory into a more complicated classification with more receptor subdivisions—in particular β1‐ and β2‐receptors—and his work suggested the possibility of further refining the selectivity of β‐blockers. Subsequently, additional β‐blockers were developed. To date, 17 different β‐blockers have been approved in the United States. 6
Therapeutic Applications of β‐Blockers
Although initially developed as antianginal agents, β‐blockers were subsequently studied for the treatment of arrhythmias, hypertension, and heart failure. Rapidly, these medications became widely used as initial antihypertensive agents in the general population. Table I lists the approved indications for β‐blockers in the United States. 6
Table I.
β‐Blockers Approved in the United States 7
| Generic Name | Indications |
|---|---|
| Acebutolol | Angina pectoris; ventricular arrhythmias |
| Atenolol | Angina pectoris; hypertension; cardiovascular mortality prophylaxis in post‐MI patients |
| Betaxolol | Hypertension; glaucoma (topical formulation) |
| Bisoprolol | Hypertension |
| Carteolol | Hypertension; glaucoma (topical formulation) |
| Carvedilol | Hypertension; moderate and severe congestive heart failure |
| Esmolol | Supraventricular arrhythmias (IV only) |
| Labetalol | Hypertension; hypertensive emergencies (IV) |
| Metoprolol | Angina pectoris; hypertension; moderate congestive heart failure; cardiovascular mortality prophylaxis in post‐MI patients |
| Nadolol | Hypertension; angina pectoris |
| Nebivolol | Hypertension |
| Oxprenolol | Hypertension |
| Penbutolol | Hypertension |
| Pindolol | Hypertension |
| Propranolol | Angina pectoris; arrhythmias; hypertension; migraine prophylaxis; essential tremor; hypertrophic cardiomyopathy; cardiovascular mortality prophylaxis in post‐MI patients |
| Sotalol | Ventricular and atrial arrhythmias |
| Timolol | Hypertension; cardiovascular mortality prophylaxis in post‐MI patients; glaucoma (topical formulation) |
Abbreviations: IV, intravenous; MI, myocardial infarction.
Differences in Pharmacologic Effects
β‐Blockers differ with respect to their β‐receptor selectivity, intrinsic sympathomimetic (or partial agonist) activity (ISA), membrane‐stabilizing activity, lipophilicity, vasodilatory mechanisms, and pharmacokinetic characteristics (Table II). 6 Lipophilic agents, such as metoprolol and atenolol, can cross the blood‐brain barrier, resulting in a greater incidence of central nervous system effects such as lethargy, confusion, and depression. 7 Agents such as atenolol, sotalol, and nadolol are renally excreted, and their doses should be modified accordingly. 7 β1‐selectivity is of clinical interest because at clinically used doses, β1‐selective agents block cardiac β‐receptors while having minor effects on bronchial and vascular β‐receptors.
Table II.
| Drug | β1/β2‐Selectivity | ISA | Lipophilicity | MSA | Ancillary Effects |
|---|---|---|---|---|---|
| Nonselective β‐adrenergic antagonists | |||||
| Carteolol | 0 | + | Low | 0 | N/A |
| Nadolol | 0 | 0 | Low | 0 | N/A |
| Penbutolol | 0 | + | Moderate | 0 | N/A |
| Pindolol | 0 | ++ | High | + | N/A |
| Propranolol | 0 | 0 | High | ++ | N/A |
| Sotalol | 0 | 0 | Low | 0 | Additional antiarrhythmic effects |
| Timolol | 0 | 0 | High | 0 | N/A |
| Selective β‐adrenergic antagonists | |||||
| Acebutolol | + | + | Moderate | + | N/A |
| Atenolol | + | 0 | Low | 0 | N/A |
| Betaxolol | ++ | 0 | Moderate | 0 | N/A |
| Bisoprolol | ++ | 0 | Moderate | 0 | N/A |
| Esmolol (IV only) | ++ | 0 | Low | 0 | N/A |
| Metoprolol | ++ | 0 | High | 0 | N/A |
| α1‐Adrenergic and β‐adrenergic antagonists | |||||
| Labetalol | + | 0 | Low | 0 | α1‐adrenergic–blocking activity; direct β2‐vasodilatory activity |
| Carvedilol | 0 | 0 | Moderate | ++ | α1‐adrenergic–blocking activity; vasodilation |
| Selective β‐adrenergic antagonists with ancillary properties | |||||
| Nebivolol | +++ | 0 | Moderate | 0 | Endothelium‐dependent, NO‐mediated vasodilation |
Abbreviations: ISA, intrinsic sympathomimetic activity; IV, intravenous; MSA, membrane‐stabilizing activity; N/A, not applicable; NO, nitric oxide.
Earlier β‐blockers, such as propranolol, are nonselective, blocking both β1‐ and β2‐adrenoceptors. Later, selective for β1‐adrenoceptors, known also as cardioselective, were developed, and most recently β‐blockers with vasodilatory effects have emerged. Among these, labetalol, carvedilol, and nebivolol are approved by the Food and Drug Administration for use in the United States.
Carvedilol is a lipophilic nonselective β‐blocker with vasodilatory actions and no ISA. It is rapidly absorbed orally and extensively metabolized by the liver. 8 In hypertensive patients, carvedilol decreases blood pressure (BP), heart rate, and cardiac index, with an associated decrease in the peripheral vascular resistance. Its vasodilatory effects are considered to be secondary to α1‐receptor antagonism. 9 As it is known that conventional β‐blockers are detrimental for glucose control and insulin sensitivity, effects of carvedilol on insulin sensitivity have also been a target of interest. The Glycemic Effects in Diabetes Mellitus: Carvedilol‐Metoprolol Comparison in Hypertensives (GEMINI) study was a double‐blind trial that examined 1235 patients with diabetes mellitus and hypertension already using a renin‐angiotensin system blocker agent. Participants were randomized to carvedilol or metoprolol tartrate. The analysis showed a reduction in insulin resistance (measured by homeostasis model assessment of insulin resistance) and hemoglobin A1C in the carvedilol‐treated group when compared with the metoprolol‐treated arm. 10 In addition, carvedilol has antioxidant effects that depend on its tricyclic carbazole moiety and produce free‐radical scavenging and prevention of ferric ion–induced oxidation effects. 11
Nebivolol, the newest‐generation β‐blocker, is a racemate that exhibits both high β1‐selectivity and vasodilating effects (Table II). It was approved recently for the treatment of hypertension in the United States, and it has already been approved for treatment of both hypertension and chronic heart failure (CHF) in Europe. This medication has the highest β1‐selectivity compared with other β‐blockers commonly used in clinical practice. 12 The vasodilation associated with administration of nebivolol is thought to be due to increased bioavailability of nitric oxide (NO). A study in human endothelial cells obtained from African American and white women demonstrated that nebivolol enhanced NO bioavailability and reduced oxidative stress. Furthermore, nebivolol prevented endothelial NO synthase uncoupling and restored NO bioavailability in endothelial cells from black donors to a greater extent than in their white counterparts. The antioxidant effect seems to be related, at least in part, to inhibition of nicotinamide adenine dinucleotide phosphate (reduced) oxidase activity. 13 This is clinically significant, because it has been recognized that black patients have higher rates of endothelial dysfunction than white patients, which might contribute to a higher incidence of hypertension and cardiovascular disease. 14 , 15 A randomized, double‐blind, crossover study in a general hypertensive population demonstrated that the combination of nebivolol plus a thiazide diuretic, given for 3 months enhanced endothelium‐mediated vasodilation, whereas the combination of atenolol and a diuretic did not, despite comparable BP control in the two cohorts. 16
The hemodynamic profile of nebivolol is different from that of other β‐blockers. In a double‐blind randomized study of 25 hypertensive patients, nebivolol 5 mg administered once daily significantly increased stroke volume compared with atenolol 100 mg once daily. The BP effects of nebivolol were associated with a reduction in peripheral resistance, while cardiac output was preserved. 17 Such differences in the mechanism of action of nebivolol may lead to differences in clinical effects, safety, and tolerability compared with older β‐blockers.
Efficacy and Tolerability of β‐Blockers
Efficacy
Major trials have demonstrated the efficacy of β‐blockers in the treatment of hypertension, angina, acute MI, arrhythmias (including sudden death), and heart failure. 18 , 19 , 20 , 21 , 22 , 23 , 24
Myocardial Infarction
It was reported as early as 1965 that propranolol, given to patients after MI, reduced mortality. Later, the β‐Blocker Heart Attack Trial (BHAT) and the Norwegian Multicenter Study Group Trial demonstrated significant reductions in mortality with propranolol and timolol, respectively. 19 More recently, in the Carvedilol Post‐Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial, a significant reduction in all‐cause mortality in post‐MI patients with reduced left ventricular function was seen with carvedilol treatment, compared with placebo. 21 Based on these findings, international guidelines committees recommend β‐blockers for secondary prevention for all patients without contraindications who have experienced acute MI. 2 , 3
Clinical benefits have also been well documented when β‐blockers are administered during the acute phase of MI. Several major studies have evaluated β‐blockers in patients with ST‐segment elevation MI (STEMI). Prior to the advent of reperfusion therapy, 2 studies demonstrated reduced early mortality as a result of β‐blocker therapy. In the First International Study of Infarct Survival (ISIS‐1), treatment with intravenous atenolol followed by oral atenolol was compared with placebo in patients within 12 hours of presentation to the hospital. Atenolol was associated with a 15% relative reduction in mortality over 7 days (P=.05). 22 Similar reductions in mortality were seen in the Metoprolol in Acute Myocardial Infarction (MIAMI) study. 23 In patients receiving thrombolytic therapy, the benefits of β‐blockers were confirmed in the Thrombolysis in Myocardial Infarction Phase II‐B (TIMI‐IIB) trial. This study demonstrated that early treatment with metoprolol (within 2 hours of initiation of thrombolytic therapy) in patients with acute MI resulted in lower rates of death, reinfarction, and recurrent ischemia than treatment with metoprolol 6 days after presentation. 24
Hypertension
β‐Blockers have also been shown to be effective in the primary prevention of cardiac events in hypertensive patients. Of note, the usefulness of conventional β‐blockers to reduce major cardiovascular outcomes related to hypertension in the population without heart failure or ischemic heart disease has been recently scrutinized. The Blood Pressure–Lowering Arm of the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT‐BPLA), which examined 19,257 hypertensive patients with at least 3 other cardiovascular risk factors to either an atenolol‐based treatment with a diuretic added as needed or to an amlodipine‐based treatment with an angiotensin‐converting enzyme inhibitor added as needed showed that major cardiac and stroke outcomes favored the amlodipine‐based treatment arm. 25 Furthermore, a meta‐analysis by Lindholm and colleagues 26 confirmed a negative effect of β‐blocker use for treatment of uncomplicated hypertension with respect to stroke prevention when compared with other antihypertensive therapy. The negative effects seen with β‐blocker therapy have been attributed to more frequent adverse metabolic effects (lower high‐density lipoprotein cholesterol and higher triglyceride and blood glucose levels) as well as to unfavorable hemodynamic effects (less reduction on central BP). 27 Importantly, and as mentioned earlier, newer β‐blockers have become available in the past couple of years with neutral or beneficial metabolic effects and a more favorable hemodynamic profile.
According to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), β‐blockers are recommended for initial treatment of patients with stage 1 hypertension with compelling indications (eg, previous MI, ischemic heart disease, heart failure, diabetes, or high risk of coronary disease). 3 They may also be considered for initial treatment in patients with stage 1 hypertension without compelling indications and in patients with stage 2 hypertension who require a combination of drugs to achieve BP control. Nevertheless, taking into consideration the unsatisfactory cardiovascular outcomes obtained with older β‐blockers, it would be useful to carefully examine the role of β‐blockers in the treatment of hypertension in the general population and the potential advantages that new vasodilatory β‐blockers might bring in terms of cardiovascular disease prevention and management.
Heart Failure
All patients with stable, mild, moderate, and severe congestive heart failure in whom there is no contraindication, should receive β‐blocker treatment. 2 Recent trials with extended‐release metoprolol, bisoprolol, and carvedilol have demonstrated significant morbidity and mortality benefits in β‐blocker–treated patients compared with placebo‐treated patients, with a mortality reduction of approximately 35% across trials. 28 Nebivolol was recently studied in patients 70 years and older with heart failure independent of left ventricular ejection fraction at study entry. After a mean follow‐up of 21 months, there was a significant reduction in all‐cause mortality or cardiovascular hospitalization in the nebivolol‐treated patients compared with placebo‐treated patients. 29 The Figure shows the comparative range of effect from various β‐blocker heart failure trials. 29 , 30 , 31 , 32 , 33
Figure.
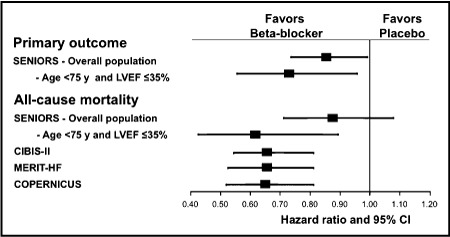
Summary of the effects from various β‐blocker heart failure trials. Primary outcome in the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure (SENIORS) was defined as a composite of all‐cause mortality or cardiovascular hospital admission (time to first event). Mean age was 61 years in the Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II); 63 years in the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial; 64 years in the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT‐HF); and 76 years in SENIORS. LVEF indicates left ventricular ejection fraction; CI, confidence interval.
Utilization of β‐Blockers
In spite of compelling evidence of benefit from β‐blocker treatment in a number of disease states, there is still underutilization of β‐blockers in clinical practice. Gottlieb and colleagues 34 reviewed medical records of 201,752 patients with MI and found that only 34% received β‐blockers. The percentage was even lower among high‐risk populations such as patients with type 1 diabetes, patients with elevated serum creatinine concentrations, lowest ejection fractions, and heart failure. Importantly, mortality was lower in every subgroup of patients treated with β‐blockade when compared with untreated patients. As mentioned previously, the underutilization of β‐blockers may partially result from doubts that have recently been raised about their efficacy.
Potential differences in the effects of the newer vasodilating agents, such as carvedilol and nebivolol, on arterial stiffness and central aortic pressure, compared with nonvasodilating β‐blockers may be an important consideration in this regard. 28 Two recent studies have shown that while both nebivolol and atenolol have similar effects on brachial BP and aortic stiffness, nebivolol has a more pronounced effect on reducing aortic pulse pressure and wave reflection as well as on increasing pulse pressure amplification. 35 , 36 Whether these differences will translate into differential effects in clinical outcomes will require further investigation. A recent meta‐analysis of 21 heart failure trials, however, demonstrated that vasodilating β‐blockers were associated with a greater reduction in mortality relative to nonvasodilating agents, especially in patients with nonischemic heart disease. 20
Suboptimal use of β‐blockers is more pronounced in some patient subgroups. In African American patients with compelling indications, β‐blockers should be used according to current guidelines. Newer‐generation β‐blockers, such as nebivolol with its NO‐mediated vasodilatory effects, may be particularly efficacious in reducing BP in black patients. 37
Adverse Events
The reluctance of some clinicians to prescribe β‐blockers may be due to concerns about tolerability and adverse effects such as depression, fatigue, and sexual dysfunction. 38 Analysis of 15 large randomized trials of β‐blocker treatment of 35,000 patients with MI, heart failure, or hypertension revealed that although β‐blockers were not associated with an increased risk of depression, they were associated with small but significant increases in fatigue and sexual dysfunction. The risk of fatigue was significantly higher in patients treated with early‐generation β‐blockers compared with later‐developed drugs. Studies in hypertensive patients that compared nebivolol with atenolol demonstrated that nebivolol was associated with fewer adverse events. 39
Sexual dysfunction has been reported as an adverse drug effect in 20% of cases. 40 This is particularly problematic, since real or perceived drug‐related erectile dysfunction may result in noncompliance with medical therapy. A study of 44 hypertensive patients receiving β‐blocker monotherapy found that substitution of nebivolol for prior β‐blocker therapy resulted in a significant improvement in erectile function. 40 In another study, 131 male patients with no history of erectile dysfunction (mean age, 47.3±4.6 years) were randomized to receive 12 weeks of therapy with nebivolol 5 mg/d (n=43), atenolol 50 mg/d (n=44), or atenolol 50 mg/d plus chlorthalidone 12.5 mg/d (n=44). 41 Erectile function, as measured by the number of instances of successful intercourse per month, was assessed by a questionnaire. At 12 weeks, the mean number of episodes of satisfactory sexual intercourse per month was significantly decreased from baseline in the groups receiving atenolol (from 7.0 to 3.7; P<.01) and atenolol plus chlorthalidone (from 6.4 to 2.8; P<.01), but not with nebivolol (6.4 at baseline vs 6.0 at follow‐up). The authors concluded that the NO‐mediated vasodilatory effects of nebivolol may contribute to sustained erectile function.
Finally, there have also been concerns about the effects of β‐blockers on quality of life. A study comparing nebivolol with losartan in hypertensive patients demonstrated that there were no significant differences between the 2 drugs on quality‐of‐life scales that measured symptomatic well‐being. 42 The cardioselectivity and NO‐mediated vasodilation of nebivolol may make it better tolerated than earlier‐generation β‐blockers.
Conclusions
Since the discovery of the first‐approved β‐blocker, this class of drugs has evolved to be a cornerstone of cardiovascular medicine. Improvements in pharmacokinetic and pharmacodynamic effects with newer agents have expanded the β‐blocker class of drugs. Likewise, studies have demonstrated the wide variety of diseases for which this class is beneficial. Nevertheless, despite clear evidence supporting their use, there is continued underutilization of β‐blockers, which may be due to clinician concerns regarding efficacy and tolerability. The current generation of β‐blockers could overcome concerns about efficacy, metabolic and symptomatic adverse effects, and tolerability. Carvedilol is approved for the treatment of heart failure and hypertension. The newest β‐blocker is nebivolol, which, due to its NO‐induced vasodilatory effects and high β1‐selectivity, has demonstrated a hemodynamic profile that confers beneficial effects for its approved indication of hypertension, as well as for heart failure.
Disclosure: Thomas D. Giles, MD, is a consultant and speaker for Forest Laboratories. James R. Sowers, MD, is a consultant for Forest Laboratories. Research for this paper was funded by an investigator‐initiated grant from Forest Laboratories.
References
- 1. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110:588–636. [DOI] [PubMed] [Google Scholar]
- 2. Lopez‐Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on beta‐adrenergic receptor blockers. Eur Heart J. 2004;25:1341–1362. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 4. Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. [DOI] [PubMed] [Google Scholar]
- 5. Quirke V. Putting theory into practice: James Black, receptor theory and the development of the beta‐blockers at ICI, 1958–1978. Med Hist. 2006;50:69–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frishman WH. Alpha‐ and Beta‐Adrenergic Blocking Drugs. In: Frishman WH, Sonnenblick EH, Sica DA, eds. Cardiovascular Pharmacotherapeutics. New York, NY: McGraw‐Hill; 2004:19–57. [Google Scholar]
- 7. Sica DA, Black HR. Pharmacologic considerations in the positioning of beta‐blockers in antihypertensive therapy. Curr Hypertens Rep. 2008;10:330–335. [DOI] [PubMed] [Google Scholar]
- 8. Stafylas PC, Sarafidis PA. Carvedilol in hypertension treatment. Vasc Health Risk Manag. 2008;4:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Afonso RA, Patarrao RS, Macedo MP, et al. Carvedilol action is dependent on endogenous production of nitric oxide. Am J Hypertens. 2006;19:419–425. [DOI] [PubMed] [Google Scholar]
- 10. Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. [DOI] [PubMed] [Google Scholar]
- 11. Dandona P, Ghanim H, Brooks DP. Antioxidant activity of carvedilol in cardiovascular disease. J Hypertens. 2007;25:731–741. [DOI] [PubMed] [Google Scholar]
- 12. Bristow MR, Nelson P, Minobe W, et al. Characterization of β1‐adrenergic receptor selectivity of nebivolol and various other beta‐blockers in human myocardium. Am J Hypertens. 2005;18:51A–52A. Abstract P‐121. [Google Scholar]
- 13. Mason RP, Kalinowski L, Jacob RF, et al. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation. 2005;112:3795–3801. [DOI] [PubMed] [Google Scholar]
- 14. Campia U, Choucair WK, Bryant MB, et al. Reduced endothelium‐dependent and ‐independent dilation of conductance arteries in African Americans. J Am Coll Cardiol. 2002;40:754–760. [DOI] [PubMed] [Google Scholar]
- 15. Cardillo C, Kilcoyne CM, Cannon RO III, et al. Racial differences in nitric oxide‐mediated vasodilator response to mental stress in the forearm circulation. Hypertension. 1998;31:1235–1239. [DOI] [PubMed] [Google Scholar]
- 16. Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double‐blind, crossover study. Circulation. 2001;104:511–514. [DOI] [PubMed] [Google Scholar]
- 17. Kamp O, Sieswerda GT, Visser CA. Comparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertension. Am J Cardiol. 2003;92(3):344–348. [DOI] [PubMed] [Google Scholar]
- 18. Wikstrand J, Warnold I, Tuomilehto J, et al. Metoprolol versus thiazide diuretics in hypertension. Morbidity results from the MAPHY Study. Hypertension. 1991;17:579–588. [DOI] [PubMed] [Google Scholar]
- 19. Gheorghiade M, Goldstein S. Beta‐blockers in the post‐myocardial infarction patient. Circulation. 2002;106:394–398. [DOI] [PubMed] [Google Scholar]
- 20. Bonet S, Agustí A, Arnau JM, et al. Beta‐adrenergic blocking agents in heart failure: benefits of vasodilating and non‐vasodilating agents according to patients’ characteristics: a meta‐analysis of clinical trials. Arch Intern Med. 2000;160:621–627. [DOI] [PubMed] [Google Scholar]
- 21. McMurray J, Køber L, Robertson M, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post‐Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol. 2005;45:525–530. [DOI] [PubMed] [Google Scholar]
- 22. First International Study of Infarct Survival Collaborative Group . Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS‐1. Lancet. 1986;2:57–66. [PubMed] [Google Scholar]
- 23. The MIAMI Trial Research Group . Metoprolol in acute myocardial infarction (MIAMI). A randomised placebo‐controlled international trial. Eur Heart J. 1985;6:199–226. [PubMed] [Google Scholar]
- 24. Roberts R, Rogers WJ, Mueller HS, et al. Immediate versus deferred beta‐blockade following thrombolytic therapy in patients with acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) II‐B Study. Circulation. 1991;83:422–437. [DOI] [PubMed] [Google Scholar]
- 25. Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 26. Lindholm LH, Carlberg BH, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta‐analysis. Lancet. 2005;366:1545–1553. [DOI] [PubMed] [Google Scholar]
- 27. Weber MA, Bakris GL, Giles TD, et al. Beta‐blockers in the treatment of hypertension: new data, new directions. J Clin Hypertens (Greenwich). 2008;10:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen ME, Cockcroft JR. The latest generation of beta‐blockers: new pharmacologic properties. Current Hypertens Rep. 2006;8:279–286. [DOI] [PubMed] [Google Scholar]
- 29. Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. [DOI] [PubMed] [Google Scholar]
- 30. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. New Engl J Med. 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 31. Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. [DOI] [PubMed] [Google Scholar]
- 32. Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT‐HF). JAMA. 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 33. MERIT‐HF Study Group . Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention Trial in congestive heart failure (MERIT‐HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 34. Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta‐blockade on mortality among high‐risk and low‐risk patients after myocardial infarction. New Engl J Med. 1998;339:489–497. [DOI] [PubMed] [Google Scholar]
- 35. Mahmud A, Feely K. β‐blockers reduced aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–667. [DOI] [PubMed] [Google Scholar]
- 36. Dhakam Z, Yasmin McEniery CM, Burton T, et al. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–356. [DOI] [PubMed] [Google Scholar]
- 37. Saunders E, Smith WB, DeSalvo KB, et al. The efficacy and tolerability of nebivolol in hypertensive African American patients. J Clin Hypertens (Greenwich). 2007;9(11):866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ko DT, Hebert PR, Coffey CS, et al. Beta‐blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288:351–357. [DOI] [PubMed] [Google Scholar]
- 39. Doumas M, Doumas S. The effect of antihypertensive drugs on erectile function: a proposed management algorithm. J Clin Hypertens (Greenwich). 2006;8:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boydak B, Nalbantail S, Fici F, et al. A randomised comparison of the effects of nebivolol and atenolol with and without chlorthalidone on the sexual function of hypertensive men. Clin Drug Investig. 2005;25:409–416. [DOI] [PubMed] [Google Scholar]
- 41. Grassi G, Trevano FQ, Facchina A, et al. Efficacy and tolerability profile of nebivolol vs atenolol in mild‐to‐moderate essential hypertension: results of a double‐blind randomized multicentre trial. Blood Press Suppl. 2003;2:35–40. [PubMed] [Google Scholar]
- 42. Van Bortel LM, Bulpitt CJ, Fici F. Quality of life and antihypertensive effect with nebivolol and losartan. Am J Hypertens. 2005;18:1060–1066. [DOI] [PubMed] [Google Scholar]


