Pheochromocytomas are rare neuroendocrine tumors usually associated with severe or intermittent hypertension. Pheochromocytomas derive from chromaffin cells of the sympathetic nervous system and produce excess amounts of catecholamines, which are responsible for hypertensive surges, palpitations, headache, and diaphoresis commonly found with these tumors. Less commonly, pheochromocytomas result in hypotension or cyclic hypertension and hypotension. We report a case of pheochromocytoma presenting with pulmonary hemorrhage and hemoptysis, anterior ST‐elevation pattern on electrocardiography, and severe hemodynamic instability. The management and treatment of the patient in this case report and the proposed mechanisms of his presentation are discussed.
Case Report
A 40‐year‐old man was transferred to our hospital for emergent cardiac catheterization. The patient had a history of hypertension and congestive heart failure (CHF) secondary to ephedrine use 5 years earlier. On the evening prior to presentation, he experienced several episodes of nonbloody, nonbilious emesis associated with nausea and headache. Frank hemoptysis occurred, prompting his visit to a local emergency department. The patient had been experiencing similar episodes of vomiting without hemoptysis about once a month for the previous 10 months. With these episodes, he had severe headaches, dizziness, diaphoresis, and nausea. He denied chest pain, shortness of breath, diarrhea, or palpitations. He had a known adverse drug reaction to codeine (nausea) and was taking quinapril for hypertension; montelukast, cetirizine, and triamcinolone nasal spray for seasonal allergies; and acetaminophen/aspirin/caffeine pills for headache. He reported extensive past use of dietary supplements used by bodybuilders including glutamine, branched chain amino acids, carnitine, and other unknown supplements purchased on the Internet. He denied use of anabolic steroids or ephedrine since his episode of CHF 5 years previously and denied cocaine use. His cardiac function was reported to have returned to normal after the acute episode of CHF in 2002. He was known to be negative for the human immunodeficiency virus in 2004 and denied sexual exposures. He worked as an airplane inspector and did not smoke or drink alcohol.
In the emergency department, he remained nauseated but did not have further episodes of vomiting or hemoptysis. He denied chest pain or shortness of breath. His vital signs included a heart rate of 116 beats per minute, blood pressure (BP) of 85/59 mm Hg, respiratory rate of 16 breaths per minute, and oxygen saturation of 96%. He was found to be orthostatic, with a change in his pulse rate from 100 to 122 to 138 beats per minute in the supine, sitting, and erect positions, respectively. His examination was unremarkable for cardiac abnormalities. His lungs were clear and his lower extremities were nonedematous. His initial laboratory values revealed erythrocytosis and an elevated creatinine level of 1.9 mg/dL. He also had an elevated troponin concentration of 2.3 ng/mL (Table). ECG findings revealed 1‐mm ST‐elevations in the precordial leads, with T waves flattened in leads II and III and T waves inverted in leads II and aVL. Echocardiography performed at the bedside revealed diffuse hypokinesis. He received intravenous (IV) methylprednisolone for hypotension, was started on IV heparin, and was transferred to our hospital for emergent cardiac catheterization. This catheterization revealed completely normal coronary anatomy without plaque or obstruction. During the procedure, the patient had an episode of supraventricular tachycardia (SVT) that subsided with 6 mg of adenosine. His systolic BP throughout the catheterization was in the 100 range. Heparin was discontinued.
Table.
Laboratory Values Throughout the Patient’s Hospital Course
| Patient’s Value | Normal Range | |
|---|---|---|
| Initial laboratory values on presentation | ||
| Hematocrit, % | 52.7 | 40–52 |
| Creatinine, mg/dL | 1.9 | 0.5–1.2 |
| Troponin‐I, ng/mL | 2.3 | <0.04 |
| In intensive care unit, after catheterization | ||
| Chest x‐ray | Minimal perihilar infiltrates | – |
| Hematocrit, % | 59.8 | 40–52 |
| Urinalysis | 3+ protein, large blood, 2–5 red blood cells | – |
| In intensive care unit, 6 hours later | ||
| Troponin, ng/mL | 2.22 | <0.04 |
| Lipase, U/L | 159 | <60 |
| Erythropoietin, mIU/mL | 29 | 5–15 |
| Hematocrit, % | 64.5 | 40–52 |
| Urinalysis | Normal | – |
| Erythrocyte sedimentation rate, mm/h | 2 | 0–20 |
| C‐reactive protein, mg/L | 10 | 0.1–4.9 |
| Cortisol, μg/dL | 22 | 7–25 |
| Reticulocyte count, % | 0.006 | 0.6–2.7 |
| Other laboratory values | ||
| Antineutrophil cytoplasmic antibody | Negative | – |
| Antinuclear antibody | Negative | – |
| Anti‐glomerular basement membrane antibody | Negative | – |
| Anti–double‐stranded DNA antibody | Negative | – |
| 24‐H urine, norepinephrine, μg | 75 | 0–80 |
| 24‐H urine, epinephrine, μg | 180 | 0–20 |
The patient was admitted to the cardiac intensive care unit and was noted to be hypertensive, with a BP to 208/106 mm Hg and a pulse rate of 130 beats per minute. He was given 2.5 mg of IV metoprolol and subsequently had a BP of 160/58 mm Hg with a pulse rate of 130 beats per minute. He became extremely diaphoretic and developed frank hemoptysis with labored breathing. He was unable to maintain a normal oxygen saturation level and was intubated for ventilatory support. Frank bloody secretions were continuously suctioned from his endotracheal tube. A radial arterial line was placed and the patient’s BP was found to fluctuate from peaks in the 200s to as low as the 30s and 40s (Figure 1A and 1B). A right internal jugular catheter revealed a central venous pressure of 2 mm Hg. The patient was started on fluids and received empiric antibiotics (ceftriaxone and vancomycin) and stress‐dose steroids. A chest radiograph revealed minimal perihilar infiltrates that later progressed to more extensive infiltrates. Laboratory values at this time showed progressive erythrocytosis (hematocrit, 59.8%) and a urinalysis with 3+ protein and a large amount of blood but only 2 to 5 red blood cells (Table). Results from echocardiography revealed severely depressed ejection fraction of 25% and mildly increased left ventricular cavity size.
Figure 1.
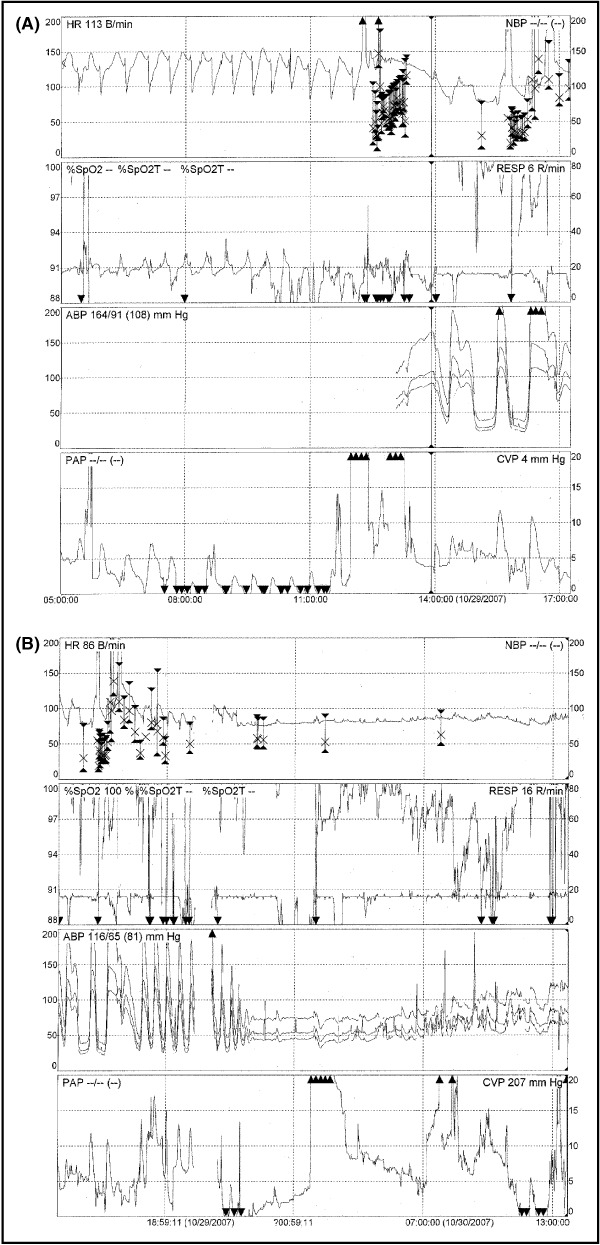
(A) Heart rate, oxygen saturation, arterial blood pressure, and pulmonary artery pressure during cycles of hypertension and hypotension. (B) Continued erratic fluctuations in the same parameters.
Initially, the patient’s differential diagnosis included the pulmonary‐renal syndromes and necrotizing pneumonia. It also included subarachnoid hemorrhage, cardiomyopathy and drug‐related toxicity from his numerous supplements including possibly anabolic steroids, and pheochromocytoma. Pheochromocytoma was suspected given the dramatic pattern of his hypertensive and hypotensive episodes. Management with α‐blockade was initially avoided due to extreme cyclic hypotension and volume depletion.
The patient developed an SVT and received adenosine, magnesium, and calcium gluconate. He was started on an amiodarone infusion. The initial cyclic pattern of his BP changed to persistent hypotension, and pressors were required. Despite concern for catecholamine resistance, associated with pheochromocytoma and downregulation of β‐receptors, the patient’s BP responded to norepinephrine. In the setting of persistent SVT, this was switched to neosynephrine for decreased β‐agonism.
At this time, his troponin remained constant at 2.22 ng/mL and his lipase level was found to be elevated at 159 U/L. An erythropoietin level was found to be 29 mIU/mL (elevated) and his hematocrit level continued to rise. He underwent phlebotomy of 200 cc of blood in an attempt to improve his large A‐a gradient. Additional laboratory results are given in the Table. A computed tomography scan of the abdomen revealed a large mass superior to the left kidney (Figure 2). An endocrine consultation was performed and he was started on metyrosine for catecholamine synthesis blockade. Although α‐blockade could have been instituted at this stage, his volume status was not yet adequately restored and his BP remained low.
Figure 2.
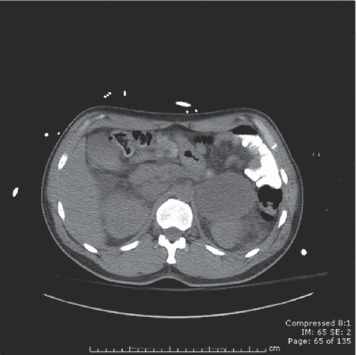
CT scan of the abdomen revealing large mass superior to the left kidney.
With sufficient volume restoration, the patient was weaned off pressors and extubated on the fifth day in the intensive care unit. He did not have further hemoptysis, and his antineutrophil cytoplasmic, antinuclear, anti‐glomerular basement membrane, and anti–double‐stranded DNA antibodies were all negative. He was started on phenoxybenzamine 10 mg twice a day and discharged with a stable systolic BP of 120 to 130 mm Hg on phenoxybenzamine 40 mg twice a day, metyrosine 250 mg every 6 hours, and amiodarone 400 mg daily. A repeat echocardiogram demonstrated normal LV function. His 24‐hour urinary catecholamine collection (off pressors but on metyrosine) revealed a norepinephrine level of 75 μg/total volume (upper level of normal 80 μg) and epinephrine level of 180 μg (upper limit of normal 20 μg). Magnetic resonance imaging confirmed a heterogeneous mass in the region of the left adrenal gland, atypical in appearance for pheochromocytoma (Figure 3). The patient underwent laparoscopic removal of his left‐sided pheochromocytoma, located on the superior aspect of the right lobe of the left kidney (Figure 4A and 4B). Pathological examination confirmed the diagnosis of pheochromocytoma and was graded as a 6 on the Pheochromocytoma of the Adrenal Gland Scaled Score (PASS) system, indicating an increased risk for metastasis.
Figure 3.
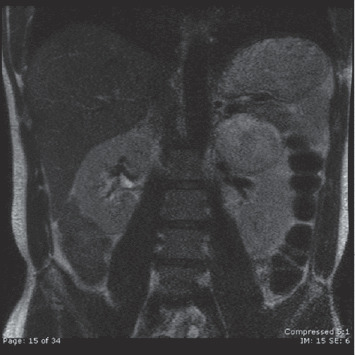
MRI confirming CT finding in the region of the left adrenal gland.
Figure 4.
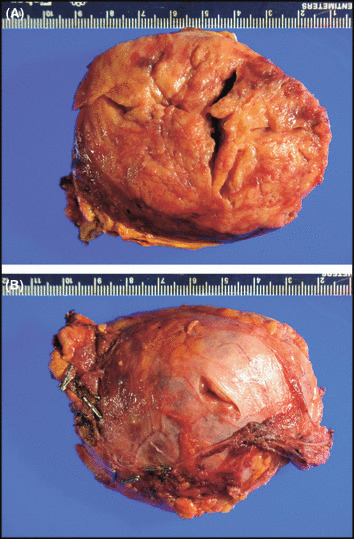
Gross specimen, patient's resected pheochromocytoma.
Discussion
Pheochromocytoma is a rare neuroendocrine tumor, occurring in 0.1% to 0.6% of patients with hypertension. 1 The clinical presentation varies widely, with hypertension, tachycardia, pallor, headache, sweating, and panic attacks being most common, and nausea, fever, and flushing occurring less commonly. 1 Hypertension is the most common presentation, but normal BP and hypotension can also be observed, typically with dopamine‐producing paragangliomas and epinephrine‐secreting tumors. Cyclic hypertension and hypotension, such as that observed in our patient, is observed rarely but is more common with epinephrine‐secreting tumors. Pheochromocytoma can also present as shock, due to extensive intravascular volume depletion, hypocalcemia, and desensitization of adrenergic receptors. 2 , 3 Myocardial infarction, cardiac arrhythmias, and heart failure due to toxic cardiomyopathy can also occur. 4 , 5 Pulmonary hemorrhage and hemoptysis as a presenting symptom have only rarely been reported. 4 , 6 Our patient had several features of pheochromocytoma that have thus far not been simultaneously reported, including pulmonary hemorrhage, cardiomyopathy, cyclic hypertension and hypotension, and renal failure. He also had a number of unusual possible precipitants of his pheochromocytoma crisis. These features are discussed below.
Pulmonary Hemorrhage
The immediate cause of the patient’s visit to the emergency department was hemoptysis, as he had had previous episodes of nausea and vomiting throughout the year. After catheterization, his hemoptysis worsened as his BPs became more severely elevated; he had been given heparin for presumed acute coronary syndrome at the outside emergency department. There have been only a few reports of hemoptysis associated with pheochromocytoma. When present, it is usually in the setting of pulmonary metastases or present at autopsy as hemorrhagic pulmonary edema secondary to severe catecholamine crises. 7 In 1986, Frymoyer and colleagues8 described hemoptysis as a presenting symptom of pheochromocytoma in a patient with poorly controlled hypertension and an isolated right adrenal pheochromocytoma. This patient’s hemoptysis resolved with resolution of hypertension, as in our patient. In 2004, Kimura and colleagues6 described a 59‐year‐old woman with a history of hypertension who presented with massive hemoptysis, moist rales in both lungs, and hypoxemia. Hemoptysis resolved with antihypertensive therapy. 6 Hemoptysis in pheochromocytoma is attributed to elevated BP and pulmonary venous hypertension leading to pulmonary edema and hemorrhage. 8 Severe systemic hypertension can also lead to pulmonary venous hypertension, which may predispose these patients to hemoptysis similar to patients with mitral stenosis and pulmonary hypertension. 8 In addition, vascular injury from catecholamines may result in abnormalities of the coagulation system, specifically activation of the coagulation cascade. 9
Cardiomyopathy
Cardiomyopathy with or without pulmonary edema and cardiac arrhythmias is a more frequent manifestation of pheochromocytoma than is hemoptysis, with greater than 150 reports in the literature dating back more than 50 years. 10 These reports have described a range of pathologies, including dilated cardiomyopathy, 11 tako‐tsubo (or stress‐related) cardiomyopathy, 12 and myocarditis. 4 The common mechanism of these injuries is catecholamine‐mediated. According to echocardiographic results, our patient had slightly enlarged ventricles and diffuse hypokinesis with basal sparing. His ejection fraction on presentation was 25%, with improvement to 50% by discharge. This pattern of tako‐tsubo cardiomyopathy has been described in a recent case of pheochromocytoma 12 and in a paper in this issue of The Journal of Clinical Hypertension (JCH). 13 This is defined as apical and midventricular akinesis or dyskinesis and hyperkinesis of the base. This pattern differs from the dilated cardiomyopathy usually observed in pheochromocytoma, described as 4‐chamber dilatation with a globally depressed left ventricular ejection fraction, 14 which can occur with or without a hypertrophic myocardium. 15 Most cases of cardiomyopathy in pheochromocytoma result in partial or complete reversal following treatment of pheochromocytoma. The majority of cases of dilated cardiomyopathy take 6 weeks to 16 months to improve. 11 The mechanism of myocardial damage secondary to pheochromocytoma is attributed to chronic secretion of catecholamines leading to increases in intracellular calcium, increased sarcomere contraction, and myofibrolysis. In most cases, hypertrophy and moderate inflammation do not result in permanent fibrous replacement of myocardium but, when this occurs, the resulting cardiomyopathy is not completely reversible. 16
Cyclic Hypertension and Hypotension and Orthostatic Hypotension
Pheochromocytoma is usually characterized by episodic hypertension. Our patient initially had orthostatic hypotension but quickly developed rapid cycling between severe hypertension and severe hypotension, with systolic BPs ranging from 200 mm Hg to 40 mm Hg. This cyclical pattern has been described rarely, with the first case report in 1958, 17 several between 1975 and 2005, one in 2008, 18 and one in this issue of JCH. 13 This cyclic pattern of BP is thought to be associated with primarily epinephrine‐secreting tumors, which our patient had. 1 The mechanism of the cyclic pattern is poorly understood but is attributed to rapid changes in blood volume and catecholamine release induced by prolonged exposure to elevated levels of circulating catecholamines. 19 Vasoconstriction experienced in the setting of excessive epinephrine and norepinephrine leads to decreased circulating blood volume and cardiac output, which leads to excessive catecholamine release and hypertension. This hypertension then triggers baroreceptors to reduce BP by a negative feedback loop, leading to decreased peripheral vascular resistance and decreased cardiac output. 18 In the setting of preexisting decreased circulating blood volume, the hypotension is especially pronounced.
Myoglobinuric Renal Failure
Our patient had myoglobinuric renal failure, demonstrated by a large amount of blood measured on urinalysis but only 2 to 5 red blood cells and an elevated creatinine level. His creatine kinase level was 682 U/L and his creatinine level was 1.9 mg/dL on presentation. This renal failure could also have been exacerbated by hypovolemia, contrast exposure, and microangiopathy. Our patient’s renal failure improved with correction of the hemodynamic crisis. In 1990, Shemin and colleagues20 described a patient with rhabdomyolysis and renal failure as a result of an isolated right adrenal pheochromocytoma, which also caused florid pulmonary edema and hypertension. The patient required hemodialysis for severe renal failure with anuria. The mechanism of injury is presumed to be a result of intense vasoconstriction induced by catecholamines, leading to muscle ischemia. 20 Our patient had no other explanation for myoglobinuria, as he had no evidence of muscle injury from prolonged exercise, seizure, or immobility, or ingestion of cocaine, sedatives, or narcotics.
Precipitants
The precipitant of our patient’s episode could not be proven, but he experienced a number of insults. First, the patient was significantly volume‐depleted, as evidenced by his low central venous pressure. A state of hypovolemia is classic for pheochromocytoma due to prolonged vasoconstriction by circulating catecholamines; further volume depletion can precipitate a crisis. 21 Second, the patient had experienced a radiographic contrast load for his cardiac catheterization, which is a known precipitant of pheochromocytoma. 1 , 22 In addition, the patient was given steroids in the setting of hypotension and empiric treatment for sepsis, which could have precipitated further conversion of norepinephrine to epinephrine. 23 Other possible precipitants of his presentation include the unusual nutritional supplements that he was taking, most of which have unknown ingredients. Tyramine‐containing compounds have been implicated in pheochromocytoma attacks and it is unknown whether the patient’s products contained derivatives of this compound. Finally, the patient received a small dose of a β‐blocker, which has also been described to precipitate attacks because of unopposed α‐vasoconstriction activity. 24
Conclusions
It is unclear how long this patient’s tumor was present. The patient described a prodrome of approximately 10 months of nausea, vomiting, and headache similar to the incident that provoked his presentation to the hospital. In addition, he had presented with CHF attributed to ephedrine a few years previously. It is possible that his tumor was already present at this time and the added insult of ephedrine precipitated a previous pheochromocytoma crisis that was unrecognized. Prompt identification of any of the several unusual features in this case may point to the appropriate diagnosis and timely treatment of pheochromocytoma.
Acknowledgments
Acknowledgments: Thank you to Dr Oliver Wang and Dr Constantine Theoharis for assistance with the images in this report.
References
- 1. Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet. 2005;366:665–675. [DOI] [PubMed] [Google Scholar]
- 2. Imperadore F, Azzolini M, Piscioli F, et al. A rare cause of cardiogenic shock: catecholamine cardiomyopathy of pheochromocytoma. Italian Heart J. 2002;3:375–378. [PubMed] [Google Scholar]
- 3. Hamrin B. Sustained hypotension and shock due to an adrenaline‐secreting phaeochromocytoma. Lancet. 1962;2:123–124. [DOI] [PubMed] [Google Scholar]
- 4. Dinckal MH, Davutoglu V, Soydinc S, et al. Phaeochromocytoma‐induced myocarditis mimicking acute myocardial infarction. Int J Clin Pract. 2003;57:842–843. [PubMed] [Google Scholar]
- 5. Attar MN, Moulik PK, Salem GD, et al. Phaeochromocytoma presenting as dilated cardiomyopathy. Int J Clin Pract. 2003;57:547–548. [PubMed] [Google Scholar]
- 6. Kimura Y, Ozawa H, Igarashi M, et al. A pheochromocytoma causing limited coagulopathy with hemoptysis. Tokai J Exp Clin Med. 2005;30:35–39. [PubMed] [Google Scholar]
- 7. Minno AM, Bennett WA, Kvaie WF. Pheochromocytoma: a study of 15 cases diagnosed at autopsy. N Engl J Med. 1954;251:959–965. [DOI] [PubMed] [Google Scholar]
- 8. Frymoyer PA, Anderson GH Jr, Blair DC. Hemoptysis as a presenting symptom of pheochromocytoma. J Clin Hypertens. 1986;2:65–67. [PubMed] [Google Scholar]
- 9. Ljungner H, Manhem P, Bergqvist D. Increased vascular plasminogen activity in patients with pheochromocytoma. Acta Chir Scand. 1983;149:767–770. [PubMed] [Google Scholar]
- 10. Sayer WJ, Moser M, Mattingly TW. Pheochromocytoma and the abnormal electrocardiogram. Am Heart J. 1954;48:42–53. [DOI] [PubMed] [Google Scholar]
- 11. Lin PC, Hsu JT, Chung CM, et al. Pheochromocytoma underlying hypertension, stroke, and dilated cardiomyopathy. Tex Heart Inst J. 2007;34:244–246. [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez‐Recalde A, Costero O, Oliver JM, et al. Images in cardiovascular medicine. Pheochromocytoma‐related cardiomyopathy: inverted Takotsubo contractile pattern. Circulation. 2006;113:e738–e739. [DOI] [PubMed] [Google Scholar]
- 13. Jindal V, Baker ML, Arangat A, et al. Pheochromocytoma: presenting with regular cyclic blood pressure and inverted takotsubo cardiomyopathy. J Clin Hypertens (Greenwich). 2009;11:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mishra AK, Agarwal G, Kapoor A, et al. Catecholamine cardiomyopathy in bilateral malignant pheochromocytoma: successful reversal after surgery. Int J Cardiol. 2000;76:89–90. [DOI] [PubMed] [Google Scholar]
- 15. Eschen O, Frobert O, Jensen V, et al. Pheochromocytoma, a rare cause of acute cardiogenic shock. Clin Res Cardiol. 2007;96:232–235. [DOI] [PubMed] [Google Scholar]
- 16. Bloom S. Catecholamine cardiomyopathy. N Engl J Med. 1987;317:900–901. [DOI] [PubMed] [Google Scholar]
- 17. Terry RB, Tobin JR Jr, O’Connor RB. Intravenous phentolamine for phaeochromocytoma and adrenaline shock. Br Med J. 1958;27:771–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobal SL, Paran E, Jamali A, et al. Pheochromocytoma: cyclic attacks of hypertension alternating with hypotension. Nat Clin Pract Cardiovasc Med. 2008;5:53–57. [DOI] [PubMed] [Google Scholar]
- 19. Cohn JN. Paroxysmal hypertension and hypovolemia. N Engl J Med. 1966;275:643–646. [DOI] [PubMed] [Google Scholar]
- 20. Shemin D, Cohn PS, Zipin SB. Pheochromocytoma presenting as rhabdomyolysis and acute myoglobinuric renal failure. Arch Intern Med. 1990;150:2384–2385. [PubMed] [Google Scholar]
- 21. Brock L. Hypovolaemia and pheochromocytoma. Ann R Coll Surg Eng. 1975;56:218. [PMC free article] [PubMed] [Google Scholar]
- 22. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138:273–281. [DOI] [PubMed] [Google Scholar]
- 23. Brown H, Goldberg PA, Selter JG, et al. Hemorrhagic pheochromocytoma associated with systemic corticosteroid therapy and presenting as myocardial infarction with severe hypertension. J Clin Endocrinol Metab. 2005;90:563–569. [DOI] [PubMed] [Google Scholar]
- 24. Sibal L, Jovanovic A, Agarwal SC, et al. Phaeochromocytomas presenting as acute crises after beta blockade therapy. Clin Endocrinol (Oxf). 2006;65:186–190. [DOI] [PubMed] [Google Scholar]


