Abstract
J Clin Hypertens(Greenwich). 2010;12:508–515. © 2010 Wiley Periodicals, Inc.
To evaluate the long‐term prognostic significance of different ranges of the percentage fall in nighttime blood pressure (BP) of the nondipping pattern, 1200 hypertensive patients (645 women, age 51±12 years) underwent ambulatory BP monitoring under stabilized therapy. The occurrence of cardiovascular (CV) events was followed for 9833 patient‐years and analyzed by the Cox hazard model. There were 152 CV fatal/nonfatal events (79 strokes, 51 coronary events, 22 others) during the 15.2 years of follow‐up. According to nighttime BP fall (%) the authors noted: <0% (reverse‐dippers [RD], n=83); 0%–4.9% (nondippers 1 [ND1], n=207); 5%–9.9% (nondippers 2 [ND2], n=311), 10%–19.9% (dippers [D], n=523); and ≥20% (extreme dippers [ED], n=76). After adjustment for confounding variables, hazard ratios (95% confidence interval) of CV event and stroke in RD vs D were 2.29 (1.31–3.99) and 2.46 (1.11–5.49); of ND1 vs D were 1.42 (1.12–1.79) and 1.62 (1.17–2.23); and of ND1 vs ND2 were 2.24 (1.33–3.75) and 2.30 (1.15–4.58). No differences were found in RD vs ND1 and ND2 vs D. Nondippers have a higher CV risk than dippers but only for a nighttime BP fall <5% suggesting that the limits for nondipping should be redefined for a stratification of CV risk.
Blood pressure (BP) has a circadian rhythm. Most normotensive and hypertensive patients present a BP fall between 10% and 20% during nighttime hours, this has been called the dipper condition. 1 , 2 However, a variety of abnormal nighttime–daytime patterns have been described, in which the nocturnal BP fall may be <10% (nondippers) or even reversed (reverse dippers). Although the pathogenetic mechanisms of the blunted nocturnal fall in BP are still unclear, it has been claimed that nondippers show an impairment in autonomic nervous drive that includes an abnormal sympathovagal balance at night. 3 , 4 , 5 It has been postulated that the lack of nocturnal BP fall in nondipper patients is associated with more serious end organ damage by arterial hypertension 6 , 7 , 8 , 9 , 10 and with a worsened CV outcome 6 , 11 , 12 , 13 than in dippers whose BP falls during the night. However, some dispute still exists on that subject, since the association between nondipping status and the excess of organ damage or CV events was not confirmed by other authors. 14 , 15 , 16 However, the short term reproducibility of the nocturnal blood pressure patterns (dipping/nondipping) has been questioned recently, 17 which could account for the conflicting data published in the literature. It was shown 17 that more than 20% of patients previously classified as nondippers changed their initial nocturnal pattern at a second ambulatory BP monitoring (ABPM) recording. Meanwhile, it would be expected that those nondipper patients showing a nighttime BP fall value closer to 9.9% would be more prone to having a misdiagnosed nondipper status than patients with a nighttime fall closer to 0%. We hypothesized that the CV risk of the latter would be more similar to that of the so‐called reverse dippers, whereas the former would present a risk more similar to that of dippers. Thus, the aim of the present study was to evaluate the long‐term CV prognostic significance of different ranges of the nighttime BP percentage fall of the nondipping profile.
Methods
Patients and Protocols
We conducted a longitudinal, retrospective cohort study of outpatients referred for ABPM between 1991 and 1998. Consecutive patients, aged 18 years and older were eligible for inclusion if (1) they had no history or clinical evidence of congestive heart failure, cerebrovascular disease, myocardial infarction, coronary bypass or angioplasty, cardiac valve disease, renal insufficiency, peripheral artery disease, atrial fibrillation or other major arrhythmias or severe hepatic disease; (2) they had no suspicion of secondary hypertension nor clinical suspicion of sleep apnea; and (3) they could be further evaluated either in a follow‐up examination or, if deceased, with a death certificate. Patients receiving antihypertensive treatment had to be on stabilized therapy for at least 3 months. All patients underwent clinic BP measurements, routine investigation (blood chemistry, urinalysis, and 12‐lead electrocardiogram at rest), and 24‐hour ambulatory BP monitoring. All patients gave their informed consent.
Clinic and ABPM
Clinic BP was evaluated at 2 different visits in the outpatient clinic. Humeral BP and heart rate were evaluated in the nondominant arm with an automated digital oscillometric sphygmomanometer (Omron, Model M6; Omron Corporation, Japan). Three readings separated by 2 minutes each were taken and the mean of the last 2 was considered as the brachial BP. Twenty‐four‐hour ABPM was performed during a work day at the entry of the study with SpaceLabs 90207 (SpaceLabs Inc, Redmond, WA) as previously described. 18 The monitor was mounted on the nondominant arm between 0800 and 0900, and was removed 24 hours later. A mercury sphygmomanometer was initially attached to the monitor through a Y‐connector to ensure agreement between both measurements. Patients were instructed to undertake their usual daily activities and were asked to go to bed no later than 2300 and arise not before 0700. BP was recorded every 20 minutes during the day (between 0700 and 2300) and every 30 minutes at night (between 2330 and 0630). These periods were considered as representatives of the awake and sleep BPs, respectively. The nocturnal systolic BP (SBP) fall (%) was calculated as 100 × [1‐sleep SBP/awake SBP ratio]. We subclassified the patients by their nocturnal SBP as: extreme dippers (ED) if their nocturnal SBP fall was ≥20%, dippers (D) if the fall was ≥10% but <20%, nondippers (ND) if it was ≥0% but <10% and reverse dippers (RD) if it was <0%. Those classified as ND were further divided into ND1 if the nocturnal SBP fall was ≥0% but <5% and ND2 if the nocturnal SBP fall was ≥5% but <10%.
Follow‐Up and Events
The patients’ medical records were reviewed at the moment of ABPM and thereafter for the use of antihypertensive drug therapy and further occurrence of CV events. Follow‐up was performed from 1991 to 2007. The mean follow‐up period was 8.2 years, ranging from 0.8 to 15.2 years. The presence or absence of CV events was assured by the examination of the patients’ medical records until the end of the follow‐up period. CV events were diagnosed either by the physician who cared for the patients at the time of the events or if death occurred, information on its cause came either from the patients’ physician or otherwise by examination of the official death certificate. CV events were classified as fatal or nonfatal and consisted of congestive heart failure, cerebrovascular disease, myocardial infarction, angor pectoris, coronary bypass or angioplasty). Strokes and coronary events were considered both integrating all CV events and also separately. Stroke events included ischemic stroke (cerebral infarction and cerebral embolism), hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage), and undefined type of stroke. Coronary events included sudden death and fatal and nonfatal myocardial infarction or angor pectoris confirmed in hospital and coronary bypass or angioplasty. Transient ischemic attack was not considered an event. For patients who experienced multiple events we confined the analysis to the first event under study.
Statistical Analysis
Statistical analysis was performed using the SPSS software version 13.0 (SPSS Inc., Chicago, IL). Values of continuous variables are presented as mean + standard deviation and differences between groups were evaluated by one‐way analysis of variance. Continuous variables were compared using parametric (Student’s t) or nonparametric (Wilcoxon‐Mann–Whitney) tests. Proportions were compared through de χ2 test or Fisher′s exact test, when possible.
Long‐term cumulative survival curves in D, ND1, ND2, and RD were estimated with the Kaplan–Meier method, and comparison between each of 2 groups was made with a log‐rank test. A multivariate Cox proportional hazard model analysis was used to evaluate the relative risk (hazard ratios) of the parameters of the ABPM and of the nighttime fall patterns on CV events, stroke, and coronary events, controlling for clinical risk factors. Follow‐up duration was calculated from the date of ABPM to either the date of the last day of follow‐up in patients without events or the date where outcome event/death occurred. Differences were considered statistically significant when P<.05.
Results
A total of 1200 Caucasian patients, 53.7% women, aged 51±12 years, body mass index 27±5 kg/m2 met the inclusion criteria. Mean follow‐up was 8.2±3.1 years (range: 0.8–15.2) and total follow‐up time amounted to 9833 patient‐years. The total number of first events during follow‐up consisted of 152 CV events, 16 of them fatal and 136 of them nonfatal, distributed by 79 strokes, 51 coronary events, and 22 other CV events. Determination of nighttime/daytime BP fall (%) permitted the classification of RD, n=83 (6.9%); ND1, n=207 (17.3%); ND2, n=311 (25.9%); D, n=523 (43.6%); and ED, n=76 (6.3%). Baseline characteristics of all these groups are shown in the Table. Compared to the other groups, RD were slightly older, more obese, and more likely to be diabetic; they showed higher levels of left ventricular mass index (LVMI), of plasma glucose, and of casual and 24‐hour SBP, and were more frequently medicated with antihypertensive drugs, particularly angiotensin‐converting enzyme inhibitors and diuretics. Nighttime BP values were significantly different among all groups being higher within the expected ranking order, ie, RD > ND1 > ND2 > D > ED. Besides the BP levels during nighttime, no significant differences were observed between ND1, ND2, and D groups for the majority of the variables, with the exception that ND1 were slightly older than ND2 and D, and 24‐hour SBP was lower in D than in the other groups. The number of events and strokes in the different groups was respectively 23 and 13 in RD, 40 and 25 in ND1, 30 and 15 in ND2, 52 and 22 in D, and 7 and 4 in ED. Based on a Cox proportional hazard analysis, Figure 1 shows the adjusted HRs and 95% confidence limits for total CV events and for stroke with the different patterns of dipping status. As shown, RD and ND1 associated significantly and positively with CV events and stroke, compared to D and ND2. However, when all ND (ND1 + ND2) were compared with D, differences did not achieve statistical significance. In addition, during the follow‐up period, the incidences of CV event and stroke per 1000 patient‐years (adjusted by the follow‐up periods) were around 3 times higher in RD than in ND2 and D, and 2.2 and 2.7 times higher in ND1 than in ND2 and D. The incidence of coronary events was also higher in RD than in ND1, ND2, and D (Figure 2). As shown in Figure 3, the cumulative CV event‐free survival rates and stroke‐free survival rates were significantly worse in RD and in ND1 than in D and ND2. In contrast cumulative CV event‐free survival rates did not differ between RD and ND1 and between ND2 and D. Cumulative CV event‐free survival rates (not shown in the figure) did not differ between D and all ND altogether (ND1 + ND2), but stroke‐free survival rates were worse in the assembly of all ND (ND1 + ND2) than in D (log rank 5.22, P=.0224).
Table.
Baseline Characteristics
| RD (n=83) | ND1 (n=207) | ND2 (n=311) | D (n=523) | ED (n=76) | ANOVA P Value | |
|---|---|---|---|---|---|---|
| Age (y) | 54.8±13.7a | 51.0±14.4c | 50.3±12.8 | 50.2±11.6 | 51.1±12.7 | .034 |
| Sex (male/female) | 41/43 | 106/103 | 143/171 | 234/281 | 30/47 | NS |
| BMI (kg/m2) | 28.1±5.8a | 26.2±4.1 | 26.9±4.6 | 27.4±4.6 | 27.2±3.9 | .021 |
| Diabetics (n/%) | 16/19.3%a | 20/9.7% | 28/9.0% | 53/10.1% | 5/6.6% | .04 |
| Current smokers (n/%) | 2/2.4% | 7/3.4% | 10/3.2% | 20/3.8% | 1/1.3% | NS |
| LVMI (g/m2) | 99.5±40.4a | 84.1±16.9 | 81.5±19.7 | 81.26±20 | 69.7±18.3a | .01 |
| Creatinine (mg/dL) | 1.05±0.5 | 0.97±0.3 | 0.92±0.23 | 0.91±0.22 | 0.88±0.26 | NS |
| Na (mmol/L) | 140.9±3.5 | 141.4±3.9 | 141.9±3.5 | 140.7±3.7 | 140.5±2.8 | NS |
| K+ (mmol/L) | 4.29±0.7 | 4.29±0.5 | 4.3±0.5 | 4.2±0.5 | 4.3±0.4 | NS |
| Glucose (mg/dL) | 119.1±47.6a | 98.1±28.3 | 100.9±31 | 100.6±27 | 97.2±15.7 | .001 |
| Uric acid (mg/dL) | 5.57±1.8 | 5.58±1.8 | 5.42±1.8 | 5.49±1.7 | 5.41±1.9 | NS |
| Total cholesterol (mg/dL) | 223±46 | 219±40 | 219±40 | 222±44 | 224±36 | NS |
| Triglycerides (mg/dL) | 155±82 | 131±67 | 140±89 | 138±83 | 129±16 | NS |
| eGFR (mL/min) | 78.16±28.4a | 81.3±23.4c | 84.5±24 | 83.4±20.3 | 89.3±23.5a | .03 |
| Follow‐up duration (y) | 8.3±2.8 | 7.9±3.3 | 8.1±3.0 | 8.2±3.2 | 8.0±3.3 | NS |
| Office BP (mm Hg) | ||||||
| SBP | 158±27a | 154±23 | 155±21 | 154±19 | 154.5±19 | .05 |
| PAD | 94.3±16.5 | 94.6±12.5 | 94.9±12.5 | 95.9±11.7 | 95.9±13.7 | NS |
| HR (beats/min) | 77.3±14.3 | 78.7±13.1 | 80.1±13.7 | 80±31.1 | 78.1±11.9 | NS |
| ABPM (mm Hg) | ||||||
| 24‐h SBP | 138.9±17.2a | 136.31±17 | 134.25±18 | 131.3±14.3d | 129.3±15.6d | .05 |
| 24‐h DBP | 82.4±12.2 | 83.5±10.8 | 82.6±11.9 | 81.7±10.3 | 80.2±10.7 | NS |
| 24‐h HR | 72.8±11.2 | 73.7±10.2 | 74.1±10.5 | 73.3±10.2 | 73.8±8.7 | NS |
| 24‐h PP | 56.58±11.8a | 52.95±13.2 | 51.8±10.5 | 49.7±9.2 | 49.1±9.6 | .03 |
| Daytime SBP | 136.8±17.1 | 137.5±17 | 137.7±17.9 | 137.6±14.9 | 139.7±17 | NS |
| Daytime DBP | 82.4±12.2 | 85.3±11 | 85.9±12.1 | 87±10.8 | 88.3±12 | NS |
| Daytime HR | 74.2±11.6 | 75.9±10.9 | 77.1±11 | 76.7±11 | 77.6±9.3 | NS |
| Daytime PP | 54.6±11.5a | 52.4±13.4 | 51.9±10.8 | 50.7±9.8 | 51.5±10.6 | .02 |
| Nighttime SBP | 143.2±18.2b | 133.8±16.7b | 127.1±16.7b | 118.1±13.3b | 107.4±13.6b | .001 |
| Nighttime DBP | 82.6±12.8b | 79.8±10.9b | 75.5±11.2b | 70.7±9.5b | 63.3±9.1b | .001 |
| Nighttime HR | 69.9±12.6 | 68.9±10.1 | 67.7±10.8 | 66.1±9.7 | 65.9±8.6 | NS |
| Nighttime PP | 61.1±13.2b | 54.3±13b | 51.7±10.2b | 47.6±8.4b | 44.3±7.7b | .001 |
| With medication (n/%) | 50/60.2%a | 94/45.4% | 161/53.7% | 280/53.5% | 44/57.9% | .01 |
| ACEI/ARBs (n/%) | 36/43.3%a | 60/28.9% | 106/34.1% | 187/35.8% | 32/42.1% | .02 |
| Calcium blockers (n/%) | 25/30.1% | 44/21.3% | 69/22.2% | 119/22.8% | 20/26.3% | NS |
| β‐blockers (n/%) | 15/18.0% | 37/17.9% | 45/14.5% | 93/17.8% | 13/17.1% | NS |
| Diuretics (n/%) | 31/37.3%a | 42/20.3% | 74/23.8% | 128/24.5% | 18/23.7% | .04 |
Data are shown as mean + standard deviation or percentages. Overall P values for 5‐group comparison of means (analysis of variance [ANOVA] F‐test) or percentages (χ2 test).
Abbreviations: ABPM, ambulatory blood pressure monitoring; ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; D, dippers; DBP, diastolic BP; ED, extreme dippers; eGFR, estimated glomerular filtration rate; HR, heart rate; K+, potassium; LVMI, left ventricular mass index; Na, sodium; ND1, nondippers with nighttime BP fall 0%–4.9%; ND2, nondippers with nighttime BP fall 5–9.9%; NS, not significant; PAD, peripheral artery disease; PP, pulse pressure; RD, reverse dippers; SBP, systolic BP.
a P<.04 RD significantly different from all other groups; b P<.02 significantly different between all groups. c P<.03 ND1 significantly different from ND2 and D groups. d P<.02 significantly different from RD, ND1, and ND2D groups.
Figure 1.
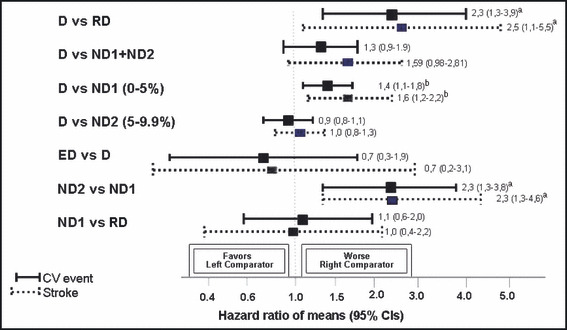
Hazard ratios and 95% confidence intervals (CIs) for cardiovascular events and strokes after adjustment for age, gender, smoking, total cholesterol, diabetes, and antihypertensive treatment between each of 2 groups (dippers [D], nondippers with nighttime blood pressure [BP] fall 5%–9.9% [ND2], nondippers with nighttime BP fall 0%–4.9% [ND1], and reverse dippers [RD]). aP<.001; bP<.01.
Figure 2.
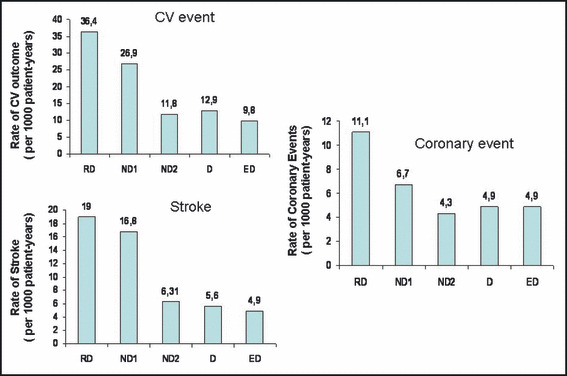
The incidence of cardiovascular (CV) outcomes (CV events, strokes, and coronary events) per 1000 patient‐years are compared between extreme dippers (ED), dippers (D), nondippers with nighttime blood pressure (BP) fall 5%–9.9% (ND2), nondippers with nighttime BP fall 0%–4.9% (ND1), and reverse dippers (RD). ND1 and RD showed an approximately 2–3 times higher rate of CV events and 2.8–3 times higher rate of strokes than D and ND2 did. RD showed an approximately 2 times higher rate of coronary events than any of the other groups.
Figure 3.
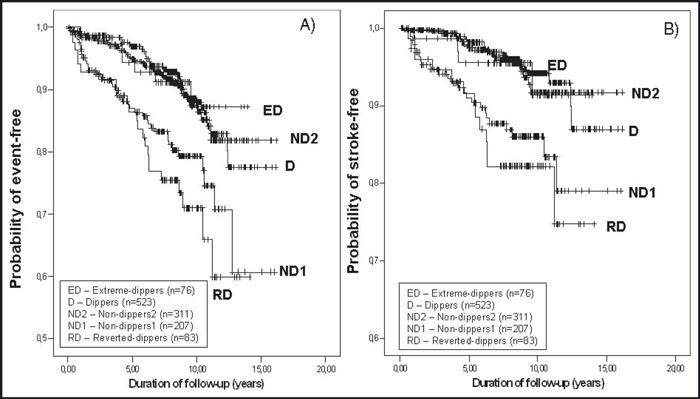
A) Event‐free survival curves. Overall log rank statistic for 5‐group comparison is 33.4 (P=.0001). Log rank statistic is 1.35 (P=.24, RD vs ND1, NS), 18.47 (P=.0001, RD vs ND2), 18.98 (P=.0001, RD vs D), 12.38 (P=.0001, ND1 vs ND2), 0.13 (P=.71, ND2 vs D, NS), 0.07 (P=.79, D vs ED, NS). B) Stroke‐free survival curves. Overall log rank statistic for 5‐group comparison is 30.26 (P=.0001). Log rank statistic is 0.51 (P=.48, RD vs ND1, NS), 13.3 (P=.0003, RD vs ND2), 16.60 (P=.0001, RD vs D), 11.22 (P=.0008, ND1 vs ND2), 0.06 (P=.83, ND2 vs D, NS), 0.06 (P=.81, D vs ED, NS). NS indicates not significant.
Discussion
Twenty‐four‐hour ABPM is a valuable tool to evaluate the circadian BP pattern and particularly the nighttime–daytime BP profile. 2 Patients who show a nocturnal BP fall of at least 10% are called dippers whereas those who show a nocturnal BP fall between 0 and 9.9% are called nondippers. 1 , 2 In the last few years, several crossover and longitudinal studies showed that the nondipping pattern in hypertensive patients is associated with an increase in total and CV mortality, 13 with an increased damage to target organs such as the heart, brain, and kidney 5 , 10 , 11 , 19 , 20 , 21 and with a higher frequency of CV events 22 (stroke, myocardial infarction, etc.) as compared to patients with normal nocturnal BP fall (dippers). Additionally, patients with reverse‐dipping, ie, showing higher BP values during the night than during daytime have been particularly associated with adverse CV events and higher risk. 13 , 22 , 23 Since the nondipping classification was accepted, it has been a current practice to classify patients with a nocturnal BP fall below 10% as nondippers, and to think of them as representing a group of higher risk. However, not all studies confirm that nondippers have a higher risk of CV events compared to dippers. 14 , 16 , 24 , 25 Among several reasons, that could be a result of the wide range of definitions of nighttime–daytime periods that have been used, of the importance of the influence of age, 25 and of the lack of reproducibility of the nondipping status when ABPM is repeated without intervention. 17 , 26
In the present study, the most striking finding was that among hypertensive patients usually classified as nondippers, those patients showing a nocturnal BP fall between 0 and 5% (ND1) encompass a much higher incidence of total CV events and stroke than the other nondipper patients whose nocturnal BP fall stays between 5% and 9.9% (ND2). Moreover, we also found that the CV outcome of ND1 was similar to that of RD, whereas that of ND2 was indistinguishable from that of D even after adjustment for other variables such as age, gender, and therapy. In our study, RD were those with higher adverse CV events and stroke in agreement with data reported by others. 13 , 22 , 23 Nevertheless, because they were older and heavier, had higher 24‐hour BP values, a slightly worse renal function, and included more diabetic patients than the other groups, all these factors could explain such an increase in the CV‐event ratio. In our study, however, adverse CV events and stroke were clearly more frequent (more than double) in ND1 as compared to ND2, although no differences in age, weight, mean 24‐hour BP, renal function, and presence of diabetes were found between these 2 groups. Our data suggest that the classification of nondipper ensembles a great variety of subjects with a large spectrum of diversity of CV risk, which perhaps may explain why some studies were enable to distinguish their CV risk from that of dippers. 14 , 16 , 24 , 25 Another argument that could be taken into account deals with the limited reproducibility of nocturnal pressure patterns. In fact, some recent studies have shown that the reproducibility of the nondipping pattern is much lower than that of the dipping pattern, because as many as 35% of hypertensive patients initially classified as nondippers did not confirm their nocturnal profile at a second ABPM recording. 17 , 26 It is plausible, although not proved, that those nondipper subjects with a nighttime BP fall closer to the limit of the definition of nondipper status, ie, 9.9% (such as our ND2), would be more prone to change their nocturnal pattern to dipper after a second ABPM recording than those with a nighttime BP fall closer to 0% (such as our ND1). In other words, a misdiagnosis of nondipper status would be more likely to occur in subjects such as our ND2 that, after a single ABPM recording, have a nighttime BP fall closer to the upper limit of the definition of nondipper status. Such a plausible misdiagnosis occurring in a high percentage of patients could explain why the CV risk of our ND2 did not differ from that of D. The only difference that we found between ND1 and ND2 groups was the BP level at the nighttime period which was higher among ND1. Some studies have found that nighttime ABPM is a significantly better predictor of an adverse CV outcome than daytime ABPM, 27 , 28 , 29 whereas the prognostic value of daytime and nighttime BP was almost similar in other studies. 24 , 25 Nevertheless, we can not rule out that such higher BP values at night could explain the higher CV risk of ND1 vs ND2. In our study, those patients classified as ED showed a relatively good CV prognosis, which contrasts with the results reported by others, 22 who showed that such a nighttime BP pattern in elderly patients is particularly related with ischemic events. However, since our group of ED included the youngest patients in our population and also those with lower nighttime BP absolute values, it is possible that these characteristics may explain the difference between our findings and those of others. 22
Our study has some limitations. Since it was a retrospective longitudinal study, we could not control any changes of hypertensive control and antihypertensive therapy that might occur during the follow‐up. Also multiple assessments of ambulatory BP were not performed which could elucidate our hypothesis determining whether the nondipping status was modified particularly among ND2. One important limitation of our study was the small number of total events so far observed during the follow‐up period, which is probably related to the fact that our population did not have a high CV risk at baseline. That could explain why we were unable to observe the expected worse outcome in all ND population (ND1 + ND2), as compared to D. An additional limitation is that because the number of coronary events was too small we did not have statistical power to optimally evaluate the influence of the nocturnal BP pattern on coronary events. In contrast, we found a clear predominance of strokes among all the events reported. However, this was not totally unexpected, since it is known that Portugal has a high mortality and a high prevalence of stroke that is much higher than that of myocardial infarction. 30
In summary we conclude that comparing to dipping, the nondipping phenomenon is closely related to a high incidence of CV disease and stroke, but only in patients with a nighttime fall below 5%. We may conclude that the classical classification of nondipper (nighttime fall 0%–9.9%) includes a broad spectrum of individuals with a wide range of different CV risk. Our data also suggest that the limits for classifying the nondipper status should be redefined in the clinical practice for CV risk stratification.
Ackowledgments: This work was partially founded by the Comissão de Fomento de Investigação Cuidados de Saúde, Ministério Saude, Portugal & Bolsa Menarini, Sociedade Portuguesa de Hipertensão, Portugal. The authors are deeply grateful to Master Maria José Brito for her English proofreading assistance.
References
- 1. Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990;81:700–702. [DOI] [PubMed] [Google Scholar]
- 2. O Brien E, Sheridan J, O Malley K. Dippers and non‐dippers [letter]. Lancet. 1988;2:397. [DOI] [PubMed] [Google Scholar]
- 3. Hojo Y, Noma S, Ohki T, et al. Autonomic nervous system activity in essential hypertension: a comparison between dippers and non‐dippers. J Hum Hypertens. 1997;11:665–671. [DOI] [PubMed] [Google Scholar]
- 4. Abate G, D’Andrea L, Battestini M, et al. Autonomic nervous activity in elderly dipper and non‐dipper patients with essential hypertension. Aging (Milano). 1997;9:408–414. [DOI] [PubMed] [Google Scholar]
- 5. Polonia J, Amaral C, Bertoquini S, et al. Attenuation of heart rate recovery after exercise in hypertensive patients with blunting of the nighttime blood pressure fall. Int J Cardiol. 2006;106:238–243. [DOI] [PubMed] [Google Scholar]
- 6. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24 (6):793–801. [DOI] [PubMed] [Google Scholar]
- 7. Verdecchia P, Porcellati C. The day‐night changes in ambulatory blood pressure: another risk indicator in hypertension? G Ital Cardiol. 1992;22:879–886. [PubMed] [Google Scholar]
- 8. Pierdomenico SD, Costantini F, Bucci A, et al. Blunted nocturnal fall in blood pressure and oxidative stress in men and women with essential hypertension. Am J Hypertens. 1999;12 (4 Pt 1):356–363. [DOI] [PubMed] [Google Scholar]
- 9. Kohno I, Takusagawa M, Yin D, et al. QT dispersion in dipper‐ and nondipper‐type hypertension. Am J Hypertens. 1998;11 (3 Pt 1):280–285. [DOI] [PubMed] [Google Scholar]
- 10. Ferrara AL, Pasanisi F, Crivaro M, et al. Cardiovascular abnormalities in never‐treated hypertensives according to nondipper status. Am J Hypertens. 1998;11 (11 Pt 1):1352–1357. [DOI] [PubMed] [Google Scholar]
- 11. White WB. How well does ambulatory blood pressure predict target‐organ disease and clinical outcome in patients with hypertension? Blood Press Monit. 1999;4 (Suppl. 2):S17–S21. [PubMed] [Google Scholar]
- 12. Shimada K, Kario K. Altered circadian rhythm of blood pressure and cerebrovascular damage. Blood Press Monit. 1997;2:333–338. [PubMed] [Google Scholar]
- 13. Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. [DOI] [PubMed] [Google Scholar]
- 14. Fagard RH, Staessen JA, Thijs L. The relationships between left ventricular mass and daytime and night‐time blood pressures: a meta‐analysis of comparative studies. J Hypertens. 1995;13:823–829. [DOI] [PubMed] [Google Scholar]
- 15. Cuspidi C, Lonati L, Sampieri L, et al. Impact of nocturnal fall in blood pressure on early cardiovascular changes in essential hypertension. J Hypertens. 1999;17:1339–1344. [DOI] [PubMed] [Google Scholar]
- 16. Bjorklund K, Lind L, Andren B, et al. The majority of nondipping men do not have increased cardiovascular risk: a population‐based study. J Hypertens. 2002;20:1501–1506. [DOI] [PubMed] [Google Scholar]
- 17. Cuspidi C, Meani S, Valerio C, et al. Reproducibility of dipping/nondipping pattern in untreated essential hypertensive patients: impact of sex and age. Blood Pressure Monit. 2007;12:101–106. [DOI] [PubMed] [Google Scholar]
- 18. Groppelli A, Omboni S, Parati G, et al. Evaluation of noninvasive blood pressure monitoring devices Spacelabs 90202 and 90207 versus resting and ambulatory 24‐h intra‐arterial blood pressure. Hypertension. 1992;20:227–232. [DOI] [PubMed] [Google Scholar]
- 19. Shimada K, Kawamoto A, Matsubayashi K, et al. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–878. [PubMed] [Google Scholar]
- 20. Timio M, Venanzi S, Lolli S, et al. “Non‐dipper” hypertensive patients and progressive renal insufficiency: a 3‐year longitudinal study. Clin Nephrol. 1995;43:382–387. [PubMed] [Google Scholar]
- 21. Lurbe E, Redon J, Pascual JM, et al. The spectrum of circadian blood pressure changes in type I diabetic patients. J Hypertens. 2001;19:1421–1428. [DOI] [PubMed] [Google Scholar]
- 22. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]
- 23. Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Eng J Med. 2003;348:2407–2415. [DOI] [PubMed] [Google Scholar]
- 25. Khattar RS, Swales JD, Dore C, et al. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001;104:783–789. [DOI] [PubMed] [Google Scholar]
- 26. Cuspidi C, Macca G, Michev I, et al. Short‐term reproducibility of nocturnal non‐dipping pattern in recently diagnosed essential hypertensives. Blood Press. 2002;11:79–83. [DOI] [PubMed] [Google Scholar]
- 27. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 28. Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282:539–546. [DOI] [PubMed] [Google Scholar]
- 29. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause‐specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 30. Barros P, De Almeida Simões J. Portugal: health system in review. Health Systems in Transition. 2007;9:1–140. [PubMed] [Google Scholar]


