Abstract
Patients with stage 2 systolic hypertension require sizable blood pressure (BP) reductions to achieve recommended targets. This randomized double‐blind study compared a single‐pill combination of the direct renin inhibitor aliskiren and hydrochlorothiazide (aliskiren/HCTZ) with HCTZ monotherapy in older patients (older than 55 years) with systolic BP ≥160 mm Hg and <200 mm Hg. After a 1‐ to 4‐week washout, 451 patients were randomized to once‐daily aliskiren/HCTZ 150/12.5 mg or HCTZ 12.5 mg for 1 week, and then double the doses for 7 weeks. Overall baseline BP was 168.8/91.4 mm Hg. At week 4 (primary) end point, aliskiren/HCTZ provided significantly greater BP reductions from baseline than HCTZ monotherapy (29.6/9.3 mm Hg vs 22.3/6.8 mm Hg) and resulted in a greater proportion of patients achieving BP goal of <140/90 mm Hg (51.1% vs 33.3%). Aliskiren/HCTZ therapy provides substantial BP reductions and may thus be a useful treatment option for older patients with stage 2 hypertension. J Clin Hypertens (Greenwich). 2011;13:162–169. © 2011 Wiley Periodicals, Inc.
Approximately a quarter of the 74.5 million adults with hypertension in the United States are affected by stage 2 hypertension (systolic blood pressure [SBP] >160 mm Hg). 1 Isolated systolic hypertension (raised SBP but normal diastolic blood pressure [DBP]) is particularly common in older patients because of the natural physiologic changes that occur during the aging process: SBP rises as the vasculature stiffens and is remodelled with age. 2 Thus, whereas most people with hypertension who are younger than 50 years have elevated DBP, those who are older than 50 years tend to have increased SBP. 3
Current treatment guidelines advise lowering blood pressure (BP) to <140/90 mm Hg (or <130/80 mm Hg for patients with diabetes). 4 , 5 , 6 To reach these BP goals, most patients require ≥2 antihypertensive agents, and therefore available treatment guidelines recommend first‐line combination therapy for patients with stage 2 hypertension. 6 Agents with complementary mechanisms of action, such as thiazide diuretics and agents that target the renin‐angiotensin system, are logical choices to be used together 4 , 5 , 6 and are available in single‐pill combinations (SPCs).
The benefits of lowering BP in older patients with hypertension have been well documented in recent years. Several studies, including the Systolic Hypertension in the Elderly Program (SHEP), have demonstrated that BP reductions are associated with improvements in cardiovascular outcomes. 7 A meta‐analysis of 8 studies of patients 60 years and older with stage 2 systolic hypertension showed that antihypertensive therapy for a median duration of 3.8 years reduced stroke by 30%, cardiovascular death by 18%, and total mortality by 13%. 8 Despite this, only 50% of treated patients older than 60 years and 31% of patients 80 years and older achieve BP goal, possibly reflecting the traditional reluctance of physicians to treat elderly patients aggressively because of the fear of treatment‐related adverse events. 9 The benefits of antihypertensive treatment, however, have clearly been shown to outweigh the risks by the landmark Hypertension in the Very Elderly Trial (HYVET). 10 With an aging population, there is a growing need for BP‐lowering therapies that combine large BP reductions with good tolerability in older patients.
Aliskiren‐based regimens demonstrated superiority over established therapies based on angiotensin‐converting enzyme (ACE) inhibitors in patients older than 60 years in the recently published Aliskiren for Geriatric Lowering of Systolic Hypertension (AGELESS) study. 11 Here, we report the results of the Aliskiren HCTZ In Older Patients With Stage 2 Hypertension (ACTION) trial, a phase 4, randomized, controlled trial that evaluated the aliskiren/hydrochlorothiazide (HCTZ) SPC compared with HCTZ monotherapy in older patients (55 years and older) with stage 2 systolic hypertension.
Patients and Methods
Patients
Men and women 55 years and older with stage 2 systolic hypertension, defined as mean sitting SBP (msSBP) ≥160 and <200 mm Hg, were eligible for inclusion in the study. The main exclusion criteria included very severe hypertension (msSBP ≥200 mm Hg and/or msDBP ≥110 mm Hg), secondary hypertension, history of severe cardiovascular or cerebrovascular disease, history of angioedema during use of an ACE inhibitor, unstable diabetes mellitus, and any condition that may alter the absorption, distribution, metabolism or excretion of study drugs. Pregnant or nursing women were excluded, and women of childbearing potential had to be using effective contraceptive methods for inclusion in the study.
All patients provided written informed consent before participating in any study procedures. The study was conducted in accordance with International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki, and received approval from the institutional review board for each center.
Study Design
This was a double‐blind, parallel‐group, active‐controlled, dose‐escalation study conducted at 80 study centers in the United States. After the initial screening visit, eligible patients entered a 1‐ to 4‐week washout period, during which their existing antihypertensive medication was withdrawn gradually. Patients were randomized to study treatment when their BP met the entry criteria, and patients not meeting the entry criteria after 4 weeks were excluded. Patients with newly diagnosed hypertension and those who had not received any antihypertensive medications for at least 1 month before screening did not have to undergo any washout and were randomized within a week of the screening visit.
After the washout, eligible patients were randomly assigned in a 1:1 ratio to once‐daily treatment with either an SPC of aliskiren plus HCTZ or HCTZ monotherapy. A double‐dummy method ensured blinding of study medication using placebo tablets matched to the SPC of aliskiren/HCTZ and placebo capsules matched to the HCTZ capsules. After 1 week of aliskiren/HCTZ 150/12.5 mg or HCTZ 12.5 mg, study medication was force‐titrated to aliskiren/HCTZ 300/25 mg and HCTZ 25 mg for a further 7 weeks. Amlodipine (AML) 5 mg once daily could be added to the treatment regimen at weeks 4 or 6 for patients with msSBP ≥160 mm Hg.
Study Assessments
The primary objective of the study was to compare the efficacy of aliskiren/HCTZ 300/25 mg therapy with HCTZ 25 mg monotherapy, as evaluated by the primary variable, change in msSBP from baseline to week 4 end point.
Secondary efficacy variables included the change in msDBP from baseline to week 4 end point, the change in msSBP and msDBP from baseline to week 8 end point, the proportion of patients reaching BP goal (msSBP/msDBP <140/90 mm Hg) at week 4 and week 8 end points, the proportion of patients requiring additional AML therapy, and changes in plasma renin activity (PRA) at week 4 and week 8.
Clinic BP was measured at screening, randomization (baseline), and weeks 1, 4, 6, and 8 using a standard mercury sphygmomanometer with the appropriate cuff size. At the first study visit, BP was measured in both arms, and the arm with the higher DBP reading was used throughout the study. Three sitting BP measurements were taken at 1‐ to 2‐minute intervals after the patient had been sitting for 5 minutes, and the average of the 3 readings was recorded as the DBP value for that visit.
PRA was measured from blood samples obtained at baseline, week 4 and week 8, or early termination visit, and was measured by means of radioimmunoassay of generated angiotensin I (DiaSorin kit; DiaSorin, Stillwater, MN). Blood samples for PRA measurements were collected after patients had fasted for at least 8 hours. Samples were centrifuged within 5 minutes of collection and stored frozen until analysis was performed using complete patient sets by CRL Global Services (Lenexa, KS).
All adverse events and serious adverse events were recorded throughout the double‐blind treatment period and assessed by the investigator for their relationship to study medication. Other safety assessments were performed at regular intervals throughout the study.
Statistical Analyses
Changes in msSBP and msDBP from baseline to week 4 and 8 end points were assessed using a 2‐way analysis of covariance (ANCOVA) model with treatment and region as factors and baseline msSBP or msDBP, respectively, as a covariate. The proportions of patients achieving BP control and of those requiring add‐on AML were analyzed using a logistic regression model with treatment and region as factors and baseline msSBP as a covariate.
Changes from baseline in msSBP and msDBP were also analyzed by subgroups of age (younger than 75 years and 75 years and older), obesity (body mass index [BMI] ≥30 kg/m2 and <30 kg/m2), and severity of hypertension (baseline SBP 160 mm Hg to <170 mm Hg, 170 mm Hg to <180 mm Hg, and 180 mm Hg to <190 mm Hg).
Summary statistics for the postbaseline measurements of PRA and changes from baseline were presented by treatment arm and time point.
The study planned to randomize 432 patients in a 1:1 ratio, with the aim of 194 patients per treatment group completing the study. With this number of patients and an assumed standard deviation of 14 mm Hg for msSBP, the study had ≥80% statistical power to detect a difference between treatment groups in msSBP of 4 mm Hg at a 2‐sided α level of 0.05.
Results
Demographics and Baseline Patient Characteristics
All 451 patients who were enrolled into the study were randomized to treatment with either aliskiren/HCTZ (n=228) or HCTZ monotherapy (n=223). One patient was randomized to treatment with aliskiren/HCTZ in error and was withdrawn from the study before receiving any study drug; this patient was not included in the analysis of safety or efficacy. A similar proportion of patients in each of the treatment groups completed the study (89.5% in the aliskiren/HCTZ group and 91.9% in the HCTZ group). The most common reason for discontinuation was adverse events (n=20 [4.4%]: aliskiren/HCTZ, n=13; HCTZ, n=7).
Demographics and baseline characteristics were generally similar between treatment groups (Table I), although there was a slightly lower proportion of men in the aliskiren/HCTZ group than in the HCTZ group (44.3% vs 55.2%). Mean age of patients in the study was 64.8 years, and almost half were older than 65 years. Mean duration of hypertension was approximately 11 years, and mean baseline SBP/DBP was 169/91 mm Hg.
Table I.
Patient Demographics and Baseline Characteristics (Randomized Seta)
| Aliskiren/HCTZ 300/25 mg (n=228) | HCTZ 25 mg (n=223) | |
|---|---|---|
| Age, y | 64.5±7.6 | 65.1±7.0 |
| ≥65, No. (%) | 103 (45.2) | 107 (48.0) |
| ≥75, No. (%) | 27 (11.8) | 26 (11.7) |
| Sex, No. (%) | ||
| Male | 101 (44.3) | 123 (55.2) |
| Race, No. (%) | ||
| Caucasian | 174 (76.3) | 179 (80.3) |
| Black | 39 (17.1) | 37 (16.6) |
| Asian | 6 (2.6) | 1 (0.4) |
| Native American | 1 (0.4) | 1 (0.4) |
| Pacific islander | 5 (2.2) | 4 (1.8) |
| Other | 3 (1.3) | 1 (0.4) |
| Duration of hypertension, y | 11.0±10.0 | 10.8±9.4 |
| History of diabetes, No. (%) | 40 (17.5) | 49 (22.0) |
| History of smoking, No. (%) | 26 (11.4) | 28 (12.6) |
| Height, cm | 167±10.9 | 169±11.5 |
| Weight, kg | 86.2±18.8 | 87.0±18.5 |
| Body mass index, kg/m2 | 31.1±6.4 | 30.3±5.1 |
| Obesity,b No. (%) | 124 (54.4) | 109 (48.9) |
| Waist circumference, cm | ||
| Men | 102.5±14.2c | 105.0±13.4c |
| Women | 97.3±14.6d | 94.8±11.8d |
| eGFR, mL/min/1.73 m2 | 75.9±17.3 | 79.9±18.8 |
| eGFR ≥60 mL/min/1.73 m2, No. (%) | 186 (81.6) | 189 (84.8) |
| Metabolic syndrome, No. (%) | 59 (25.9) | 64 (28.7) |
| Mean sitting SBP, mm Hg | 169.1±7.7 | 168.6±7.9 |
| Mean sitting DBP, mm Hg | 91.4±9.3 | 91.3±8.7 |
Data are shown as mean ± standard deviation unless otherwise stated. Abbreviations: DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide; SBP, systolic blood pressure. aRandomized set includes all patients who were randomized to study treatments regardless of whether they received study treatments. bBMI ≥30 kg/m2. cAliskiren/HCTZ, n=100; HCTZ monotherapy, n=120. dAliskiren/HCTZ, n=127; HCTZ monotherapy, n=99.
Efficacy
Aliskiren/HCTZ tablets provided a significantly greater reduction from baseline in SBP than HCTZ monotherapy at week 4 end point (least‐squares mean reductions of 29.6 mm Hg vs 22.3 mm Hg; P<.0001 [Figure 1]), with patients who received aliskiren/HCTZ gaining an additional mean reduction in SBP of 7.3 mm Hg compared with those who received HCTZ monotherapy. Mean reductions in DBP were also significantly greater with aliskiren/HCTZ than with HCTZ monotherapy (P<.005 [Figure 1]).
Figure 1.
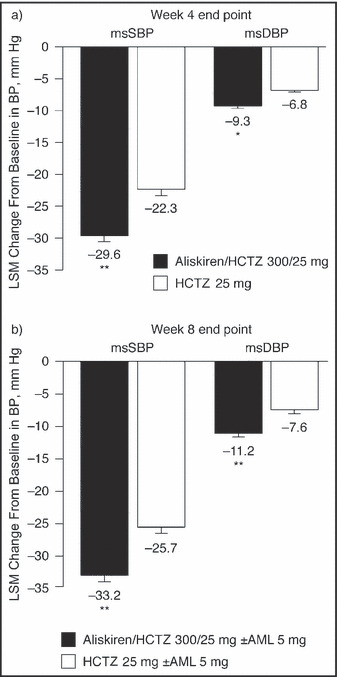
Mean changes from baseline in mean sitting systolic blood pressure (msSBP) and mean sitting diastolic blood pressure (msDBP) at week 4 end point (a) and week 8 end point (b) (full analysis seta). Data are least‐squares mean (LSM) ± standard error of the mean. aFull analysis set includes all randomized patients who received at least one dose of study medication. Patients whose blood pressure did not respond to aliskiren/hydrochlorothiazide (HCTZ) combination therapy (ie, SBP ≥160 mm Hg at week 4 or 6) received add‐on amlodipine (AML) 5 mg. *P<.005 vs HCTZ 25 mg; **P<.0001 vs HCTZ 25 mg.
As specified by the protocol, patients whose SBP remained above 160 mm Hg at weeks 4 or 6 could receive add‐on AML. A lower proportion of patients required add‐on AML in the aliskiren/HCTZ group than in the HCTZ monotherapy group (12.8% vs 22.0%; P<.01). Despite the use of add‐on AML, mean reductions in SBP and DBP at week 8 end point remained significantly greater in the aliskiren/HCTZ group than in the HCTZ monotherapy group (P<.0001 for SBP and DBP [Figure 1]).
Aliskiren/HCTZ treatment brought a significantly higher proportion of patients to BP goal than HCTZ monotherapy at week 4 end point (51.1% vs 33.3%; odds ratio [OR], 2.38; 95% confidence interval [CI]: 1.58–3.58; P=.0001). This difference remained significant at week 8 end point (62.2% vs 39.2%; OR, 2.91; 95% CI, 1.94–4.35; P<.0001), when patients who were initially nonresponders were receiving AML in addition to the aliskiren/HCTZ SPC or HCTZ.
Subgroup analyses showed that aliskiren/HCTZ treatment also lowered BP effectively in obese and nonobese patients (BMI <30 kg/m2 vs ≥30 kg/m2), patients younger than 75 years and 75 years and older, and patients with baseline SBP 160 mm Hg to <170 mm Hg, 170 mm Hg to <180 mm Hg, and 180 mm Hg to <190 mm Hg (Figure 2). At week 4 end point, all subgroups had mean SBP reductions of 28.3 mm Hg to 34.4 mm Hg with aliskiren/HCTZ compared with 19.4 mm Hg to 24.9 mm Hg with HCTZ monotherapy. All between‐treatment differences were statistically significant (P<.05; post hoc ANCOVA).
Figure 2.
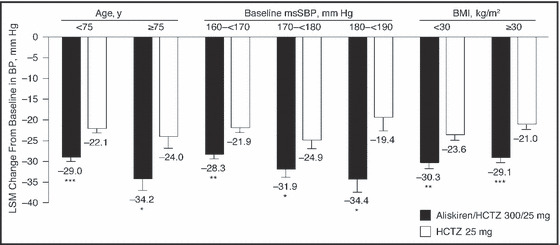
Change from baseline in mean sitting systolic blood pressure (msSBP) at week 4 end point by subgroup of age, baseline systolic blood pressure, and body mass index (BMI) (full analysis seta). Data are least‐squares mean (LSM) ± standard error of the mean. aFull analysis set includes all randomized patients who received at least one dose of study medication. *P<.05 vs hydrochlorothiazide (HCTZ) 25 mg; **P<.001 vs HCTZ 25 mg; ***P<.0001 vs HCTZ 25 mg.
Aliskiren/HCTZ treatment was associated with a reduction from baseline in PRA (by 49% at week 4 and 57% at week 8), whereas HCTZ monotherapy led to an increase from baseline (122% at week 4 and 143% at week 8).
Safety
Aliskiren/HCTZ combination therapy and HCTZ monotherapy were generally well tolerated with or without optional addition of AML. Adverse events were as expected for the study population and were generally mild in severity. A similar proportion of patients in each treatment group experienced adverse events (Table II). The most common event leading to discontinuation was dizziness (4 patients in the aliskiren/HCTZ±AML group and no patients in the HCTZ±AML group).
Table II.
Safety and Tolerability of Study Treatments (Safety Seta)
| Aliskiren/HCTZ 300/25 mg (±AML 5 mg) (n=227) | HCTZ 25 mg (±AML 5 mg) (n=223) | |
|---|---|---|
| Any adverse event | 98 (43.2) | 100 (44.8) |
| Serious adverse event | 4 (1.8) | 3 (1.3) |
| Discontinuation due to adverse event | 13 (5.7) | 7 (3.1) |
| Most frequent adverse events (≥2% of patients in any treatment group) | ||
| Dizziness | 13 (5.7) | 9 (4.0) |
| Cough | 6 (2.6) | 3 (1.3) |
| Headache | 4 (1.8) | 17 (7.6) |
| Hypokalemia | 4 (1.8) | 7 (3.1) |
| Upper respiratory tract infection | 4 (1.8) | 8 (3.6) |
| Urinary tract infection | 3 (1.3) | 5 (2.2) |
| Peripheral edema | 2 (0.9) | 5 (2.2) |
| Sinusitis | 0 | 5 (2.2) |
| Laboratory abnormalities, No. | 224b | 222b |
| Serum potassium, mmol/L | ||
| <3.5 | 22 (9.8) | 51 (23.0) |
| >5.5 | 3 (1.3) | 3 (1.4) |
| ≥6.0 | 1 (0.4) | 2 (0.9) |
| Serum creatinine | ||
| >176.8 μmol/L | 1 (0.4) | 0 |
| Blood urea nitrogen | ||
| >14.28 mmol/L | 1 (0.4) | 0 |
Data are shown as number (percentage) of patients unless otherwise indicated. Abbreviations: AML, amlodipine; HCTZ, hydrochlorothiazide. aSafety set includes all patients that received at least one dose of study medication. bNumber of patients with evaluable laboratory assessments.
There were few clinically meaningful laboratory abnormalities during the study (Table II). Three patients discontinued from the study as a result of abnormal laboratory values (elevated blood creatinine and abnormal renal function test in the aliskiren/HCTZ±AML group and low serum potassium in the HCTZ±AML group). Low potassium levels (<3.5 mmol/L) were found in a lower proportion of patients receiving aliskiren/HCTZ±AML (9.8%) than in those receiving HCTZ±AML (23.0%).
Metabolic markers (fasting blood glucose, total cholesterol, high‐ and low‐density lipoproteins, and triglycerides) were well matched between treatment groups at baseline, and no notable changes occurred during the study for either treatment regimen (Table III).
Table III.
Change in Metabolic Parameters (Safety Seta)
| Aliskiren/HCTZ 300/25 mg (±AML 5 mg) (n=204) | HCTZ 25 mg (±AML 5 mg) (n=205) | |
|---|---|---|
| Fasting plasma glucose, mmol/Lb | ||
| Baseline | 5.9±1.4 | 6.1±1.6 |
| Week 8 | 6.3±2.2 | 6.4±1.7 |
| Change from baseline | 0.4±1.8 | 0.3±1.5 |
| Total cholesterol, mmol/L | ||
| Baseline | 5.1±1.0 | 4.9±1.1 |
| Week 8 | 5.2±1.2 | 5.0±1.2 |
| Change from baseline | 0.2±0.8 | 0.1±0.7 |
| HDL cholesterol, mmol/L | ||
| Baseline | 1.4±0.4 | 1.4±0.4 |
| Week 8 | 1.4±0.4 | 1.4±0.4 |
| Change from baseline | 0.0±0.2 | 0.0±0.2 |
| LDL cholesterol, mmol/L | ||
| Baseline | 3.1±0.9 | 2.9±0.9 |
| Week 8 | 3.2±1.0 | 3.0±1.0 |
| Change from baseline | 0.1±0.6 | 0.1±0.6 |
| Triglycerides, mmol/L | ||
| Baseline | 1.8±1.3 | 1.7±1.0 |
| Week 8 | 2.1±1.8 | 1.9±1.2 |
| Change from baseline | 0.3±1.3 | 0.2±0.8 |
Data are shown as mean ± standard deviation for patients with a value at both baseline and week 8. Abbreviations: AML, amlodipine; HCTZ, hydrochlorothiazide; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. aSafety set includes all patients that received at least one dose of study medication. bAliskiren/HCTZ±AML, n=202; HCTZ±AML, n=205.
Discussion
The results of the ACTION study show that aliskiren/HCTZ SPC treatment is a highly effective option for BP reduction in older patients with stage 2 systolic hypertension. Patients receiving aliskiren/HCTZ had large SBP reductions of almost 30 mm Hg, on average, and more than half (51%) reached the recommended BP goal of <140/90 mm Hg after only 4 weeks of treatment. By contrast, patients receiving HCTZ monotherapy had significantly smaller reductions in SBP (approximately 22 mm Hg) and a significantly lower percentage reached BP goal (33%). The superiority of aliskiren/HCTZ over HCTZ monotherapy persisted after 8 weeks of treatment despite the optional addition of AML in patients with SBP ≥160 mm Hg. Aliskiren/HCTZ treatment was generally well tolerated. Adverse events were experienced by a similar proportion of patients to those receiving HCTZ monotherapy and most were mild in severity.
It is particularly noteworthy that a large proportion of aliskiren/HCTZ‐treated patients rapidly achieved BP goal, with mean SBP falling to under 140 mm Hg within 4 weeks. Previous long‐term outcome studies of other antihypertensive combinations in elderly populations, such as SHEP and the Systolic Hypertension in Europe (Syst‐Eur) trial, showed marked BP reductions with active treatment, but mean SBP generally remained above 140 mm Hg. 7 , 10 , 12 Although comparisons between studies with different enrollment criteria should be made with caution, mean baseline SBP (approximately 170 mm Hg) in these studies was similar to that in our study, as was the strategy of treatment escalation if patients did not achieve BP targets. In our study, SBP targets (<140 mm Hg) were more stringent than those of SHEP (<160 mm Hg for patients with baseline SBP of >180 mm Hg, or a reduction of 20 mm Hg for patients with baseline SBP of 160–180 mm Hg) and Syst‐Eur (<150 mm Hg).
Evidence that rapid BP reductions are associated with beneficial outcomes comes from the Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial, in which patients who responded to treatment (defined as previously untreated patients having a ≥10 mm Hg drop in SBP or previously treated patients not having an increase in BP) within a month of initiation had a 12% reduction in cardiac events (P<.01) and a 17% reduction in stroke (P<.05) compared with those who did not benefit from an immediate response to treatment. 13 Although the VALUE trial did not enroll older patients exclusively, the mean patient age was approximately 67 years. 14 The results of VALUE thus challenge the historical approach for the treatment of hypertension in older patients of “starting low and going slow” and suggest that physicians should aim to get these patients to BP goal as quickly as possible.
Importantly, our subgroup analyses showed that aliskiren/HCTZ provided large and rapid (ie, within 4 weeks) BP reductions consistently in the very elderly, those with very high BP, and those who were obese, populations that are all notoriously hard to treat effectively. 9 , 15 These patient groups are also those at particularly high cardiovascular risk and thus may have the greatest need for effective treatments with good tolerability.
Our study demonstrates robust SBP reductions of 30 mm Hg after 4 weeks’ treatment with aliskiren/HCTZ combination therapy. Notably large SBP reductions (22 mm Hg) were also observed in the HCTZ monotherapy group, as might be expected in a study performed in patients with stage 2 hypertension at baseline. These reductions are broadly consistent with those observed in a post hoc subgroup analysis of patients with stage 2 hypertension from a placebo‐controlled, multifactorial aliskiren/HCTZ study, in which aliskiren/HCTZ 300/25 mg combination therapy reduced SBP by 27.2 mm Hg and HCTZ 25 mg monotherapy reduced SBP by 18.9 mm Hg. 16 Although the effect on absolute BP of adding aliskiren to HCTZ thus appears to be incremental (consistent with findings for combination of ARBs and HCTZ 17 , 18 ), whether the effects of the two drugs in combination are additive requires the placebo effect to be taken into account. As the present study had no placebo arm, it is not possible to comment definitely on whether the addition of aliskiren to HCTZ had additive or incremental effects on BP. However, in the subgroup of patients with stage 2 hypertension in the previous multifactorial study, placebo‐adjusted SBP reductions with aliskiren 300 mg, HCTZ 25 mg, and aliskiren/HCTZ 300/25 mg were 9.3 mm Hg, 8.8 mm Hg, and 17.1 mm Hg, respectively, suggesting additive effects of aliskiren and HCTZ on BP. 16 Regarding the changes in BP at week 8 end point in the present study, it is important to note that between‐treatment differences at this time point are less meaningful than those at the week 4 end point because optional add‐on of AML at week 4 or 6 for patients who had not achieved SBP <160 mm Hg was used for a greater proportion of patients in the HCTZ group (22.0% vs 12.8% in the aliskiren/HCTZ group). The further reductions in mean BP and higher control rate in the aliskiren/HCTZ group at week 8 (62%) compared with week 4 (51%) reflect the addition of AML to the treatment regimen and demonstrate the effectiveness of the triple combination (aliskiren/HCTZ and AML) in patients who do not respond to combination therapy with two agents. The triple combination is thus likely to represent an important treatment option for patients with particularly hard‐to‐control hypertension.
Aliskiren/HCTZ combination therapy reduced PRA from baseline by 49% at week 4, and aliskiren/HCTZ±AML reduced PRA by 57% at week 8, confirming the ability of aliskiren to suppress diuretic‐ and calcium channel blocker–induced PRA increases. The present study did not evaluate the relationship between baseline PRA or on‐treatment changes in PRA and observed changes in BP. Two published aliskiren studies have shown that changes in BP with aliskiren monotherapy are not related to PRA levels at baseline. 19 , 20 Andersen and colleagues19 showed that the BP‐lowering effect of aliskiren monotherapy was similar in patients with low (≤0.65 ng/mL/h) and high (>0.65 ng/mL/h) baseline PRA (−13.0/−12.5 and −13.7/−12.1 mm Hg, respectively). In addition, Richter and colleagues 20 showed that the proportion of patients reaching target BP (<140/90 mm Hg [<130/80 mm Hg in patients with diabetes]) with aliskiren monotherapy, or aliskiren/HCTZ or aliskiren/HCTZ/AML combination therapy, was similar in patients with baseline PRA ≤0.65 ng/mL/h and >0.65 ng/mL/h. With regard to the relationship between changes in PRA and changes in BP, a modelling analysis of pooled data from 9 studies of aliskiren monotherapy in patients with stage 1 or 2 hypertension showed that the magnitude of the change in PRA on aliskiren treatment is predictive of the magnitude of the change in BP. 21
Conclusions
Aliskiren/HCTZ treatment provides superior BP reductions and significantly improves rates of BP goal attainment compared with HCTZ monotherapy, with or without the optional addition of AML, in older patients with stage 2 hypertension. With more than 50% of patients with stage 2 hypertension achieving the BP goal of <140/90 mm Hg within 4 weeks of treatment, aliskiren/HCTZ represents a highly effective and well‐tolerated treatment option in this patient group.
Acknowledgements: All authors participated in the development and writing of the paper, and approved the final manuscript for publication. The authors take full responsibility for the content of the paper and thank Dr Annette Keith (Oxford PharmaGenesis Ltd) for medical writing support, editorial assistance and collation, and incorporation of comments from all authors.
Disclosures: This work was funded by Novartis Pharma‐ceuticals Corporation, East Hanover, NJ. Jan Basile has received research support from Novartis, The National Heart, Lung and Blood Institute and Boehringer‐Ingelheim; has acted as a consultant for Novartis, Abbott Laboratories, Boehringer‐Ingelheim, Forest Laboratories, Daiichi‐Sankyo and Takeda Pharmaceuticals; and has been a member of the speakers’ bureau for Novartis, Abbott Laboratories, AstraZeneca, Boehringer‐Ingelheim, Forest Laboratories, GSK, Merck, Pfizer and Daiichi‐Sankyo. Michael Lillestol has received research support from Novartis. Jaco Botha, Carol Yurkovic, and Richard Weitzman are employees of Novartis and are therefore eligible for Novartis stock and stock options.
References
- 1. Qureshi AI, Suri MF, Kirmani JF, et al. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit. 2005;11:CR403–CR409. [PubMed] [Google Scholar]
- 2. Dao HH, Essalihi R, Bouvet C, et al. Evolution and modulation of age‐related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–317. [DOI] [PubMed] [Google Scholar]
- 3. Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 4. Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 5. Mancia G, Laurent S, Agabiti‐Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 7. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 8. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet. 2000;355:865–872. [DOI] [PubMed] [Google Scholar]
- 9. Ostchega Y, Dillon CF, Hughes JP, et al. Trends in hypertension prevalence, awareness, treatment, and control in older U.S. adults: data from the National Health and Nutrition Examination Survey 1988 to 2004. J Am Geriatr Soc. 2007;55:1056–1065. [DOI] [PubMed] [Google Scholar]
- 10. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 11. Duprez DA, Munger MA, Botha J, et al. Aliskiren for Geriatric Lowering of Systolic Hypertension (AGELESS): a randomized controlled trial. J Hum Hypertens. 2010;24:600–608. [DOI] [PubMed] [Google Scholar]
- 12. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 13. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 14. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 15. Bramlage P, Pittrow D, Wittchen HU, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17:904–910. [DOI] [PubMed] [Google Scholar]
- 16. Calhoun D, Villamil A, Chrysant SG, et al. Antihypertensive efficacy of aliskiren/hydrochlorothiazide (HCT) combinations in patients with stage 2 hypertension: subgroup analysis of a randomized, double‐blind, factorial trial. Hypertension. 2008;52:e97 (P209). [Google Scholar]
- 17. Pool JL, Glazer R, Weinberger M, et al. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double‐blind, placebo‐controlled study followed by long‐term combination therapy in hypertensive adults. Clin Ther. 2007;29:61–73. [DOI] [PubMed] [Google Scholar]
- 18. Chrysant SG, Weber MA, Wang AC, et al. Evaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. Am J Hypertens. 2004;17:252–259. [DOI] [PubMed] [Google Scholar]
- 19. Andersen K, Weinberger MH, Constance CM, et al. Comparative effects of aliskiren‐based and ramipril‐based therapy on the renin system during long‐term (6 months) treatment and withdrawal in patients with hypertension. J Renin Angiotensin Aldosterone Syst. 2009;10:157–167. [DOI] [PubMed] [Google Scholar]
- 20. Richter D, Mickel C, Acharya S, et al. Aliskiren‐based treatment controls blood pressure independent of baseline plasma renin activity. J Hypertens. 2010;28:e429. [Google Scholar]
- 21. Sarangapani R, Ebling W, Bush CA, et al. Integrated analysis of PRA, PRC and BP relationship in hypertensive patients on aliskiren treatment: a pooled analysis using data from nine clinical trials. J Hypertens. 2009;27:S143. [Google Scholar]


