Abstract
Use of β‐blockers in hypertensive obese patients remains controversial because of concerns about potential influences on weight, lipids, and glucose metabolism. The authors examined a pooled analysis of 3 multicenter randomized placebo‐controlled trials. Patients were randomized to placebo or an increasing dose of nebivolol for 12 weeks. Primary outcome was the mean baseline to end point change in trough mean sitting diastolic blood pressure (SiDBP). Secondary outcomes were baseline to end point changes in trough sitting systolic blood pressure (SiSBP); trough standing and peak supine diastolic blood pressure and systolic blood pressure. Nebivolol reduced SiDBP significantly compared with placebo at all doses ≥2.5 mg in obese and non‐obese patients. Reductions in SiSBP with nebivolol were higher than controls at all studied doses ≥5 mg in non‐obese and ≥2.5 mg in obese patients. These findings and nebivolol’s neutral effects on lipid and carbohydrate metabolism suggest that it is one option for blood pressure control in the moderately obese population.
The prevalence of overweight and obesity is increasing in the United States. Recent data show that 65% of the adult population is overweight and 30% is obese. 1 , 2 The presence of obesity is strongly correlated with well‐established risk factors for cardiovascular disease morbidity and mortality and is itself a risk factor. 3 , 4 One such modifiable risk factor, hypertension, is frequently observed in obese individuals and has been documented in up to 60% of overweight or obese patients. 4 , 5 This association is now supported by sufficient animal and human studies that confirm a strong relationship between obesity and hypertension. 4 Observations have demonstrated that the age‐adjusted prevalence of hypertension rises progressively with increasing body mass index (BMI) in both men and women. 5 Obese individuals have been observed to have a 3.5‐fold higher risk for developing hypertension compared with their non‐obese counterparts. 3 , 4 , 5 Several possible mechanisms may contribute to the development of hypertension in obese individuals. Increased activation of the renin‐angiotensin‐aldosterone system (RAAS) and the sympathetic nervous system (SNS) have been linked to impaired pressure‐induced natriuresis. 5 , 6 , 7 , 8 In addition, obesity is characterized by endothelial dysfunction with decreased nitric oxide (NO) production and impaired vascular responsiveness. 9 , 10
Because of the important role of the SNS and the RAAS in the pathogenesis of hypertension in overweight and obese persons, a therapeutic strategy that suppresses both systems would appear to have utility in the treatment of overweight/obese patients with hypertension. In this context, β‐blockers are known to suppress both the SNS and RAAS. 11 These agents have demonstrated efficacy in lowering blood pressure (BP) in obese individuals and use of these agents predicts better control in the obese population. 5 However, the use of β‐blockers in the treatment of hypertension in overweight and obese patients still remains controversial, based on the propensity of some β‐blocking agents to cause weight gain and adverse effects on lipid and glucose metabolism. 11
Various β‐blockers have been developed with neutral metabolic and weight effects. Nebivolol is highly selective for cardiac β1‐adrenoceptors and lacks intrinsic sympathomimetic activity. The ratio of β1‐ to β2‐competitive antagonism has been demonstrated to be 321:1. 12 , 13 Additionally, nebivolol has demonstrated endothelium‐dependent vasodilation through the l‐arginine/NO pathway in different regional vascular beds. 10 , 14 , 15 , 16 This agent displays a hemodynamic profile, similar to another vasodilating β‐blocker, carvedilol, reducing systemic vascular resistance without significant impact on cardiac output, stroke volume, and left ventricular function. 17
Clinical studies have shown that nebivolol provides significant BP reductions across a broad range of patients and does not adversely affect glucose or lipid metabolism or weight. The drug appears to be well tolerated. 11 , 16 The data presented are from a pooled analysis of the intention‐to‐treat populations of 3 similarly designed randomized placebo‐controlled nebivolol monotherapy trials in patients with mild to moderate hypertension, in which the antihypertensive efficacy of nebivolol in obese and non‐obese patients was evaluated.
Methods
Study Populations
Eligible patients included men and nonpregnant women aged 18 years and older with stage I or stage II hypertension, defined as an average sitting diastolic BP (SiDBP) ≥90 mm Hg and ≤109 mm Hg at baseline. Patients were excluded if they had secondary or malignant hypertension, a BMI ≥35 kg/m2 or obesity as measured by waist circumference >102 cm in men or >88 cm in women, chronic obstructive pulmonary disease, a myocardial infarction or stroke within 6 months of study entry, clinically significant renal or hepatic dysfunction, hemodynamically significant valvular disease, clinically relevant arrhythmias, uncontrolled type 2 diabetes mellitus (hemoglobin A1C≥10%), or a history of hypersensitivity to β‐blockers.
Study Design
All 3 trials (NEB‐202 [N=300], NEB‐302 [N=909], and NEB‐305 [N=807]) 17 , 18 , 19 included in this pooled analysis were randomized, placebo‐controlled, 12‐week, double‐blind, parallel‐group, multicenter studies conducted at 188 sites in the United States, in accordance with both Good Clinical Practice Guidelines and the Declaration of Helsinki revisions. Protocols and written informed consents were reviewed and approved by a central institutional review board/independent ethics committee prior to enrollment into the studies.
Following a 4‐ to 6‐week screening/washout/single‐blind, placebo run‐in phase, eligible patients were randomized to receive either placebo or once‐daily nebivolol 1.25 to 40 mg. No other antihypertensive medications were given to patients during the study. Patients were stratified at randomization by subgroups, including oxidative genotype (extensive metabolizer vs poor metabolizer of nebivolol), race, age (younger than 65 years vs 65 years and older), diabetes mellitus status, and sex. Study NEB‐202 did not include stratification by race because all patients in this study were black. Obesity (defined as a BMI ≥30 kg/m2) was not used for stratification but was a prespecified subgroup analysis.
End Points
The primary efficacy end point was the change in trough mean SiDBP between baseline and end of the study (week 12). Secondary efficacy end points included baseline to end point changes in trough sitting systolic BP (SiSBP); trough standing diastolic BP (DBP) and systolic (SBP); peak supine and standing DBP and SBP; and a responder analysis, where a response to treatment was defined as the proportion of patients with mean trough SiDBP <90 mm Hg or an absolute reduction of ≥10 mm Hg from baseline at the end of the study.
Efficacy and Safety Assessments
BP was measured using a calibrated mercury sphygmomanometer and appropriately sized cuff in the supine, sitting, and standing positions at medication trough. Three separate measurements were taken 2 minutes apart, and the mean value was calculated and recorded. Safety was assessed by monitoring reports of clinical adverse events (AEs) and by evaluating changes in vital signs, 12‐lead electrocardiographic findings, and laboratory parameters including biochemistry, hematology, and urinalysis. All AEs from initial screening to the end of the study were recorded, and their severity and possible relationship to the study drug were assessed by the investigators.
Statistical Methods
The primary population for efficacy and safety analyses, both for each study and for this pooled analysis, was the intent‐to‐treat population, which included all randomized patients who took at least 1 dose of the study medication. Missing values were imputed using a last‐observation‐carried‐forward method. The primary statistical method of treatment comparison was a step‐down dose‐response test using a linear contrast in the analysis of covariance model with treatment as main effect and baseline BP and dichotomous baseline variables as covariates. Response rates of the treatment groups were analyzed using a logistic regression model, with responder as the response variable and baseline dichotomous covariates and baseline DBP as a continuous covariate. Response rates were compared using the Wald χ2 test. For pooled data, a linear trend analysis was performed comparing the antihypertensive efficacy of nebivolol in obese and non‐obese patients across all treatment groups.
Results
Patient Disposition and Baseline Characteristics
A total of 1136 non‐obese patients and 878 obese patients were included in the pooled analysis (N=2016); 154 non‐obese (13.55%) and 123 obese (14%) patients prematurely discontinued the study. The distribution of patients across the dosing groups at baseline was similar in obese and non‐obese patients (Table I). Mean baseline SiDBP and SiSBP were also comparable among the treatment groups (Table II).
Table I.
Demographics and Baseline Characteristicsa
| BMI, kg/m 2 | Placebo | Nebivolol 1.25 mg | Nebivolol 2.5 mg | Nebivolol 5 mg | Nebivolol 10 mg | Nebivolol 20 mg | Nebivolol 30/40 mg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <30 (n=133) | ≥30 (n=92) | <30 (n=43) | ≥30 (n=40) | <30 (n=71) | ≥30 (n=60) | <30 (n=269) | ≥30 (n=189) | <30 (n=273) | ≥30 (n=188) | <30 (n=263) | ≥30 (n=196) | <30 (n=104) | ≥30 (n=113) | |
| Age, y | 54±11 | 51±9 | 57±11 | 54±12 | 53±12 | 51±11 | 55±12 | 52±10 | 55±12 | 52±10 | 54±11 | 53±11 | 55±13 | 52±11 |
| Male sex | 61 (54.0) | 47 (51.1) | 25 (58.1) | 21 (52.5) | 47 (66.2) | 32 (53.3) | 149 (55.4) | 99 (52.4) | 154 (56.4) | 92 (48.9) | 141 (53.6) | 103 (52.3) | 55 (52.9) | 59 (52.2) |
| Race | ||||||||||||||
| Caucasian | 80 (70.8) | 54 (58.7) | 35 (81.4) | 35 (87.5) | 39 (54.9) | 30 (50.0) | 206 (76.6) | 143 (75.7) | 210 (76.9) | 136 (72.3) | 203 (77.2) | 45 (73.6) | 69 (66.3) | 69 (61.1) |
| Non‐ Caucasian | 33 (29.2) | 38 (41.3) | 8 (18.6) | 5 (12.5) | 32 (45.1) | 30 (50.0) | 63 (23.4) | 46 (24.3) | 63 (23.1) | 52 (27.7) | 60 (22.8) | 52 (26.4) | 35 (33.7) | 44 (38.9) |
| Black | 33 (100.0) | 38 (100.0) | 7 (87.5) | 5 (100.0) | 32 (100.0) | 30 (100.0) | 59 (93.7) | 45 (97.8) | 55 (87.3) | 52 (100.0) | 55 (91.7) | 50 (96.2) | 33 (94.3) | 43 (97.7) |
| Asian | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 4 (6.3) | 1 (2.2) | 3 (4.8) | 0 | 3 (5.0) | 2 (3.8) | 0 | 1 (2.3) |
| Weight, kg | 75.6±12.4 | 98.1±11.3 | 76.7±12.4 | 95.2±12.6 | 78.3±12.5 | 97.8±11.2 | 77.2±12.4 | 96.0±12.1 | 76.8±12.2 | 96.2±13.2 | 77.0±12.4 | 95.5±11.9 | 77.0±12.7 | 97.5±12.1 |
Values are expressed as mean ± standard deviation or No. (%). aPatients with a body mass index (BMI) ≥35 kg/m2 were excluded.
Table II.
Baseline Patient and Clinical Characteristics
| Treatment Subgroup, mg | No. | SiDBP, mm Hg | SiSBP, mm Hg | |||
|---|---|---|---|---|---|---|
| BMI, kg/m 2 | BMI, kg/m 2 | BMI, kg/m 2 | ||||
| <30 | ≥30 | <30 | ≥30 | <30 | ≥30 | |
| Placebo | 113 | 92 | 99.5 | 100.2 | 152.2 | 152.4 |
| Nebivolol, 1.25 | 43 | 40 | 99.0 | 98.8 | 151.7 | 152.7 |
| Nebivolol, 2.5 | 71 | 60 | 99.5 | 99.9 | 149.0 | 150.2 |
| Nebivolol, 5 | 269 | 189 | 99.3 | 99.6 | 152.0 | 152.1 |
| Nebivolol, 1 | 273 | 188 | 99.1 | 99.6 | 153.1 | 152.4 |
| Nebivolol, 20 | 263 | 196 | 99.4 | 99.6 | 152.8 | 151.9 |
| Nebivolol, 30/40 | 104 | 113 | 98.3 | 99.9 | 150.8 | 154.3 |
Abbreviations: BMI, body mass index; SiDBP, mean sitting diastolic blood pressure; SiSBP, mean sitting systolic blood pressure.
Effects on BP
Nebivolol reduced mean trough SiDBP significantly from baseline to study end compared with placebo at all doses ≥2.5 mg in both obese and non‐obese patients (1, 2). Baseline‐adjusted reductions in mean trough SiSBP with nebivolol were larger than controls at all studied doses ≥5 mg in non‐obese patients and ≥2.5 mg in obese patients (1, 2). Trends for BP reduction across all treatment groups were not significantly different in obese and non‐obese patients (P=.327 for SiDBP; P=.859 for SiSBP), indicating that nebivolol had similar antihypertensive efficacy in obese and non‐obese patients.
Figure 1.
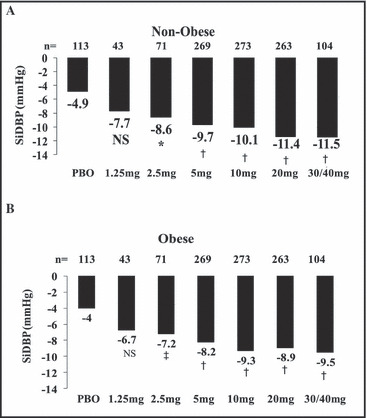
Least squares mean reduction in trough sitting diastolic blood pressure (SiDBP) in obese and non‐obese patients from baseline to end of study. *P=.003 vs placebo (PBO). † P<.001 vs PBO. ‡ P=.013 vs PBO. NS indicates not significant.
Figure 2.
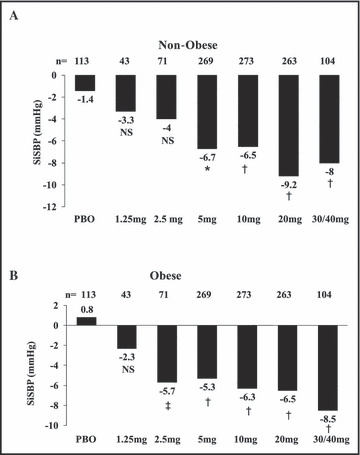
Least squares mean reduction in trough sitting systolic blood pressure (SiSBP) in obese and non‐obese patients from baseline to end of study. *P=.003 vs placebo (PBO). † P<.001 vs PBO. ‡ P=.004 vs PBO. NS indicates not significant.
Response Rates
Compared with placebo, response rates at the end of the treatment period were significantly higher for all nebivolol doses ≥2.5 mg in non‐obese patients (P<.001) and ≥5 mg in obese patients (P<.001) (Figure 3). Trends in response rates across treatment groups were similar between obese and non‐obese patients (P=.991), indicating that obese and non‐obese patients exhibited similar responses to nebivolol therapy (Figure 3).
Figure 3.
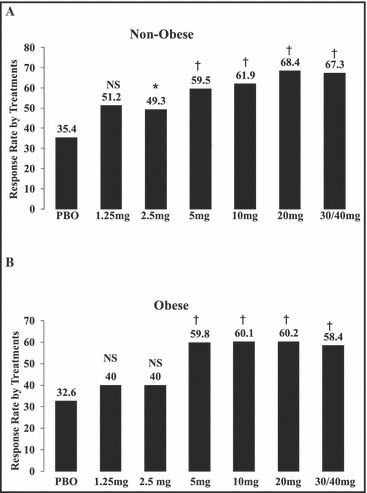
Response rates by treatment. *P=.024 vs placebo (PBO). † P<.001 vs PBO. NS indicates not significant.
Safety and Tolerability
Nebivolol was well tolerated at all doses studied in the total pooled analysis population. There were no marked differences in the rate of AEs with nebivolol (44.5%) and placebo (38.5%) groups (P=.101 by chi‐square test). The most frequently reported treatment‐related AEs were headache, nausea, diarrhea, and dizziness. AEs are summarized in Table III. There were no significant differences in laboratory values, including metabolic parameters, glucose, and lipids between the nebivolol and placebo‐treated groups (data not shown).
Table III.
Treatment Emergent Adverse Events
| Placebo Group | Nebivolol | |||
|---|---|---|---|---|
| Non‐Obese (n=113) | Obese (n=92) | Non‐Obese (n=1023) | Obese (n=787) | |
| Any event | 41 (36.3) | 38 (41.3) | 429 (41.9) | 377 (47.9) |
| Any cardiac event | 0 | 3 (3.3) | 26 (2.5) | 19 (2.4) |
| Myocardial infarction | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Bradycardia | 0 | 1 (1.1) | 14 (1.4) | 5 (0.6) |
| Palpitations | 0 | 0 | 5 (0.6) | 4 (0.5) |
| Any gastrointestinal disorder | 8 (7.1) | 8 (8.7) | 91 (8.9) | 65 (8.3) |
| Constipation | 2 (1.8) | 3 (3.3) | 10 (1.0) | 2 (0.3) |
| Dyspepsia | 1 (0.9) | 2 (2.2) | 7 (0.7) | 9 (1.1) |
| Diarrhea | 3 (2.7) | 1 (1.1) | 25 (2.4) | 21 (2.7) |
| Nausea | 0 | 1 (1.1) | 21 (2.1) | 10 (1.3) |
| Vomiting | 1 (0.9) | 0 | 3 (0.3) | 3 (0.4) |
| Any neurological disorder | 12 (10.6) | 11 (12) | 112 (10.9) | 105 (13.3) |
| Dizziness | 3 (2.7) | 1 (1.1) | 25 (2.4) | 28 (3.6) |
| Headache | 7 (6.2) | 7 (7.6) | 76 (7.4) | 60 (7.6) |
| Somnolence | 0 | 1 (1.1) | 2 (0.2) | 5 (0.6) |
| Any psychiatric disorder | 2 (1.8) | 0 | 24 (2.3) | 20 (2.5) |
| Insomnia | 1 (0.9) | 0 | 12 (1.2) | 9 (1.1) |
| Anxiety | 0 | 0 | 2 (0.2) | 5 (0.6) |
| Depression | 0 | 0 | 5 (0.5) | 3 (0.4) |
| Other | ||||
| Peripheral edema | 0 | 1 (1.1) | 5 (0.5) | 12 (1.5) |
| Fatigue | 2 (1.8) | 1 (1.1) | 40 (3.9) | 25 (3.2) |
Values are expressed as No. (%).
Discussion
The association between excess body weight and hypertension is well established. Overall, 50% of overweight and obese patients are hypertensive, with an increasing prevalence of hypertension among groups with higher BMIs. 4 , 5 The probability of suboptimal BP control in the obese population is approximately 50% higher than in hypertensive patients of normal body weight. 18 This pooled analysis demonstrates that the use of nebivolol in this short‐term 12‐week study in moderately obese patients is well tolerated and safe in a range of 1.25 mg to 40 mg.
Previous studies have demonstrated acceptable hypertension control with β‐blockers as well as with other antihypertensive agents, ie, diuretics, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, in obese patients. 19 The side effect profile of nonvasodilating β‐blockers, however, includes weight gain and some metabolic abnormalities. These agents may therefore not be preferred as antihypertensive agents in this population, although in patients with coronary heart disease they have proven beneficial in obese as well as nonobese patients. 19 In the present pooled analysis, once‐daily nebivolol, as expected, significantly lowered BP at most studied doses. BP reductions were similar in both obese and non‐obese patients, and response rates were high and comparable between both cohorts. A possibly distinctive mechanism of action of β‐blockade in obese patients might control the increased activation of the renal sympathetic nervous system and secondary impairment of pressure‐induced natriuresis observed. In addition, increased endothelial NO release and vasodilation induced by nebivolol might also contribute to its efficacy in hypertensive patients who are overweight or obese. 9 , 10 , 20
Our findings in these short‐term trials confirm previous reports regarding the neutral effects of nebivolol on lipid profile and carbohydrate metabolism. 21 , 22 Recent data suggest that compared with metoprolol, nebivolol at a comparable dose improved oxidative stress and insulin sensitivity, decreased plasma soluble P‐selectin, and increased adiponectin levels in hypertensive patients. Data comparing another vasodilator β‐blocker, carvedilol, with metoprolol have shown similar findings, ie, decrease in insulin resistance. Our findings are important, since nonvasodilating β‐blockers have been associated with weight gain and adverse effects on lipid and glucose metabolism, 11 a trait apparently not shared by some vasodilating β‐blockers such as nebivolol and carvedilol. 23 These effects may be of particular clinical relevance in obese patients, in whom there is frequently an increase in the incidence of dyslipidemia and insulin resistance. 4 , 24
Conclusions
Finally, the composite of all treated patients suggests that nebivolol is safe and well tolerated, with an AE rate similar to that of placebo, at all the doses studied. Thus, the results of our pooled analysis indicate that nebivolol is an effective antihypertensive medication independently of the patient’s weight, with short‐term neutral effects on lipid and carbohydrate metabolism in an obese population. Although there are limitations in these conclusions, the data suggest that a vasodilating β‐blocker may be a better choice of therapy than a β‐blocker without these properties in patients where this type of therapy is indicated.
Acknowledgments and disclosures: The authors would like to acknowledge Brenda Hunter for help with manuscript preparation. Some statistical analysis and editorial support were performed by Forest Laboratories, Inc/Forest Research Institute. The component hypertension studies were sponsored by Mylan‐Bertek. Dr James Sowers currently holds grants from Forest Laboratories and Boehringer Ingelheim Corporation and belongs to the Forest Laboratories advisory board. Dr Whaley‐Connell currently holds a grant from Forest Laboratories.
References
- 1. Hedley AA, Cynthia LO, Clifford LJ, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. [DOI] [PubMed] [Google Scholar]
- 2. Parikh NI, Michael JP, Thomas JW, et al. Increasing trends in incidence of overweight and obesity over 5 decades. Am J Med. 2007;120:242–250. [DOI] [PubMed] [Google Scholar]
- 3. Baker JL, Lina WO, Thorkild IAS. Childhood body‐mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sowers JR. Obesity as a cardiovascular risk factor. Am J Med. 2003;115(suppl 8A):37S–41S. [DOI] [PubMed] [Google Scholar]
- 5. Bramlage P, David P, Hans‐Ulrich W, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17:904–910. [DOI] [PubMed] [Google Scholar]
- 6. Tuck ML, Sowers JR, Dornfeld L, et al. Reductions in plasma catecholamines and blood pressure during weight loss in obese subjects. Acta Endocrinol. 1983;102:252–257. [DOI] [PubMed] [Google Scholar]
- 7. Sowers JR, Whitfield LA, Catania RA, et al. Role of the sympathetic nervous system in blood pressure maintenance in obesity. J Clin Endocrinol Metab. 1982;54:1181–1186. [DOI] [PubMed] [Google Scholar]
- 8. Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. [DOI] [PubMed] [Google Scholar]
- 9. Rahmouni K, Marcelo LGC, William GH, et al. Obesity‐associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. [DOI] [PubMed] [Google Scholar]
- 10. De Jongh RT, Erik HS, Richard GI, et al. Impaired microvascular function in obesity: implications for obesity‐associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. [DOI] [PubMed] [Google Scholar]
- 11. Weber MA. The role of the new beta‐blockers in treating cardiovascular disease. Am J Hypertens. 2005;18:169S–176S. [DOI] [PubMed] [Google Scholar]
- 12. Van de Water A, Janssens W, Neuten JV, et al. Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent, and selective beta 1‐adrenergic antagonist. J Cardiovasc Pharmacol. 1988;11:552–563. [DOI] [PubMed] [Google Scholar]
- 13. Bristow MR, Nelson P, Minobe W, et al. Characterization of [beta]1‐adrenergic receptor selectivity of nebivolol and various other beta‐blockers in human myocardium. Am J Hypertens. 2005;18:A51–A52. [Google Scholar]
- 14. Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double‐blind, crossover study. Circulation. 2001;104:511–514. [DOI] [PubMed] [Google Scholar]
- 15. Bowman AJ, Chen CP, Ford GA. Nitric oxide mediated venodilator effects of nebivolol. Br J Clin Pharmacol. 1994;38:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veverka A, Nuzum DS, Jolly JL. Nebivolol: a third‐generation {beta}‐adrenergic blocker. Ann Pharmacother. 2006;40:1353–1360. [DOI] [PubMed] [Google Scholar]
- 17. Kamp O, Sieswerda GT, Visser CA. Comparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertension. Am J Cardiol. 2003;92:344–348. [DOI] [PubMed] [Google Scholar]
- 18. Lloyd‐Jones DM, Evans JC, Larson MG, et al. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594–599. [DOI] [PubMed] [Google Scholar]
- 19. Schmieder RE, Gatzka C, Schächinger H, et al. Obesity as a determinant for response to antihypertensive treatment. BMJ. 1993;307:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Korkmaz H, Ilgin K, Koç M, et al. Early effects of treatment with nebivolol and quinapril on endothelial function in patients with hypertension. Endothelium. 2008;15:149–155. [DOI] [PubMed] [Google Scholar]
- 21. Celik T, Atila I, Hurkan K, et al. Comparative effects of nebivolol and metoprolol on oxidative stress, insulin resistance, plasma adiponectin and soluble P‐selectin levels in hypertensive patients. J Hypertens. 2006;24:591–596. [DOI] [PubMed] [Google Scholar]
- 22. Rizos E, Bairaktari E, Kostoula A, et al. The combination of nebivolol plus pravastatin is associated with a more beneficial metabolic profile compared to that of atenolol plus pravastatin in hypertensive patients with dyslipidemia: a pilot study. J Cardiovasc Pharmacol Ther. 2003;8:127–134. [DOI] [PubMed] [Google Scholar]
- 23. Bakris GL, Vivian F, Richard EK, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. [DOI] [PubMed] [Google Scholar]
- 24. Rader DJ. Effect of insulin resistance, dyslipidemia, and intra‐abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S12–S18. [DOI] [PubMed] [Google Scholar]


