Abstract
Subgroup analyses were performed for the diabetic and nondiabetic cohorts from 3 randomized clinical trials that had evaluated the systolic blood pressure (SBP)–lowering efficacy and tolerability of an angiotensin receptor blocker, valsartan, alone or in combination with hydrochlorothiazide to determine when and how to initiate combination therapy in hypertensive patients with diabetes. Blood pressure reductions achieved with monotherapy were compared with combination therapy in the diabetic and nondiabetic cohorts. In addition, multivariate models were developed to predict the likelihood of the goal SBP of <130 mm Hg being reached in a diabetic patient with monotherapy or combination therapy across the range of baseline SBP values. In 2 of the 3 trials, comparable reductions in SBP were seen in the diabetic and nondiabetic cohorts. In all 3 studies, however, combination therapy provided greater blood pressure–lowering efficacy than monotherapy. The probability of achieving goal SBP was greater for diabetic patients started on combination therapy compared with monotherapy.
As many as 60% of patients with diabetes also have hypertension. 1 Blood pressure (BP) control rates are poor among diabetic persons, and BP targets are not reached in approximately two‐thirds of these patients. 2 Recent data suggest that the percentage of all hypertensive patients with controlled BP is increasing and may be as high as 50% to 60%. 3 Less than ideal control in diabetics often results from an inability to decrease systolic BP (SBP) to the more recently established goals of <130 mm Hg. Given the severe cardiovascular and renal complications associated with both diabetes and hypertension, the lack of BP control is a major concern. 4 Cardiovascular disease accounts for 86% of all premature deaths among patients with diabetes. 1 , 5 The presence of hypertension in individuals with diabetes greatly increases their risk of retinopathy and nephropathy. 1
Clinical trials have demonstrated that control of hypertension in patients with diabetes lowers the risk of cardiovascular disease and death and prevents or slows the progression of diabetic complications. 6 , 7 The United Kingdom Prospective Diabetes Study (UKPDS) found that among patients with type 2 diabetes, treatment of BP to <150/85 mm Hg (achieved BP of 144/82 mm Hg) compared with <180/105 mm Hg (achieved BP of 154/87mm Hg) was associated with risk reductions of 32% in deaths related to diabetes, 37% in microvascular outcomes, and 44% in stroke. 6 The degree of BP control appeared to be more important in determining outcome than the medications used. Adequate BP control in patients with diabetes may reduce the incidence of cardiovascular disease by one‐third to one‐half. 7 , 8 It has been estimated that for every 10‐mm Hg decrease in SBP, the risk of any complication of diabetes is reduced by 12%. 7 Evidence from studies of chronic renal disease suggests that control of mean arterial BP significantly slows the decline in glomerular filtration rate, particularly in patients with proteinuria. 9 , 10 , 11 For these reasons, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommended a BP target of <130/80 mm Hg for diabetic patients; this is consistent with 2007 treatment guidelines from the American Diabetes Association (ADA). 4 , 8
Some investigators believe that early and aggressive treatment of BP in diabetics and other high‐risk hypertensive patients to bring it to goal within 6 months of initiating therapy may be a determinant of success in slowing the progression of diabetes and atherosclerosis and preventing death from cardiovascular disease. 12 The blood pressure arm of a large, multicenter trial, the Action to Control Cardiovascular Risk in Diabetes (ACCORD), is under way to determine whether more aggressive SBP control (<120 mm Hg) will produce greater reductions in cardiovascular disease events in patients with diabetes than less intensive control (<140 mm Hg). 13 In this regard, the Stop Atherosclerosis in Native Diabetics Study (SANDS) trial 14 has shown that aggressively reducing low‐density lipoprotein cholesterol and SBP to lower targets resulted in regression of carotid intima‐media thickness and greater decrease in left ventricular mass in individuals with type 2 diabetes. However, clinical events showed lower than expected results that did not differ significantly between groups. The authors have concluded that further follow‐up is needed to determine whether these improvements will result in lower long‐term cardiovascular disease event rates and costs and favorable risk‐benefit outcomes.
Multiple‐drug therapy is usually required to achieve BP goals in hypertensive patients with diabetes. 4 , 8 The JNC 7 recommends consideration of 2‐drug therapy when BP is ≥20/10 mm Hg above goal levels (ie, if levels are ≥150/90 mm Hg in a diabetic patient). The ADA recommends including an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker (ARB) in any antihypertensive regimen for patients with both diabetes and elevated BP because these agents, usually when given with a diuretic, have been shown to reduce the risk of cardiovascular events in patients with type 2 diabetes. 4 ARB‐based therapy has also been shown to delay the progression of nephropathy in hypertensive patients with type 2 diabetes, microalbuminuria, and renal insufficiency. 4
The ideal treatment strategy in hypertensive patients with diabetes remains to be defined. An exploratory analysis was undertaken comparing results in the diabetic and nondiabetic cohorts from 3 randomized multicenter trials that evaluated different BP‐lowering treatment strategies with ARB monotherapy and/or ARB plus hydrochlorothiazide (HCTZ) combination therapy. The objective of these analyses was to provide some clarification about the value of a more aggressive approach in managing diabetic hypertensive patients.
Methods
Patient data were used from 3 trials: Valsartan‐Managing Blood Pressure Aggressively and Evaluating Reductions in hsCRP (Val‐MARC) trial, 15 Valsartan/HCTZ Combination Therapy in Patients With Moderate to Severe Systolic Hypertension (VALOR), 16 and the Valsartan Effectiveness in Lowering Blood Pressure Comparative Study (VELOCITY). 17 The primary objective of Val‐MARC was to compare the effects of monotherapy and combination therapy on change in plasma levels of the inflammatory biomarker high‐sensitivity C‐reactive protein (hsCRP) and the correlation between hsCRP and BP reduction in 1668 patients with stage 2 hypertension. 15 VALOR was designed to assess the efficacy and tolerability of treatment with valsartan alone compared with valsartan/HCTZ on SBP in 774 patients with stage 2 or 3 systolic hypertension (SBP >160 mm Hg) with or without other cardiovascular risk factors. 16 VELOCITY was a randomized, double‐blind, parallel‐group study designed primarily to evaluate whether initial treatment with high‐dose valsartan monotherapy or valsartan/HCTZ combination therapy would achieve higher BP control rates in a shorter period of time compared with conventional lower‐dose therapy in 648 patients with hypertension. 17
The study designs for the 12‐week Val‐MARC trial, 8‐week VALOR trial, and 6‐week VELOCITY trial are shown in Figure 1. All were multicenter randomized studies that included adult patients with stage 2 hypertension. Val‐MARC used forced titration at week 2 but allowed investigators to titrate doses at their discretion at week 6, VALOR force‐titrated all patients, and VELOCITY force‐titrated patients not at goal. Key exclusion criteria for all 3 trials included secondary or malignant hypertension, significant renal or hepatic disease, type 1 diabetes, or uncontrolled type 2 diabetes. In both Val‐MARC and VALOR, type 2 diabetes was identified through the patient’s medical history; VELOCITY used the criterion of fasting plasma glucose ≥126 mg/dL. In each of the trials, BP control was originally defined as BP <140/90 mm Hg, regardless of diabetes status. Patients were excluded if they had chronic renal disease.
Figure 1.
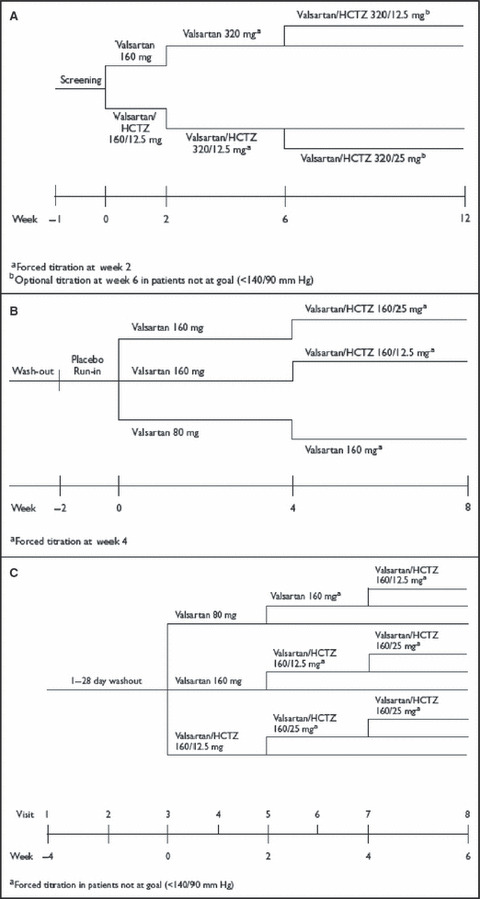
Study designs for the Val‐MARC (A), VALOR (B), and VELOCITY (C) trials. Inclusion criteria for Val‐MARC were age 18 to 75 years and systolic blood pressure 160 to 185 mm Hg or diastolic blood pressure 100 to 109 mm Hg. Inclusion criteria for VALOR were age 18 years or older and systolic blood pressure 160 to 200 mm Hg. Inclusion criteria for VELOCITY were age 18 years or older, systolic blood pressure 150 to 179 mm Hg, and diastolic blood pressure 90 to 109 mm Hg. See text for study name expansions. HCTZ indicates hydrochlorothiazide.
Statistics and Analytical Methods
We constructed the analyses to reflect the various treatment strategies that are currently being used in clinical practice: patients who started with monotherapy and remained on monotherapy, those who were up‐titrated from monotherapy to 2‐drug therapy, and those who started and remained on combination treatment. For the purposes of these specific analyses, the 2 arms in the VALOR trial that involved up‐titration from monotherapy to combination therapy (valsartan/HCTZ 160/0 mg→160/12.5 mg and→160/25 mg) were pooled into a single arm as they represented one treatment strategy. The time point for evaluating response in these analyses was defined for each trial as the last available visit that enabled comparison of the treatment strategies of interest: week 4 for VELOCITY, week 6 for Val‐MARC, and week 8 for VALOR.
To evaluate the comparability of the groups included in these analyses, a statistical test for homogeneity of baseline factors between treatment regimens was performed using Fisher’s exact test for categorical variables and analysis of variance for continuous baseline variables.
SBP change from baseline was estimated for each combination of trial, treatment strategy, and diabetic status, with multivariate adjustment for baseline SBP, diastolic BP, age, and body mass index (BMI). The model included a term for interaction between treatment strategy and diabetes to allow for nonadditive effects.
To estimate the probability of the SBP goal of <130 mm Hg being met in diabetic patients, a repeated‐measures logistic regression model was fit for the diabetic cohort with baseline SBP, age, BMI, treatment, visit, and treatment‐by‐visit interaction as explanatory variables.
Adverse event data from the diabetic cohorts were pooled from all 3 studies by treatment regimen. The monotherapy safety population consisted of all diabetic patients who received valsartan monotherapy throughout the length of the study, a combination group consisted of all diabetic patients who received the valsartan/HCTZ combination as initial therapy, and a monotherapy‐to‐combination therapy group consisted of all the diabetic patients who received monotherapy initially but were up‐titrated to combination therapy during the study.
Results
Patient Characteristics
Patient demographics and characteristics at baseline for the diabetic and nondiabetic cohorts in Val‐MARC, VALOR, and VELOCITY are listed in Table I. Approximately 10% to 12% of the total study populations had diabetes. Compared with nondiabetic patients with hypertension, patients in the diabetic subgroups tended to be older, with higher BMIs and higher SBP values. Formal comparisons were not made among the 3 studies; however, the diabetic subgroups were generally comparable in terms of mean age, BMI, and SBP. Comparisons between treatment arms in demographic variables within each study showed no significant differences for VALOR and VELOCITY. However, in Val‐MARC, the baseline SBP for diabetic patients was lower and the diastolic BP higher in the valsartan monotherapy arm compared with the valsartan/HCTZ combination arm; these differences were statistically significant (P=.03 for both). The percentage of diabetic patients in the monotherapy arm of Val‐MARC was 11.8%, whereas diabetic patients represented 8.2% of patients in the combination therapy arm.
Table I.
Characteristics of Patients With and Without Diabetes by Study and Treatment Group
| Demographic Variable | Val‐MARC | VALOR | VELOCITY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAL 160→320 mg | VAL/HCTZ 160/12.5→320/12.5 mg | VAL 80→160 mg | VAL/HCTZ 160/0→ (160/12.5, 160/25 mg) | VAL 80 mg→VAL/HCTZ 160/12.5 mg | VAL 160 mg→VAL/HCTZ 160/25 mg | VAL/HCTZ 160/12.5→160/25 mg | ||||||||
| Diabetes Status | Yes (n=95) | No (n=712) | Yes (n=66) | No (n=740) | Yes (n=26) | No (n=235) | Yes (n=51) | No (n=455) | Yes (n=23) | No (n=195) | Yes (n=30) | No (n=189) | Yes (n=30) | No (n=180) |
| Sex | ||||||||||||||
| Male, No. (%) | 58 (61.1) | 390 (54.8) | 35 (53.0) | 399 (53.9) | 15 (57.7) | 115 (48.9) | 29 (56.9) | 252 (55.4) | 13 (56.5) | 106 (54.4) | 19 (63.3) | 102 (54.0) | 17 (56.7) | 93 (51.7) |
| Race | ||||||||||||||
| White, No. (%) | 57 (60.0) | 497 (69.8) | 41 (62.1) | 505 (68.2) | 24 (92.3) | 219 (93.2) | 45 (88.2) | 428 (94.1) | 17 (73.9) | 135 (69.2) | 17 (56.7) | 132 (69.8) | 15 (50.0) | 120 (66.7) |
| Black, No. (%) | 23 (24.2) | 154 (21.6) | 14 (21.2) | 181 (24.5) | 0 (0.0) | 5 (2.1) | 2 (3.9) | 6 (1.3) | 3 (13.0) | 34 (17.4) | 9 (30.0) | 28 (14.8) | 7 (23.3) | 32 (17.8) |
| Age | ||||||||||||||
| Mean age, y (SD) | 53.3 (10.9) | 50.4 (11.6) | 55.2 (11.3) | 50.7 (11.8) | 63.7 (9.5) | 60.0 (10.7) | 63.6 (9.1) | 60.4 (11.7) | 53.6 (8.4) | 52.0 (11.0) | 53.0 (8.8) | 53.1 (9.0) | 54.1 (7.4) | 52.6 (10.7) |
| ≥65 y, No. (%) | 17 (17.9) | 85 (11.9) | 15 (22.7) | 99 (13.4) | 11 (42.3) | 82 (34.9) | 23 (45.1) | 172 (37.8) | 1 (4.3) | 20 (10.3) | 3 (10.0) | 21 (11.1) | 4 (13.3) | 26 (14.4) |
| Baseline BMI | ||||||||||||||
| No. | 93 | 703 | 66 | 731 | 26 | 229 | 50 | 446 | 23 | 195 | 30 | 189 | 30 | 180 |
| Mean, kg/m2 | 35.1 | 32.3 | 36.5 | 32.4 | 32.0 | 30.1 | 32.1 | 29.9 | 34.6 | 31.7 | 35.4 | 32.3 | 33.6 | 32.3 |
| Baseline SBP | ||||||||||||||
| No. | 95 | 712 | 66 | 740 | 26 | 235 | 51 | 445 | 23 | 195 | 30 | 189 | 30 | 180 |
| Mean, mm Hg (SD) | 167.6 (11.7) | 164.1 (12.5) | 171.4 (10.6)a | 164.5 (13.3) | 167.7 (7.5) | 167.9 (8.1) | 167.1 (6.8) | 167.3 (8.2) | 160.2 (5.6) | 160.8 (7.6) | 162.9 (8.7) | 160.7 (7.8) | 163.6 (8.3) | 160.6 (7.7) |
| Baseline DBP | ||||||||||||||
| No. | 95 | 712 | 66 | 740 | 26 | 235 | 51 | 445 | 23 | 195 | 30 | 189 | 30 | 180 |
| Mean, mm Hg (SD) | 98.0 (10.6) | 100.0 (8.7) | 94.4 (10.1)a | 100.1 (8.4) | 89.2 (10.3) | 93.7 (8.6) | 90.8 (8.6) | 93.8 (9.3) | 95.9 (4.8) | 97.9 (5.5) | 97.3 (3.6) | 98.4 (5.3) | 97.3 (5.7) | 98.3 (5.0) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; SBP, systolic blood pressure; VAL, valsartan. a P=.03 for VAL 160→320 mg vs VAL/HCTZ 160/12.5→320/12.5 mg. See text for study name expansions.
Efficacy Analyses
Table II shows mean changes from baseline in SBP for the diabetic compared with nondiabetic group by study and treatment strategy. Generally, BP reductions in diabetic patients were comparable to those of nondiabetic patients, with the exception of the VALOR trial, in which smaller reductions with monotherapy and larger reductions with combination therapy were observed in diabetic compared with nondiabetic patients. Across all 3 studies, combination treatment was, as expected, consistently more effective compared with monotherapy in both diabetic and nondiabetic patients.
Table II.
Estimated Difference in Adjusted Mean Change From Baseline in MSSBP by Treatment Strategy in Diabetic and Nondiabetic Subgroups of Val‐MARC, VALOR, and VELOCITY
| Study | Strategy | LSM Change From Baseline in MSSBP (mm Hg), Diabetic Patients | LSM Change From Baseline in MSSBP (mm Hg), Nondiabetic Patients | Mean Difference, Diabetics vs Nondiabetics | 95% CI for Mean Difference |
|---|---|---|---|---|---|
| VALOR (week 8) | Combination therapy | −30.6 | −27.7 | −2.8 | −7.0 to 1.3 |
| Monotherapy | −16.4 | −20.9 | 4.6 | −1.2 to 10.3 | |
| Val‐MARC (week 6) | Combination therapy | −24.4 | −24.8 | 0.3 | −3.9 to 4.6 |
| Monotherapy | −16.6 | −17.9 | 1.4 | −2.3 to 5.0 | |
| VELOCITY (week 4) | Combination therapy (high)a | −24.2 | −26.2 | 2.0 | −3.1 to 7.1 |
| Combination therapy (low)b | −21.5 | −21.6 | 0.1 | −5.0 to 5.1 | |
| Monotherapy | −14.6 | −15.2 | 0.6 | −5.1 to 6.2 |
Abbreviations: CI, confidence interval; LSM, least‐squares mean; MSSBP, mean sitting systolic blood pressure. a160/12.5→160/25 mg; b160/0→160/12.5 mg. See text for study name expansions.
In the diabetic cohort of the Val‐MARC study, the adjusted mean difference in SBP at week 6 was −7.9 mm Hg (P=.0034; 95% confidence interval [CI], −13.1 to −2.6) in favor of combination therapy. In VALOR, the adjusted mean difference at week 8 was −14.2 mm Hg (P<.0001; 95% CI, −20.8 to −7.6) in favor of combination therapy. In VELOCITY, in which only those patients in whom the goal was not met were up‐titrated, the adjusted mean difference in SBP was −6.9 mm Hg (P=.0576; 95% CI, −14.0 to 0.2) between the monotherapy arm and the combination therapy arm that started with valsartan 160 mg monotherapy. In comparing the monotherapy arm with the initial combination therapy arm, greater reductions also occurred with combination therapy; the adjusted mean difference in SBP was −9.5 mm Hg (P=.0089; 95% CI, −16.6 to −2.4), which represented an incremental reduction of nearly 3 mm Hg for initial use of combination therapy compared with combination treatment as a second‐line strategy.
Figure 2 shows the estimated probability of meeting an SBP goal of <130 mm Hg as a function of baseline SBP. Across the entire range of baseline SBP values, patients in the diabetic subgroups were more likely to reach SBP goal with combination therapy than with monotherapy in all 3 studies. In general, approximately 3 times as many patients reached an SBP level <130 mm Hg with combination therapy compared with monotherapy, with some variation across baseline SBP and studies.
Figure 2.
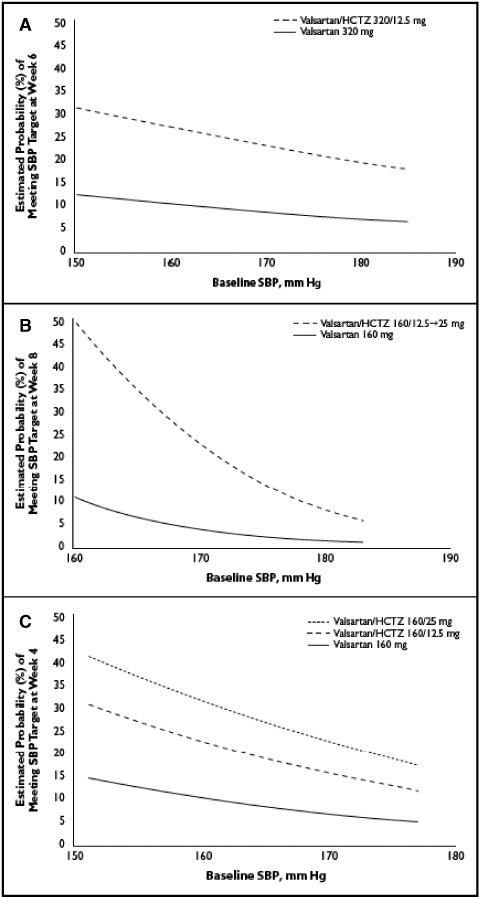
Estimated probability of achieving systolic blood pressure (SBP) goal (<130 mm Hg) at study end as a function of baseline SBP in the diabetic cohorts in the Val‐MARC (A), VALOR (B), and VELOCITY (C) trials. Adjusted for mean age and body mass index (BMI) in each trial (Val‐MARC: mean age, 54.1 years; mean BMI, 35.7 kg/m2; VALOR: mean age, 63.6 years; mean BMI, 32.1 kg/m2; VELOCITY: mean age, 53.6 years; mean BMI, 34.5 kg/m2). See text for study name expansions. HCTZ indicates hydrochlorothiazide.
Tolerability
The most common adverse events for the population pooled from the 3 studies are listed in Table III. The frequency of dizziness was higher in the patients who received initial combination therapy than in those who were started on monotherapy. Groups differed in the frequency of headache by only 1 to 2 percentage points, and rates of fatigue were comparable. There were no incidences of hyperkalemia, hypokalemia, or changes in creatinine concentration.
Table III.
Ten Most Frequent Adverse Events for Pooled Population of Diabetic Patients in the Val‐MARC, VALOR, and VELOCITY Studies (Groups Determined by Study Treatment Types Start to End of Study)
| Monotherapy (n=82) no. (%) | Combination Therapy (n=97) no. (%) | Monotherapy→ Combination Therapy (n=147) no. (%) | |
|---|---|---|---|
| Upper respiratory tract infectiona | 9 (11.0) | 9 (9.3) | 12 (8.2) |
| Headache | 6 (7.3) | 6 (6.2) | 12 (8.2) |
| Dizziness | 2 (2.4) | 8 (8.2) | 9 (6.1) |
| Fatigueb | 4 (4.9) | 4 (4.1) | 7 (4.8) |
| Gastroenteritisc | 2 (2.4) | 7 (7.2) | 5 (3.4) |
| Nausea | 3 (3.7) | 5 (5.2) | 3 (2.0) |
| Arthralgia | 4 (4.9) | 0 (0.0) | 2 (1.4) |
| Back pain | 1 (1.2) | 2 (2.1) | 3 (2.0) |
| Cough | 2 (2.4) | 0 (0.0) | 3 (2.0) |
| Blood glucose increase | 0 (0.0) | 2 (2.1) | 2 (1.4) |
aUpper respiratory tract infection: influenza, nasopharyngitis, pharyngitis, pharyngolaryngeal pain, sinusitis. bFatigue: asthenia, fatigue. cGastroenteritis: diarrhea gastroenteritis, Helicobacter infections, gastroenteritis, gastroenteritis not otherwise specified, gastroenteritis viral. See text for study name expansions.
Discussion
The current analyses of the antihypertensive efficacy of valsartan monotherapy compared with the combination of valsartan/HCTZ demonstrated that combination therapy more effectively lowers SBP in hypertensive patients with and without diabetes. In the 3 trials examined, combination therapy resulted in 8‐ to 14‐mm Hg greater reductions in SBP compared with monotherapy; this difference translated into approximately 3 times as many patients reaching the JNC 7 and ADA‐recommended SBP goal of <130 mm Hg. Moreover, while switching patients to combination therapy as a second‐line strategy helped to produce greater SBP reductions, the initial use of combination treatment offered an incremental SBP reduction of nearly 3 mm Hg. Thus, the present results support the concept that initial use of combination therapy in patients with hypertension and diabetes may offer better BP control with only a slight increase in adverse effects.
Clinical trials have demonstrated the benefits of ARBs as well as angiotensin‐converting enzyme inhibitors, usually given with a diuretic in patients with diabetes and hypertension. 8 , 18 Hypertension in persons with type 2 diabetes may have a somewhat different pathogenesis than that in persons without diabetes. Increased insulin resistance, endothelial dysfunction, enhanced vascular oxidative stress, and inflammation may be contributors to vascular disease in diabetics. 5 , 19 These appear to promote deleterious effects of the renin‐angiotensin‐aldosterone system (RAAS). 5 , 19 , 20 , 21 The use of ARBs as well as other agents that block the RAAS results in fewer cases of new‐onset diabetes than other medications, most likely by inhibiting the adverse effects of angiotensin II on glucose metabolism and increasing insulin sensitivity. 5 , 18 , 21 Guidelines therefore recommend that multiple‐drug regimens in hypertensive patients with diabetes should include an agent that blocks the RAAS, such as an angiotensin‐converting enzyme inhibitor or an ARB. 4 , 8 , 22 , 23 , 24 The combination of an ARB with a thiazide diuretic has been effective in BP reduction, with a discontinuation rate similar to that of ARB monotherapy. 5 The benefits of thiazide diuretics in the treatment of hypertension are well established, and when used in combination with an ARB, an angiotensin‐converting enzyme inhibitor, or a β‐blocker, their complementary mechanisms of action appear to enhance efficacy. 5
The results of the present analyses are consistent with findings from previous research and current recommendations for treating patients with concurrent hypertension and diabetes. 4 , 8 , 22 Hypertensive patients with diabetes typically need >2 drugs to achieve BP goals; for example, approximately one‐third of patients in the UKPDS required treatment with ≥3 drugs to achieve a BP of <150/85 mm Hg. 6 , 25 In another trial, patients with diabetes required the addition of a second drug 40% more often, and a third drug 100% more often, than patients without diabetes to achieve a BP of <140/90 mm Hg and/or a 20/10 mm Hg reduction. 26 The low BP control rates with monotherapy observed in this analysis are not unexpected, given that these patients had diabetes with SBP >20 mm Hg above goal, were older, and were obese (BMI ≥30 kg/m2).
When considering the concept that hypertension is more difficult to treat in patients with diabetes, it is interesting to note that in 2 of 3 studies analyzed, BP reductions with monotherapy and with combination therapy were comparable in diabetic and nondiabetic patients. In VALOR, however, diabetic patients treated with valsartan monotherapy had only 78% of the BP‐lowering response seen in nondiabetic patients. In the same study, combination therapy resulted in greater BP reductions in diabetic than in nondiabetic patients.
These results in VALOR are supported by the findings of a subgroup analysis of the diabetic cohort in the 18‐week Irbesartan/Hydrochlorothiazide Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) trial, 27 which evaluated the efficacy of fixed‐dose combinations of irbesartan/HCTZ in hypertensive patients. Patients with type 2 diabetes achieved a mean change in SBP of −18 mm Hg compared with −23 mm Hg for nondiabetic patients (79% of the BP‐lowering response seen in nondiabetic patients). In addition, SBP goal (<130 mm Hg) was attained in 56% (95% CI, 49%–62%) of diabetic patients at study end, while an SBP of <140 mm Hg was reached in 87% (95% CI, 84%–90%) of patients without diabetes. 27
Reaching BP goals may be more difficult in diabetic patients, due at least in part to the lower JNC 7 BP target goal in these patients (SBP <130 mm Hg) compared with nondiabetic patients (SBP <140 mm Hg). Despite the known risks of elevated BP, recommended BP goals are met in only 33% of all patients with diabetes who are receiving antihypertensive treatment. 2 Undertreatment to goal levels in this patient population is common 28 and may be due to both physician and patient inertia and adherence, respectively. 29 For example, a prospective cohort study found that among patients with type 2 diabetes undergoing treatment for elevated SBP, only 36% of these patients had their treatments adjusted when they failed to meet BP goals within a year. 29 Fixed‐dose combinations may help overcome treatment failures because they simplify the regimen and produce greater BP reductions more rapidly than monotherapy. 30
Analysis of pooled adverse event data from the 3 studies demonstrated that combination therapy with valsartan/HCTZ was generally well tolerated in diabetic patients. Therefore, rather than initiating treatment with monotherapy and then up‐titrating, it may be useful to prescribe combination regimens initially in this high‐risk patient population.
Results of this analysis should be interpreted considering the limitations inherent in all post hoc subgroup analyses. These were secondary analyses of randomized trial data initially collected for other purposes. Diabetes was defined differently among the trials. The 3 separate studies were not designed or powered to assess the comparative efficacy of valsartan and valsartan/HCTZ in the treatment of hypertension in the cohort of patients with type 2 diabetes. Furthermore, dose titration in the individual study designs were based on a desired SBP goal of <140 mm Hg; therefore, higher rates of goal achievement might have been expected if the studies had been designed for patients with diabetes who would receive more aggressive dose titration to reach an SBP goal of <130 mm Hg. Finally, none of the studies included patients with SBP values <20 mm Hg above goal or patients with existing renal impairment; therefore, the results of the present analysis may not extend to diabetic patients with less severe hypertension and chronic renal disease.
Conclusions
Combination therapy with valsartan/HCTZ is effective and well tolerated in both diabetic and nondiabetic patients with hypertension. Aggressive treatment with initial use of this combination leads to SBP goals being achieved in more diabetic patients than with monotherapy, regardless of baseline SBP. Thus, initiating therapy with 2‐drug therapy and titrating to higher doses seems to be a useful strategy to achieve better BP control in hypertensive patients with diabetes.
Acknowledgments
Acknowledgments: Dr Sowers is a consultant to Novartis Pharmaceuticals, Merck, and Forest Laboratories and has an investigator‐initiated grant from Novartis Pharmaceuticals. Dr Rocha, Dr Seifu, Ms Crikelair, and Dr Levy are employees of Novartis Pharmaceuticals Corporation. Although the data were collected and analyzed by coauthors from Novartis, Dr Sowers wrote the manuscript with help from Dr Lastra, a fellow in the division of endocrinology at the University of Missouri‐Columbia.
References
- 1. Arauz‐Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes. Diabetes Care. 2002;25:134–147. [DOI] [PubMed] [Google Scholar]
- 2. Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- 3. Moser M, Franklin SS. Hypertension management: results of a new national survey for the Hypertension Education Foundation: Harris Interactive. J Clin Hypertens (Greenwich). 2007;9(5):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Standards of medical care in diabetes: 2007. Diabetes Care. 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 5. Sowers JR. Treatment of hypertension in patients with diabetes. Arch Intern Med. 2004;164:1850–1857. [DOI] [PubMed] [Google Scholar]
- 6. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 7. Bakris GL. The importance of blood pressure control in the patient with diabetes. Am J Med. 2004;116(suppl 5A):30S–38S. [DOI] [PubMed] [Google Scholar]
- 8. Chobanian AV, Bakris GL, Black HR, et al. National High Blood Pressure Education Program Coordinating Committee . Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 9. Ritz E, Dikow R. Hypertension and antihypertensive treatment of diabetic nephropathy. Nat Clin Pract Nephrol. 2006;2:562–567. [DOI] [PubMed] [Google Scholar]
- 10. Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease study. Ann Intern Med. 1995;123:754–762. [DOI] [PubMed] [Google Scholar]
- 11. Klahr S, Levey AS, Beck GJ, et al. Modification of Diet in Renal Disease Study Group . The effects of dietary protein restriction and blood‐pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. [DOI] [PubMed] [Google Scholar]
- 12. Mugo MN, Sowers JR. Early and aggressive treatment of complex hypertension. J Clin Hypertens (Greenwich). 2005;7:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cushman WC, Grimm RH Jr, Cutler JA, et al. ACCORD Study Group . Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(suppl):44i–55i. [DOI] [PubMed] [Google Scholar]
- 14. Howard BV, Roman MJ, Devereux RB, et al. Effect of Lower Targets for Blood Pressure and LDL Cholesterol on Atherosclerosis in Diabetes: the SANDS Randomized Trial. JAMA. 2008;299(14):1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ridker PM, Danielson E, Rifai N, et al. Val‐MARC Investigators . Valsartan, blood pressure reduction, and C‐reactive protein: primary report of the Val‐MARC trial. Hypertension. 2006;48:73–79. [DOI] [PubMed] [Google Scholar]
- 16. Lacourcìre Y, Poirier L, Hebert D, et al. Antihypertensive efficacy and tolerability of two fixed‐dose combinations of valsartan and hydrochlorothiazide compared with valsartan monotherapy in patients with stage 2 or 3 systolic hypertension: an 8‐week, randomized, double‐blind, parallel‐group trial. Clin Ther. 2005;27:1013–1021. [DOI] [PubMed] [Google Scholar]
- 17. Jamerson KA, Zappe DH, Collins L, et al. The time to blood pressure (BP) control by initiating antihypertensive therapy with a higher dose of valsartan (160 mg) or valsartan/hydrochlorothiazide compared to low‐dose valsartan (80 mg) in the treatment of hypertension: the VELOCITY study [ASH abstract P‐400]. J Clin Hypertens (Greenwich). 2007;9(suppl A):A166–A167. [Google Scholar]
- 18. Chrysant SG, Chrysant GS. The pleiotropic effects of angiotensin receptor blockers. J Clin Hypertens (Greenwich). 2006;8:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cubbon RM, Rajwani A, Wheatcroft SB. The impact of insulin resistance on endothelial function, progenitor cells and repair. Diab Vasc Dis Res. 2007;4:103–111. [DOI] [PubMed] [Google Scholar]
- 20. Rocchini AP, Moorehead C, DeRemer S, et al. Hyperinsulinemia and the aldosterone and pressor responses to angiotensin II. Hypertension. 1990;15:861–866. [DOI] [PubMed] [Google Scholar]
- 21. Cooper SA, Whaley‐Connell A, Habibi J, et al. Renin‐angiotensin‐aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–H2023. [DOI] [PubMed] [Google Scholar]
- 22. Bakris GL. A practical approach to achieving recommended blood pressure goals in diabetic patients. Arch Intern Med. 2001;161:2661–2667. [DOI] [PubMed] [Google Scholar]
- 23. Giunti S, Cooper M. Management strategies for patients with hypertension and diabetes: why combination therapy is critical. J Clin Hypertens (Greenwich). 2006;8:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hollenberg NK, Parving H‐H, Viberti G, et al. Albuminuria response to very high‐dose valsartan in type 2 diabetes mellitus. J Hypertens. 2007;25:1921–1926. [DOI] [PubMed] [Google Scholar]
- 25. Flack JM, Hamaty M. Difficult‐to‐treat hypertensive populations: focus on African‐Americans and people with type 2 diabetes. J Hypertens Suppl. 1999;17(1):S19–S24. [PubMed] [Google Scholar]
- 26. Brown MJ, Castaigne A, De Leeuw PW, et al. Influence of diabetes and type of hypertension on response to antihypertensive treatment. Hypertension. 2000;35:1038–1042. [DOI] [PubMed] [Google Scholar]
- 27. Sowers JR, Neutel JM, Saunders E, et al. INCLUSIVE Investigators . Antihypertensive efficacy of irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes. J Clin Hypertens (Greenwich). 2006;8:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malik S, Lopez V, Chen R, et al. Undertreatment of cardiovascular risk factors among persons with diabetes in the United States. Diabetes Res Clin Pract. 2007;77:126–133. [DOI] [PubMed] [Google Scholar]
- 29. Grant RW, Cagliero E, Dubey AK, et al. Clinical inertia in the management of type 2 diabetes metabolic risk factors. Diabet Med. 2004;21:150–155. [DOI] [PubMed] [Google Scholar]
- 30. Basile J, Black HR, Flack JM, et al. The role of therapeutic inertia and the use of fixed‐dose combination therapy in the management of hypertension. J Clin Hypertens (Greenwich). 2007;9:636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]


