Pheochromocytomas are rare catecholamine‐producing neuroendocrine tumors arising from the chromaffin cells of the adrenal medulla (80%–85%) or extra‐adrenal paraganglia (15%–20%). The secretory profiles of these tumors vary but predominately secrete norepinephrine (NE) with lesser amounts of epinephrine (EPI). The prevalence of pheochromocytoma in the outpatient hypertensive population is reported to be somewhere between 0.1% and 0.9%; about 25% are discovered incidentally during imaging studies for unrelated disorders. 1
The clinical presentation of pheochromocytomas can vary greatly but is typically described as spells with the classic triad of tachycardia, headaches, and diaphoresis occurring in at least 25% of cases. Severe hypertension is usually present during the attacks. Other symptoms can include pallor; tremulousness; orthostatic hypotension, especially in the morning (that may reflect a low plasma volume); visual blurring; papilledema; weight loss; polyuria; and polydipsia. Several medications, including opiates, metoclopramide, tricyclic antidepressants, and anesthesia can induce pheochromocytoma‐related hypertension. Hypertension and/or hypertensive spikes are the result of catecholamines on the cardiovascular system probably coupled with enhanced sympathetic nervous system activity.
The diagnosis is often not made for many years because of the fleeting episodes and nonspecific symptoms. It is usually entertained when there is a history of spells with sweating and palpitations, resistant hypertension, family history of pheochromocytoma, or incidentally discovered adrenal mass. There is no consensus on the best chemical test for diagnosing pheochromocytoma, but high sensitivities are achieved by measuring fractionated metanephrines and catecholamines in a 24‐hour urine collection (97%) or fractionated plasma free metanephrines (99%). Tumor localization with computed tomography or magnetic resonance imaging should normally take place after biochemical confirmation of the pheochromocytoma.
Treatment with α‐blockers, usually combined with a β‐blocker, prior to tumor removal decreases mortality associated with surgery. 2 There is no consensus on the preferred agent, but competitive (doxazosin or prazosin) or noncompetitive and longer‐acting (phenoxybenzamine) α‐blockers are usually used. Alternatives are the mixed α/β‐blocker labetalol, dihydropyridine calcium channel blockers, or metirosine. 1
We report on a rare presentation of pheochromocytoma with rapid alternating cycles of hypertension and hypotension successfully treated with fluid expansion and metirosine prior to tumor removal.
Case Report
A 47‐year‐old woman presented for an EPS for evaluation of palpitations. She had hypertension diagnosed 10 years earlier (initial systolic blood pressure (SBP)/diastolic blood pressure (DBP), 150/96 mm Hg) and was started on an angiotensin receptor blocker (ARB), valsartan 80 mg/d. She was followed yearly, and office BP recordings varied from 122–160/82–100 mm Hg. Five years ago, her regimen was changed to hydrochlorothiazide 25 mg/d. Four years ago, during an episode of smoke inhalation, her SBP was apparently in the 200‐mm Hg range, and a different ARB, olmesartan 20 mg, was added to her regimen. Thereafter, her hypertension was well controlled. Two years ago, she stopped all antihypertensive medications because of multiple episodes of low BP.
One year ago, the patient had an initial cardiology evaluation for episodes of palpitations, which had been occurring for the past 2 to 3 years. The episodes had an abrupt onset and offset and lasted from 1 to 45 minutes. They were associated with some or all of the following symptoms: nausea, vomiting, diarrhea, weakness, shortness of breath, chest tightness, sweating, light‐headedness, tremulousness, and headache. She was noted to be pale during and immediately after the attacks. Long‐acting metoprolol was started at 25 mg/d and up‐titrated to 100 mg/d without symptomatic improvement. The episodes gradually became more frequent and intense, with multiple daily episodes that interfered with normal activities. During one documented episode lasting 45 minutes, her BP went from 270/120 to 160/100 to 146/80 mm Hg and she experienced headache, nausea, and dizziness. An event recorder showed that the palpitations were predominately sinus tachycardia with a heart rate (HR) of 130 beats/min. There was one documented self‐terminating episode of atrial fibrillation with rapid ventricular response during a visit to the emergency department, after which the metoprolol was increased to 200 mg daily.
During the evaluation of palpitations, a diagnosis of Graves’ disease was made and methimazole 5 mg three times daily was started, with normalization of thyroid function tests. Type 2 diabetes mellitus was also diagnosed, and the patient was placed on long‐acting metformin 1000 mg once daily.
The EPS was planned as an outpatient procedure. In the electrophysiology laboratory, the patient had some fluctuations in BP ranging from 102–157/53–94 mm Hg. She received anesthesia with propofol 100 mcg/kg/min, midazolam 5 mg IV, and fentanyl 50 mcg IV. During sheath insertion and catheter positioning, BP continued to be labile, with swings from 125/56 to 242/129 mm Hg, and an arterial line was placed. After basic measurements and several runs of atrial stimulation, the EPS was aborted. Cyclic fluctuations in BP continued and ranged from 52/34 to 344/170 mm Hg (Figure 1). These fluctuations occurred over repeated cycles lasting approximately 14 minutes each. Oxygen saturation dropped to 72%, and she was intubated. She received intravenous fluid. Phentolamine was considered but not administered due to periods of severe hypotension. Emergent abdominal computed tomography without contrast demonstrated a 4.2×3.8‐cm well‐defined heterogeneous mass in the right adrenal gland and patchy ground‐glass opacities in the lungs consistent with pulmonary edema (Figure 2 shows the mass on an abdominal magnetic resonance imaging done 6 days later). A diagnosis of pheochromocytoma complicated by pulmonary edema was made.
Figure 1.
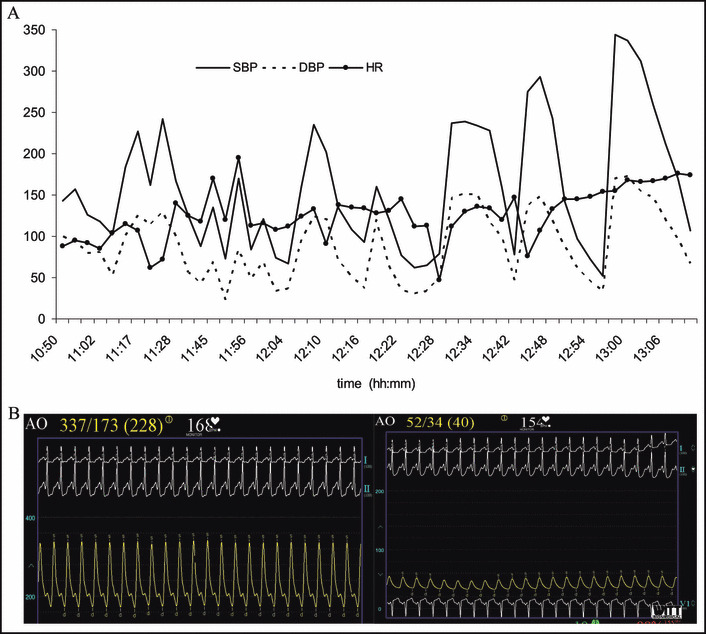
Arterial blood pressure (BP) monitoring shows the cyclic fluctuations of BP during a hemodynamic crisis in the electrophysiology laboratory. (A) A 2‐hour period is shown with fluctuating BP with cycles every 14 minutes. (B) Images of the recording screen with continuous arterial BP and heart rate (HR) monitoring show 2 episodes of severe hypertension and hypotension. SBP indicates systolic blood pressure; DBP, diastolic blood pressure; AO, radial arterial line blood pressure value.
Figure 2.
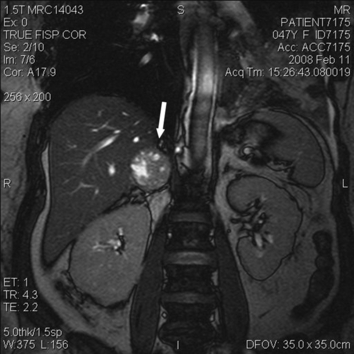
Coronal view of the abdomen on magnetic resonance imaging with gadolinium contrast. The arrow points toward the right adrenal mass, which measures 4×3.7×4.2 cm; its proximity to the liver is evident. The lesion is predominantly solid but also contains multiple foci of high T2 signal intensity. The left adrenal gland is normal.
The hemodynamic crisis lasted for 10 hours after which the patient remained severely hypotensive with BP recordings as low as 50/35 mm Hg and tachycardic with a mean HR of 110 beats/min. Phenylephrine was used to maintain a mean arterial pressure >65 mm Hg and was discontinued after 24 hours. While the patient was intubated, sedation was maintained with continuous intravenous fentanyl and intermittent intravenous midazolam. Due to episodic hypotension, α‐blockers were not used and the patient was started on metirosine (Table I). Metirosine inhibits tyrosine hydroxylase, which is the key enzyme in DOPA formation, the dopamine precursor. The patient was successfully extubated on hospital day 3. A diltiazem drip at 10 mg/h was initiated for persistent tachycardia (HR, 110–130 beats/min) with stable BP (mean, 120/60 mm Hg). Her vital signs remained stable for the next 48 hours and diltiazem was changed to 60 mg orally every 6 hours.
Table I.
Catecholamine Measurements in 24‐Hour Urine Collection and Metirosine Treatment During Hospitalization
| Day | EPI (<20 μg/24 h) | NE (<80 μg/24 h) | Total (<100 μg/24 h) | Dopamine (<500 μg/24 h) | Creatinine (mg/dL) | Urine volume (cc) | Metirosine |
|---|---|---|---|---|---|---|---|
| 1 | 185 | 145 | 330 | 399 | 3300 | 500 mg TID | |
| 2 | 500 mg QID | ||||||
| 3 | 110 | 53 | 163 | 128 | 1150 | 500 mg QID | |
| 4 | 76 | 43 | 119 | 96 | 1630 | 500 mg QID | |
| 5–10 | 750 mg QID | ||||||
| 11 | 11 | 24 | 35 | 33 | 3600 | 1000 mg QID | |
| 12 | 31 | 26 | 57 | 420 | 26 | 4400 | 1000 mg QID |
| 13 and14 | 1000 mg QID |
Abbreviations: EPI, epinephrine; NE, norepinephrine; Total, total catecholamines.
On hospital day 7, doxazosin was started at 1 mg at night and increased to 2 mg the next day. On the morning of her 10th hospital day, the patient had an episode of tremulousness, pallor, anxiety, nausea, and vomiting. Her vital signs (SBP [mm Hg]/DBP [mm Hg]/HR [beats/min]) were 106/67/102 supine, 108/82/115 seated, and 83/63/130 standing. Doxazosin and diltiazem were discontinued and intravenous normal saline was initiated.
Catecholamine levels were consistent with pheochromocytoma with predominant EPI secretion (I, II). Magnetic resonance imaging with gadolinium contrast showed no other abdominal masses. The patient was aggressively hydrated for 48 hours with intravenous normal saline in preparation for surgery. Treatment with metirosine led to normalization of urinary NE level and a significant decrease in EPI level, though the latter still remained 1.5 times elevated compared with the normal reference range. On hospital day 14, the patient underwent an open right adrenalectomy without complications. During anesthesia, apart from ramifentanyl, midazolam, and propofol, she received diltiazem and labetalol at the start; they were then used throughout the procedure at the discretion of the anesthesiologist. Tumor manipulation produced swings in BP similar to those in Figure 1. Pathology findings were consistent with pheochromocytoma. The tumor weight was 35 g, and it was confined within the adrenal capsule. There were no signs of necrosis, but hemorrhage was noted. Postresection, BP stabilized in the range of 120–140/60–70 mm Hg, with a progressive increase in the next day to 155–170/80–90 mm Hg.
Table II.
Metanephrine Measurements in 24‐Hour Urine Collection During Initial Days of Hospitalization
| 24‐Hour Urine Metanephrines | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Total metanephrines (≤900 μg/24 h) | 18,843 | 7387 | 3583 |
| Metanephrines (≤300 μg/24 h) | 15,576 | 6121 | 2881 |
| Normetanephrines (≤600 μg/24 h) | 3267 | 1266 | 702 |
| Creatinine (g/24 h) | 2.5 | 1.5 | 1.6 |
The patient was discharged after 17 days of hospitalization on methimazole 20 mg/d and long‐acting diltiazem 240 mg/d. Six months postresection, she continued to be free of symptoms but required antihypertensive medications. Plasma metanephrine levels, checked on several occasions, remained in the normal reference range.
Discussion
There is accumulating evidence that pheochromocytoma, apart from the classic hypertensive cycles, can also present with rapidly cycling paroxysms of hypertension alternating with hypotension. 3 , 4 , 5 Kobal and associates 3 reviewed 7 cases of this unusual form of pheochromocytoma and proposed that the BP fluctuations are associated with tumors that predominantly secrete EPI. They argued that EPI and NE released in large amounts could result in vasoconstriction and decreased blood volume, resulting in decreased cardiac output that would act as a further stimulus for the release of catecholamines. This would raise BP with activation of baroreceptors, which by negative feedback through the sympathetic and parasympathetic systems would decrease BP, resulting in the alternating waves of hypertension and hypotension. This arc reflex would also explain the inverse relationship between the HR and BP during the attacks (Figure 1A). Using a beat‐to‐beat finger BP monitoring system and software reconstruction algorithms, Guzik and colleagues 4 found that the first changes were in the vascular tone; these seemed to be responsible for the alternating cycles of hypertension and hypotension.
In contrast, Oishi and associates 5 documented fluctuation in BP that was associated with cyclic changes in NE secretion. In their review of 16 patients, the majority had a single right‐sided adrenal tumor (12/16). They hypothesized that the liver might exert pressure over the tumor with subsequent release of catecholamines, similar to physical manipulation of the tumor. They described a patient with wide fluctuation in BP while laying supine with his legs stretched, with normalization upon leg bending. They proposed that the intra‐abdominal pressure would be responsible for the catecholamine release. Other mechanisms proposed to explain the development of rapidly fluctuating BP were ischemic response of the central nervous system and intravascular volume contraction. 6
BP oscillations are generally followed by hypotension; this has been described after experimental infusion of catecholamines as well as with adrenergic shock. 7 Massive release of catecholamines could result in decreased receptor activity. Shock could be the result of hemorrhagic necrosis in the tumor, with initial severe hypertension followed by hypotension due to abrupt cessation of catecholamine secretion. It follows that the cyclic crisis could be self‐limiting. Of note, the recorded peripheral hypotension could be falsely low secondary to severe vasoconstriction with concomitant severe central hypertension. Femoral arterial BP monitoring may be more accurate in this setting.
Treatment of this type of pheochromocytoma is not straightforward. The literature has conflicting data about the utility of fluid resuscitation and α‐blockade in these cases. 3 , 6 , 7 Previous reports have shown improvement and stabilization in BP following phentolamine administration, 3 while others have not. 6 Our patient was initially treated with normal saline, but there is no universal acceptance of the benefits of this approach. 7
Despite “near‐normalization” of the 24‐hour catecholamine levels before the surgery with 14 days of treatment with metirosine, life‐threatening BP fluctuations occurred intraoperatively. Unfortunately, we did not measure the circulating level of catecholamines during surgery. Thus, it is impossible to correlate circulating catecholamine levels and the cyclic fluctuations in BP during surgery. The discrepancy between the urine catecholamine level and intraoperative BP instability demonstrates the complexity of cardiovascular regulatory mechanisms in patients with pheochromocytoma.
The unique presentation of our case during an elective outpatient procedure is a reminder that anesthesia is likely to be a trigger of a pheochromocytoma crisis. Although the diagnosis of pheochromocytoma was strongly considered during the episode and the imaging study supported the diagnosis, pharmacologic treatment was not instituted because of the hypotensive periods interspersed between hypertensive peaks. This case suggests that treatment with fluid and metirosine is feasible in a pheochromocytoma associated with rapid fluctuations in BP.
References
- 1. Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet. 2005;366:665–675. [DOI] [PubMed] [Google Scholar]
- 2. Plouin PF, Duclos JM, Soppelsa F, et al. Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: analysis of 165 operations at a single center. J Clin Endocrinol Metab. 2001;86:1480–1486. [DOI] [PubMed] [Google Scholar]
- 3. Kobal SL, Paran E, Jamali A, et al. Pheochromocytoma: cyclic attacks of hypertension alternating with hypotension. Nat Clin Pract Cardiovasc Med. 2008;5:53–57. [DOI] [PubMed] [Google Scholar]
- 4. Guzik P, Wykretowicz A, Wesseling IK, et al. Adrenal pheochromocytoma associated with dramatic cyclic hemodynamic fluctuations. Int J Cardiol. 2005;103:351–353. [DOI] [PubMed] [Google Scholar]
- 5. Oishi S, Sasaki M, Ohno M, et al. Periodic fluctuation of blood pressure and its management in a patient with pheochromocytoma. Case report and review of the literature. Jpn Heart J. 1988;29:389–399. [DOI] [PubMed] [Google Scholar]
- 6. Ganguly A, Grim CE, Weinberger MH, et al. Rapid cyclic fluctuations of blood pressure associated with an adrenal pheochromocytoma. Hypertension. 1984;6:281–284. [PubMed] [Google Scholar]
- 7. Bergland BE. Pheochromocytoma presenting as shock. Am J Emerg Med. 1989;7:44–48. [DOI] [PubMed] [Google Scholar]


