Abstract
J Clin Hypertens (Greenwich).2010;12:487–494. © 2010 Wiley Periodicals, Inc.
This post hoc analysis of a 7‐week, randomized, double‐blind trial evaluated the efficacy and safety of initial irbesartan/hydrochlorothiazide treatment in 468 patients with severe, uncontrolled, hypertension (diastolic blood pressure [DBP] ≥100 mm Hg) at high cardiovascular risk. Systolic blood pressure (SBP)/DBP reductions ranged from 28.0 to 42.9/22.9 to 27.2 mm Hg in patients with obesity, diabetes, baseline SBP ≥180 mm Hg, and in the elderly. Blood pressure control to <140/90 mm Hg in the age and obesity subgroups ranged from 32.1% to 39.2% while control to <130/80 mm Hg in patients with diabetes was 11.5%. After 1 week of therapy, 72.5% of patients no longer had SBP ≥180 mm Hg; by 7 weeks, 51.3% had SBP 140 to 159 mm Hg and 26.5% had SBP <140 mm Hg. Treatment was well tolerated regardless of the subgroup. No excess of prespecified events was noted. Thus, initial treatment with irbesartan/hydrochlorothiazide was rapidly effective in high‐risk, difficult‐to‐treat, severely hypertensive patients.
Patients with severe hypertension are at high risk of having a cardiovascular (CV) event. 1 , 2 , 3 , 4 The risk of death from ischemic heart disease and stroke doubles for every 20 mm Hg increase in systolic blood pressure (SBP) or 10 mm Hg increase in diastolic blood pressure (DBP). 2 , 3 Additionally, hypertensive emergencies, such as hospitalization for very high blood pressure (BP) levels, cerebral infarction, acute pulmonary edema, hypertensive encephalopathy, and rupture of aneurysms, occur at a rate of 1 in 5 to 10 patient years of exposure to severe hypertension. 1 , 2
CV risk further increases with the presence of additional risk factors such as advanced age, obesity, and type 2 diabetes mellitus. 5 In diabetic patients, for example, the risk of CV complications doubles when SBP increases from <120 mm Hg to >160 mm Hg. 6 Conversely, risk decreases with treatment strategies aimed at controlling BP. In the United Kingdom Prospective Diabetes Study (UKPDS) of diabetic patients with a mean baseline BP of 160/94 mm Hg, fewer clinical complications (32% fewer diabetes‐related deaths, 44% fewer strokes, and 37% fewer microvascular end points) occurred in the group in which the treatment goal was SBP/DBP <150/85 mm Hg than in the group where the treatment goal was SBP/DBP <180/105 mm Hg. 7
In addition to increasing risk, CV risk factors, such as age, obesity, and diabetes mellitus, create a physiologic environment which decreases a patient’s responsiveness to antihypertensive treatment and can make BP normalization a challenge. 7 , 8 , 9 , 10 In a large German study, for example, advanced age, high body mass index (BMI), and increased waist circumference were associated with poor BP control. 9 Not surprisingly, these difficult‐to‐treat patients often need to take multiple therapies in order to reach and maintain BP control. Indeed, many studies have documented the need for high‐risk patients to take multiple antihypertensive medications in order to reach treatment goals. 7 , 11 , 12 In the UKPDS 38 study, for example, 29% of diabetic patients required 3 or more treatment agents to lower BP to <150/85 mm Hg after 9 years of follow‐up. 7 Similarly, in the high‐risk patients with hypertension in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) trial, 32% of patients were taking 2 to 3 antihypertensive medications by the end of the study. 12
US and European hypertension guidelines now highlight the challenges associated with BP control in these difficult‐to‐treat patients. 5 , 13 They recommend initial treatment with combination antihypertensive therapies that target complementary pathways in patients with stage 2/grade 2 hypertension or above, (or ≥20/10 mm Hg above BP goal in high‐risk patients with goal BP of <130/80 mm Hg) for whom rapid BP reduction is essential, and in patients at high CV risk due to comorbidities, for whom BP normalization is particularly difficult. 5
Combination of an angiotensin receptor blocker (ARB), such as irbesartan, with a diuretic, such as hydrochlorothiazide (HCTZ), is one of the available drug associations that target complementary pathways in the treatment of hypertension. In particular this combination has been shown to be safe and effective in diverse populations of difficult‐to‐treat patients including severely hypertensive patients, 14 patients with diabetes, 15 , 16 elderly patients, 17 obese patients, 9 and patients with both moderate or severe hypertension and additional risk factors. 18 In this post hoc analysis of a prospective trial of 697 patients with severe hypertension, 14 we evaluated the efficacy and tolerability of irbesartan/HCTZ in subsets of patients expected to be particularly hard to treat due to the added presence of older age, diabetes mellitus, obesity, or very high SBP.
Materials and Methods
The study design and patient population of this prospective, randomized, 7‐week, double‐blind, multicenter trial in adult patients with uncontrolled severe hypertension, have been described elsewhere. 14
In brief, after a 1‐week placebo run‐in period, 697 adult patients were randomized in a 2:1 ratio to receive irbesartan/HCTZ (n=468) or irbesartan (n=229). The dosing schedule for irbesartan/HCTZ was 150/12.5 mg for 1 week with a forced titration to irbesartan/HCTZ 300/25 mg for an additional 6 weeks. The dosing schedule for irbesartan was 150 mg for 1 week with a forced titration to 300 mg for an additional 6 weeks. Severe uncontrolled hypertension at baseline was defined as (1) a seated DBP ≥110 mm Hg in untreated patients or (2) a seated DBP ≥100 mm Hg in patients treated with an antihypertensive monotherapy for at least 4 weeks.
Patients were excluded if they were being treated with a fixed‐dose combination antihypertensive therapy or if they had a seated SBP ≥220 mm Hg, a seated DBP ≥130 mm Hg, known or suspected secondary hypertension, any condition that required immediate BP lowering, concomitant CV or cerebrovascular disease, significant chronic renal impairment or renovascular disease, or hepatic disease. 14
In this post hoc analysis, the efficacy and safety of treatment with irbesartan/HCTZ were analyzed according to: age (≥65 or <65 years), obesity (BMI ≥30 or <30 kg/m2), type 2 diabetes mellitus (with or without/unknown), and baseline SBP (≥180 mm Hg or <180 mm Hg).
Prospectively defined end points for the post hoc analysis were (1) change from baseline in seated SBP and seated DBP at 7 weeks, and (2) BP control rate at 7 weeks. In patients with a baseline SBP ≥180 mm Hg, change in SBP to the following ranges was noted: stage 2 hypertension, SBP 160 to 179 mm Hg; stage 1 hypertension, SBP 140 to 159 mm Hg; and normotension, SBP <140 mm Hg.
All adverse events (AEs) were analyzed by subgroup and categorized according to frequency of occurrence, severity, and relationship to study medication. Prespecified events identified as particularly relevant to initial combination antihypertensive treatment with diuretics and ARBs were dizziness, headache, hypotension, syncope, hyperkalemia, hypokalemia, and serum potassium level >6.0 mmol/L.
Statistical Analysis
Mean changes from baseline in SBP and DBP were determined and t‐tests were used to calculate 95% confidence intervals for between‐subgroup differences.
Changes from baseline in mean SBP were adjusted for baseline age, BMI, type 2 diabetes mellitus, sex, race, cholesterol, target organ damage, and acute coronary syndrome using an analysis of covariance model.
Results
Four hundred sixty‐eight patients treated with irbesartan/HCTZ were stratified according to CV risk factors yielding the following subgroups: age: ≥65 years (n=53) and <65 years (n=415); obesity: BMI ≥30 kg/m2 (n=236) and BMI <30 kg/m2 (n=229); type 2 diabetes mellitus: with (n=52) and without or unknown (n=416); baseline SBP: ≥180 mm Hg (n=135) and <180 mm Hg (n=332).
Baseline SBP and DBP of patients with evaluable efficacy data are presented by subgroup in Table I. Baseline SBP was similar regardless of presence or absence of type 2 diabetes mellitus or obesity, but was statistically lower (P<.0001) in patients <65 years of age than in patients ≥65 years of age (SBP of 170 mm Hg vs 180 mm Hg). Baseline DBP was similar in all subgroups, regardless of age, obesity, type 2 diabetes mellitus, and baseline SBP. Statistical significance was not calculated for other subgroups due to low statistical test power as sample size was determined to power primary endpoints.
Table I.
Baseline SBP and DBP by Subgroup
| N | SBP in mm Hg Mean (SD) | DBP in mm Hg Mean (SD) | |
|---|---|---|---|
| Age | |||
| ≥65 y | 47 | 180.0b (16.7) | 113.0 (2.0) |
| <65 y | 379 | 170.2 (16.0) | 113.4 (3.7) |
| Obesity | |||
| BMI ≥30 kg/m2 | 195 | 170.1 (17.1) | 113.3 (3.4) |
| BMI <30 kg/m2 | 229 | 172.5 (15.5) | 113.4 (3.7) |
| Diabetes mellitus, type 2 | |||
| With | 46 | 173.0 (18.5) | 112.8 (4.3) |
| Withouta | 380 | 171.1 (16.0) | 113.4 (3.4) |
| Baseline SBP | |||
| ≥180 mm Hg | 135 | 191.2 (10.3) | 115.1 (5.0) |
| <180 mm Hg | 332 | 163.5 (10.3) | 112.7 (2.8) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; N, number of patients with evaluable efficacy data; SBP, systolic blood pressure; SD, standard deviation. aOr unknown. b P<.0001; statistical significance was not calculated for other subgroups due to low statistical test power as sample size was determined to power primary endpoints.
Changes From Baseline in Seated SBP and Seated DBP
Irbesartan/HCTZ treatment provided significant reductions in SBP and DBP from baseline, regardless of age, obesity, type 2 diabetes mellitus, and baseline SBP (P<.001 all groups) (Figure 1). Reductions in SBP ranged from 28.0 to 42.9 mm Hg depending on the risk factor subgroup. The greatest decrease in SBP (42.9 mm Hg) was noted in patients with a baseline SBP ≥180 mm Hg; the smallest decrease in SBP was noted in patients with diabetes mellitus. Reductions in DBP varied from 22.9 to 27.2 mm Hg depending on the subgroup. After adjustment for baseline SBP, age, BMI, type 2 diabetes mellitus, sex, race, cholesterol, target organ damage, and acute coronary syndrome, reductions in SBP were similar within each subgroup analyzed (Table II).
Figure 1.
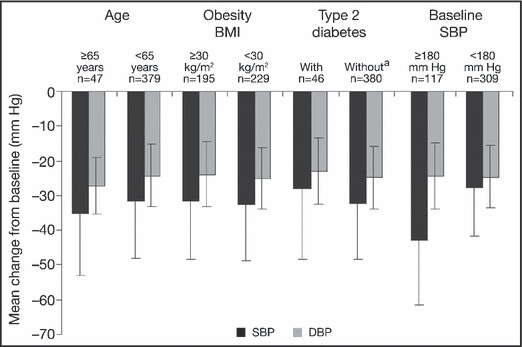
Reduction from baseline in seated systolic blood pressure (SBP) and seated diastolic blood pressure (DBP) following 7 weeks of treatment with fixed‐dose irbesartan/hydrochlorothiazide in severely hypertensive patients with additional cardiovascular risk factors. Means and standard deviations are presented. aOr unknown. BMI indicates body mass index.
Table II.
Effect of Age, Obesity, and Type 2 Diabetes Mellitus on Seated SBP Following 7 Weeks of Treatment With Fixed‐Dose Irbesartan/Hydrochlorothiazide in Severely Hypertensive Patients
| N | Seated SBP | ||||
|---|---|---|---|---|---|
| Adjusted Change From Baseline | |||||
| Mean (SD) | Difference | P Value | 95% CI | ||
| Age | |||||
| ≥65 y | 47 | −24.6 (2.7) | 2.4 | .287 | −7.0 to 2.1 |
| <65 y | 379 | −27.0 (2.0) | |||
| Obesity | |||||
| BMI ≥30 kg/m2 | 195 | −25.7 (2.3) | 0.3 | .824 | −2.4 to 3.1 |
| BMI <30 kg/m2 | 229 | −26.0 (2.1) | |||
| Diabetes mellitus, type 2 | |||||
| With | 46 | −24.2 (2.6) | 3.2 | .152 | −1.2 to 7.7 |
| Withouta | 380 | −27.4 (2.1) | |||
Using an analysis of covariance model, data were adjusted for baseline age, body mass index (BMI), type 2 diabetes mellitus, sex, race, cholesterol, target organ damage, and acute cornary syndrome.
Abbreviations: CI, confidence interval; SBP, systolic blood pressure; SD, standard deviation. aOr unknown.
BP Control Rates
DBP control rates to <90 mm Hg varied depending on the subgroup (Table III). The highest control rate (60.4%) was achieved in patients ≥65 years of age and the lowest (48.4%) was reached in patients with BMI ≥30 kg/m2. The lower DBP target of <80 mm Hg for patients with type 2 diabetes mellitus was achieved in 15.4% of these patients. Control rates to SBP/DBP <140/90 mm Hg ranged from 32.1% to 39.2% (Table III). Presence or absence of obesity did not affect SBP/DBP control rates (∼38%); whereas fewer older patients (32.1% vs 38.6% of patients <65 years of age) reached their target BP of SBP/DBP <140/90 mm Hg. The lower BP goal of <130/80 mm Hg for patients with diabetes was achieved in 11.5% of these patients.
Table III.
BP Control Following 7 Weeks of Treatment With Fixed‐Dose Irbesartan/Hydrochlorothiazide in Severely Hypertensive Patients
| N | Seated DBP <90 mm Hg a | Seated SBP/DBP <140/90 mm Hg a | |||
|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | ||
| Age | |||||
| ≥65 y | 47 | 32 (60.4%) | 0.5–0.7 | 17 (32.1%) | 0.2–0.4 |
| <65 y | 379 | 211 (50.8%) | 0.5–0.6 | 160 (38.6%) | 0.3–0.4 |
| Obesity | |||||
| BMI ≥30 kg/m2 | 195 | 107 (48.4%) | 0.4–0.6 | 85 (38.5%) | 0.3–0.4 |
| BMI <30 kg/m2 | 229 | 135 (55.3%) | 0.5–0.6 | 92 (37.7%) | 0.3–0.4 |
| Diabetes mellitus, type 2 | |||||
| Witha | 46 | 8 (15.4%) | 0.06–0.25 | 6 (11.5%) | 0.03–0.20 |
| Withoutb | 380 | 223 (53.6%) | 0.5–0.6 | 163 (39.2%) | 0.3–0.4 |
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; SBP systolic blood pressure. aBP control was to seated DBP <80 mm Hg, seated SBP/DBP <130/80 mm Hg in patients with diabetes. bOr unknown.
Change in Hypertension Severity
In patients with a baseline SBP ≥180 mm Hg, SBP decreased rapidly over time (Figure 2). By week 1, 72.5% of patients no longer had SBP ≥180 mm Hg. By week 7, only 7.7% of patients still had SBP ≥180 mm Hg, 51.3% had SBP in the range 140 to 159 mm Hg, and 26.5% had reached their goal SBP of <140 mm Hg.
Figure 2.
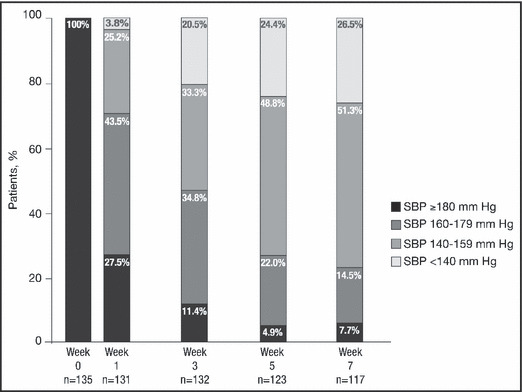
Percentage of patients with a baseline systolic blood pressure (SBP) ≥180 mm Hg achieving lower SBP ranges during treatment with fixed‐dose irbesartan/hydrochlorothiazide.
Safety and Tolerability
Treatment emergent AE rates were similar regardless of age, obesity, type 2 diabetes mellitus, and baseline SBP (Table IV). AEs varied from 25% to 32% depending on the subgroup. Slightly fewer patients ≥65 years of age experienced AEs than patients <65 years of age (26.4% vs 30.4%). Slightly fewer patients with type 2 diabetes mellitus experienced AEs than patients without type 2 diabetes mellitus (25.0% vs 30.5%). The most frequently reported events were headache and dizziness in all subgroups except in patients with type 2 diabetes mellitus who reported most frequently bronchitis, upper respiratory tract infection, and increased blood glucose.
Table IV.
Treatment‐Emergent AEs Occurring in ≥2% of Patients in Any Group and Prespecified AEs
| N | Age | Obesity BMI | Diabetes Mellitus Type 2 | Baseline SBP | ||||
|---|---|---|---|---|---|---|---|---|
| ≥65 y | <65 y | ≥30 kg/m 2 | <30 kg/m 2 | With | Without a | ≥180 mm Hg | <180 mm Hg | |
| 53 | 415 | 236 | 229 | 52 | 416 | 135 | 332 | |
| Total subjects with AEs, % | 26.4 | 30.4 | 28.2 | 31.7 | 25.0 | 30.5 | 31.1 | 29.5 |
| Headacheb | 3.8 | 4.1 | 3.3 | 5.0 | 1.9 | 4.3 | 6.7 | 3.0 |
| Dizzinessb | 1.9 | 3.6 | 3.3 | 3.6 | 1.9 | 3.6 | 3.7 | 3.3 |
| Dizziness posturalb | 0 | 0.2 | 0.4 | 0 | 0 | 0.2 | 0 | 0.3 |
| Bronchitis | 0 | 1.4 | 0.8 | 1.8 | 3.8 | 1.0 | 0 | 1.8 |
| Upper respiratory tract infection | 0 | 1.4 | 0.8 | 1.8 | 3.8 | 1.0 | 1.5 | 1.2 |
| Blood glucose increased | 1.9 | 0.2 | 0.4 | 0.5 | 3.8 | 0 | 1.5 | 0 |
| Somnolence | 0 | 0.7 | 0.8 | 0.5 | 0 | 0.7 | 2.2 | 0 |
| Nasopharyngitis | 1.9 | 1.7 | 2.0 | 1.4 | 0 | 1.9 | 1.5 | 1.8 |
| Migraineb | 0 | 0.2 | 0 | 0.5 | 0 | 0.2 | 0.7 | 0 |
| Hyperkalemiab | 0 | 0.2 | 0.4 | 0 | 0 | 0.2 | 0 | 0.3 |
| Hypokalemiab | 0 | 0.2 | 0 | 0.5 | 1.9 | 0 | 0 | 0.3 |
| Blood potassium decreasedb | 0 | 0.5 | 0.8 | 0 | 0 | 0.5 | 0.7 | 0.3 |
| Hypotensionb | 0 | 0.5 | 0.8 | 0 | 0 | 0.5 | 0.7 | 0.3 |
| Orthostatic hypotensionb | 0 | 0.2 | 0.4 | 0 | 0 | 0.2 | 0 | 0.3 |
| Syncopeb | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: AEs, adverse events; BMI, body mass index; SBP, systolic blood pressure. aOr unknown. bPrespecified AEs.
With the exception of dizziness and headache, no single prespecified event was reported in more than 2 patients in any subgroup. No patients reported syncope. There were no treatment‐related serious AEs or deaths.
Discussion
In this post hoc analysis, we evaluated the efficacy and safety of initial treatment with irbesartan/HCTZ in difficult‐to‐treat stage 2 hypertensive patients at high or very high CV risk. This therapeutic approach, which is endorsed by regulatory agencies that recommend initial treatment with combination therapies for patients with stage 2 hypertension and for patients at high CV risk, 5 , 13 was effective and well tolerated regardless of age, obesity, type 2 diabetes mellitus, and baseline SBP.
After 7 weeks of treatment, mean SBP reductions ranged from 28 to 43 mm Hg, mean DBP reductions ranged from 23 to 27 mm Hg, depending on the subgroup. These changes in BP are clinically significant as they suggest a drop in hypertension severity stage for most patients and therefore imply a reduction in morbidity and mortality. Indeed, when the stage of hypertension was assessed in patients with a baseline SBP ≥180 mm Hg, after 1 week of therapy, <30% of patients still had an SBP ≥180 mm Hg; and after 7 weeks of treatment only 7.7% of patients still had an SBP ≥180 mm Hg. Thus, the challenge of rapidly lowering BP in severely hypertensive patients, which has been imparted to first‐line combination therapies, was met by the combination of irbesartan with HCTZ. The benefit of this combination therapy is that it provides effective and similar BP reductions in all subgroups of hypertensive patients, and because of the complementary nature of irbesartan and HCTZ, efficacy is matched with a side effect profile which is equal to or better than monotherapy. 14 , 18
Although hypertensive patients with additional risk factors are harder to treat, BP control to <140/90 mm Hg in patients with comorbidities was high (32.1% to 38.5%) and efficacy comparisons within each subgroup showed that after covariate adjustment, initial treatment with irbesartan/HCTZ was equally effective in patients regardless of age, obesity, and diabetes mellitus. These data may illustrate the benefits associated with the irbesartan‐mediated blockade of the renin‐angiotensin‐aldosterone system, such as microalbuminuria reduction and renal outcome rate reduction. 19 , 20 , 21 This hypothesis is consistent with the current understanding of the pathophysiology of diabetes and obesity, both of which render patients particularly sensitive to kidney damage from systemic arterial hypertension.
Safety considerations with initial combination treatment are paramount as concerns about unnecessary exposure to AEs with initial combination therapies are often raised. In this study, the 2 main concerns associated with initial ARB/HCTZ treatment were not borne out. The initial BP response did not cause syncope and only one patient experienced orthostatic hypotension. In addition, no excess of other rare AEs, such as hypokalemia and hyperkalemia, which are associated with 12.5 and 25 mg doses of HCTZ, were observed. These data are consistent with those observed in other irbesartan/HCTZ studies. 9 , 14 , 15 , 16 , 22
Interestingly, AE rates did not increase with age as might have been expected. As the elderly often take multiple medications, their AE rates are often in part due to higher rates of drug–drug interactions and difficulties in drug metabolism. Irbesartan, which is eliminated predominantly by hepatic and biliary routes, 23 is not subjected to extensive metabolism and consequently drug–drug interactions are uncommon. These data may explain why older patients did not experience more AEs.
Overall, the data reported here are consistent with those reported in other trials in which irbesartan/HCTZ was effective in patients with type 2 diabetes mellitus, 15 in obese patients, 9 and in the very elderly (over the age of 75 years). 24 Although the present study was not long enough to assess the impact of treatment on clinical end points, irbesartan and irbesartan/HCTZ have been shown to have beneficial effects on CV risk, as well as on albuminuria, nephropathy, and metabolic parameters. 16 , 21 , 25 Longer‐term studies would be needed to quantify the effect of the BP reductions reported herein on longer‐term clinical end points.
Although traditionally sequential monotherapy or progressive titration approaches have been favored because they ensure that patients take the smallest number of medications needed, these strategies are associated with significant delays in achieving BP control 26 and therefore higher morbidity and mortality rates. 27 These traditional strategies are particularly ineffective in patients with comorbidities in whom pathophysiology of hypertension is complex and for whom multiple therapies are generally needed. Indeed, the UKPDS 38 study illustrated this point by noting that 29% of diabetic patients required 3 or more treatments to lower BP to <150/85 mm Hg, a treatment goal which is well above the now recommended 130/80 mm Hg. 7 Furthermore, as physicians are generally slow to titrate medication, 28 high‐risk patients are good candidates for initial combination treatments as long as treatment safety profiles are acceptable.
Thus, initial treatment with irbesartan/HCTZ, which offers good efficacy while remaining well tolerated, may provide significant morbidity and mortality benefits for severely hypertensive patients with comorbidities. Furthermore, the addition of a third and even fourth antihypertensive drug will often be necessary therapeutic BP goal attainment in a significant number of high‐risk patients.
Conclusions
Guidelines, which endorse the use of initial combination therapy in patients with stage 2 hypertension and in high‐risk patients, reflect the importance of an early reduction in BP and underscore the difficulties associated with controlling BP in severely hypertensive patients with added comorbidities. In this short 7‐week trial, initial treatment with irbesartan/HCTZ was rapidly effective regardless of age, obesity, type 2 diabetes mellitus, and baseline SBP. Furthermore, treatment was well tolerated and AE rates were similar between groups. Thus, in this analysis, initial treatment of severely hypertensive patients with irbesartan/HCTZ offered good efficacy while remaining well tolerated.
Disclosures: Editorial support for this article was provided by Bristol‐Myers Squibb and sanofi‐aventis.
References
- 1. Zampaglione B, Pascale C, Marchisio M, et al. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27:144–147. [DOI] [PubMed] [Google Scholar]
- 2. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 4. Anderson TW. Re‐examination of some of the Framingham blood‐pressure data. Lancet. 1978;2:1139–1141. [DOI] [PubMed] [Google Scholar]
- 5. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 6. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 8. Roux O, Chapellier M, Czernichow S, et al. Determinants of hypertension control in a large French population of treated hypertensive subjects. Blood Press. 2006;15:6–13. [DOI] [PubMed] [Google Scholar]
- 9. Sharma AM, Bramlage P, Kirch W. Antihypertensive effect of irbesartan and predictors of response in obesity‐associated hypertension: a prospective, open‐label study. Clin Drug Investig. 2005;25:765–776. [DOI] [PubMed] [Google Scholar]
- 10. Ostchega Y, Yoon S, Hughes J, et al. Hypertension Awareness, Treatment, and Control – Continued Disparities in Adults: United States, 2005–2006. National Center for Health Statistics Data Brief No. 3; 2008. [PubMed]
- 11. Mancia G, Grassi G. Systolic and diastolic blood pressure control in antihypertensive drug trials. J Hypertens. 2002;20:1461–1464. [DOI] [PubMed] [Google Scholar]
- 12. Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000;356:366–372. [DOI] [PubMed] [Google Scholar]
- 13. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 14. Neutel JM, Franklin SS, Oparil S, et al. Efficacy and safety of irbesartan/HCTZ combination therapy as initial treatment for rapid control of severe hypertension. J Clin Hypertens (Greenwich). 2006;8:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sowers JR, Neutel JM, Saunders E, et al. Antihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes. J Clin Hypertens (Greenwich). 2006;8:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bramlage P, Pittrow D, Kirch W. The effect of irbesartan in reducing cardiovascular risk in hypertensive type 2 diabetic patients: an observational study in 16,600 patients in primary care. Curr Med Res Opin. 2004;20:1625–1631. [DOI] [PubMed] [Google Scholar]
- 17. Cushman WC, Neutel JM, Saunders E, et al. Efficacy and safety of fixed combinations of irbesartan/hydrochlorothiazide in older vs younger patients with hypertension uncontrolled with monotherapy. Am J Geriatr Cardiol. 2008;17:27–36. [DOI] [PubMed] [Google Scholar]
- 18. Weir MR, Neutel JM, Bhaumik A, et al. The efficacy and safety of initial use of irbesartan/hydrochlorothiazide fixed‐dose combination in hypertensive patients with and without high cardiovascular risk. J Clin Hypertens (Greenwich). 2007;9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sasso FC, Carbonara O, Persico M, et al. Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension: a randomized double‐blind placebo‐controlled crossover study. Diabetes Care. 2002;25:1909–1913. [DOI] [PubMed] [Google Scholar]
- 20. De Alvaro F, Velasco O, Honorato J, et al. Microalbuminuria in hypertensive patients: evaluation of one‐year treatment with irbesartan. Kidney Int Suppl. 2005;Jan:S29–S34. [DOI] [PubMed] [Google Scholar]
- 21. Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–3037. [DOI] [PubMed] [Google Scholar]
- 22. Neutel JM, Saunders E, Bakris GL, et al. The efficacy and safety of low‐ and high‐dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trial. J Clin Hypertens (Greenwich). 2005;7:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnston CI. Pharmacology of irbesartan. Expert Opin Investig Drugs. 1999;8:655–670. [DOI] [PubMed] [Google Scholar]
- 24. Fogari R, Zoppi A, Mugellini A, et al. Efficacy and safety of two treatment combinations of hypertension in very elderly patients. Arch Gerontol Geriatr. 2009;48:401–405. [DOI] [PubMed] [Google Scholar]
- 25. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. [DOI] [PubMed] [Google Scholar]
- 26. Mourad JJ, Waeber B, Zannad F, et al. Comparison of different therapeutic strategies in hypertension: a low‐dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped‐care approach. J Hypertens. 2004;22:2379–2386. [DOI] [PubMed] [Google Scholar]
- 27. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 28. Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–1963. [DOI] [PubMed] [Google Scholar]


