Many authors have decried the lack of appropriate use of diuretics in the treatment of hypertension, despite their proven benefits and evidence that these agents are as (or even more) effective in preventing one or more forms of major cardiovascular disease in most hypertensive patients than other available agents. This commentary summarizes data suggesting that an appropriate diuretic should be a part of the hypertension regimen for most patients. If the estimated glomerular filtration rate (using the simplified Modification of Diet in Renal Disease equation) is <40 mL/min/1.73 m2, most authorities suggest that a loop diuretic (eg, furosemide twice daily at a dose that is half the sum of the blood urea nitrogen level (in mg/dL) + patient age (in years) twice daily) be used, based on fluid balance and lowering blood pressure. Otherwise, the preferred diuretic is chlorthalidone at 12.5 mg/d to start (a dosage not approved by the US Food and Drug Administration), rather than hydrochlorothiazide. Most of the hypertension treatment trials performed in the United States that reported beneficial results used chlorthalidone as the diuretic. Most American physicians instead have used hydrochlorothiazide, but it is neither as potent in lowering blood pressure nor as evidence‐based in preventing cardiovascular disease as chlorthalidone is. Most doctors abandoned chlorthalidone in the 1980s because the dosages considered moderate then (50–200 mg/d) are by today’s standards actually high and caused hypokalemia (potassium <3.5 mEq/L) in a number of patients. More recent clinical trial data using dosages of 12.5–25 mg/d have shown that hypokalemia is rarely a problem, particularly when this medication is used with an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB). While all drugs, and indeed, all diuretics, have the potential for side effects and drug‐drug interactions, blood pressure control and prevention of cardiovascular events would likely be improved if more physicians were aware of the strong evidence base for chlorthalidone.
Diuretics have a long and distinguished history in the treatment of hypertension. Although some recent treatment guidelines from international or foreign sources are not as specific, 1 , 2 , 3 the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) recommends, in the absence of a compelling indication, a low‐dose thiazide‐like (eg, chlorthalidone or indapamide) or thiazide‐type diuretic for initial treatment of most stage 1 hypertension and a combination of such a diuretic and another antihypertensive drug for stage 2 hypertension. 4 This recommendation was based on outcomes data from several landmark clinical trials, particularly the Antihypertensive and Lipid‐Lowering to Prevent Heart Attack Trial (ALLHAT). 5 , 6 In ALLHAT, as in many other trials, diuretics were particularly beneficial in black and older hypertensive persons. 5 , 6 After the existing data are combined, a diuretic‐based regimen was significantly better than ACE inhibitor–based treatment in preventing stroke (by 14%; 95% confidence interval [CI], 2%–27%; P<.03) or heart failure (by 16%; 95% CI, 5%–29%; P<.004). 7 Similarly, a diuretic‐based program was significantly better than a calcium channel blocker–based regimen in preventing heart failure (by 41%; 95% CI, 27%–56%; P<.0001). 7 There were no significant differences between initial treatment with a diuretic or either comparator for the end points of all‐cause mortality, cardiovascular death, coronary heart disease, or first major adverse cardiovascular event.
The “compelling indications” for specific medications listed by JNC 7, as well as many of those established by research studies since then, also involve diuretics. Heart failure can seldom be successfully treated without a diuretic. Although the American Diabetes Association recommends either an ACE inhibitor or an ARB as the first‐step antihypertensive drug in diabetics, 8 a diuretic was typically used with such an agent in the Captopril Cooperative Study Group’s regimen, 9 the Irbesartan Diabetic Nephropathy Trial, 10 and the Reduction of Endpoints in Non–Insulin‐Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan trial. 11 In patients with nondiabetic chronic kidney disease, an ACE inhibitor reduced the time to doubling of serum creatinine level or end‐stage renal disease, 12 but a diuretic was typically given with the ACE inhibitor. In all of these trials, thiazide diuretics were typically and appropriately replaced by loop diuretics when renal function declined. Of importance, the ALLHAT comparisons of chlorthalidone compared to lisinopril or amlodipine in patients with initial serum creatinine levels ≤2.0 mg/dL showed no significant differences in the composite end point of end‐stage renal disease or a 50% decline in renal function 13 nor in cardiovascular outcomes across all initial stages of chronic kidney disease. 14
A diuretic (chlorthalidone 12.5–25 mg/d) was used as initial therapy in the Systolic Hypertension in the Elderly Program (SHEP), 15 which was the first study to show a significant reduction in stroke among older persons with isolated systolic hypertension; a diuretic was the third drug used in both the Systolic Hypertension in Europe and Systolic Hypertension in China studies. 16 , 17 Many authorities will not make a diagnosis of resistant hypertension unless an appropriate diuretic has been prescribed for the patient. 18 Perhaps most indicative of the importance of a diuretic as part of the regimen was the Perindopril Protection Against Recurrent Stroke Study (PROGRESS). 19 In PROGRESS, a secondary stroke prevention study, 6105 participants with a prior history of a neurologic event in the preceding 5 years were randomized to either placebo ± placebo or an ACE inhibitor (perindopril) ± a diuretic (indapamide). The latter group had significant reductions in blood pressure (by 9/5 mm Hg), recurrent stroke (by 28%), and cardiovascular events (by 26%; P<.001 in each case). The major benefits, however, were seen in those who received both the ACE inhibitor and the diuretic (12/5 mm Hg, 43%, and 40%, respectively, compared with placebo ± placebo), whereas those who received only the ACE inhibitor had nonsignificant improvements in outcomes (5/3 mm Hg, 5%, and 4%, respectively) compared with individuals given placebo.
Which Diuretic?
The only diuretic suitable for patients with a verified allergy to “sulfa drugs” is ethacrynic acid, which became available again in 2003 after not being available for several years. It is usually used in dosages of 25 to 50 mg twice daily. 20
In the absence of the rare true sulfa allergy, the choice of a diuretic primarily depends on the patient’s renal function (Figure 1). If the estimated glomerular filtration rate is lower than about 40 mL/min/1.73 m2, a loop diuretic such as furosemide (twice daily), bumetanide (twice daily), or torsemide (which is more expensive but sometimes can be given just once daily) is generally more effective than a low‐dose thiazide for diuresis and lowering blood pressure. 23 Most physicians forget that the trade name for furosemide is derived from the fact that its diuretic effect “lasts 6 hours.” Furosemide is therefore relatively ineffective as a once‐daily medication, simply because fluid and sodium retention occur during the remaining 18 hours of the day, especially if a major salt load occurs during the evening meal and furosemide is taken in the morning. Of course, there is more to blood pressure control than fluid balance, but in patients with chronic kidney disease, they are often related. The appropriate daily dose of furosemide is typically the sum of the patient’s age in years plus the blood urea nitrogen concentration (in mg/dL) (eg, 40 years + 80 mg/dL = 120 mg/d). 24
Figure 1.
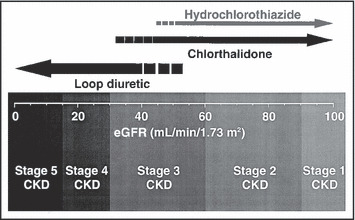
Selection of a diuretic based on the patient’s estimated glomerular filtration rate (eGFR). Note that low‐dose hydrochlorothiazide is typically less effective when the eGFR is less than about 45 mL/min/1.73 m2 (using the simplified Modification of Diet in Renal Disease equation), 21 whereas low‐dose chlorthalidone begins to lose its antihypertensive efficacy at a somewhat lower threshold. In general, a loop diuretic is recommended for patients with an eGFR<35 mL/min/1.73 m2. The stages of chronic kidney disease (CKD) are based on the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative guidelines. 22
If the estimated glomerular filtration rate is ≥40 mL/min/1.73 m2, the most common choice in clinical practice in the United States is hydrochlorothiazide, which ranked fifth in total prescriptions (with 45,124,000) dispensed by retail pharmacists in 2006, after hydrocodone/acetaminophen, atorvastatin, lisinopril, and amoxicillin). 25 Hydrochlorothiazide has been commonly paired in fixed‐dose combination products with most β‐blockers, several centrally acting drugs, most ACE inhibitors, and every currently marketed ARB. With the exception of the latter, these products are generally inexpensive, generically available, and sometimes available in a full range of doses.
The National Heart, Lung, and Blood Institute, however, has used chlorthalidone in their large clinical trials. Since about 1980, these trials have started with about half of a 25‐mg pill daily (a dose not approved by the US Food and Drug Administration). Today, 50 mg/d of chlorthalidone is seldom required. The major advantage of hydrochlorothiazide over chlorthalidone is that the former has been combined with approximately 21 different antihypertensive drugs, whereas only 3 fixed‐dose combination products are available with chlorthalidone. Ironically, they are the 3 “step 2” treatments available for participants in ALLHAT (atenolol, clonidine, or reserpine), which may not be completely coincidental. A strong evidence‐based case can be made for the use of chlorthalidone instead of hydrochlorothiazide.
Chlorthalidone is More Effective for Lowering Blood Pressure Compared With Hydrochlorothiazide
Thirty years ago, when doses of diuretics were much higher than today, 4 independent, head‐to‐head clinical trials were performed that compared the blood pressure–lowering effects of chlorthalidone and hydrochlorothiazide. 26 , 27 , 28 , 29 Despite differences in study design (crossover 26 vs parallel‐group 27 ), duration of treatment (4 27 –12 29 weeks), adjuvant potassium‐sparing diuretic with hydrochlorothiazide, 28 , 29 and baseline blood pressures (152 27 –181 26 /100 28 –110 26 mm Hg), patients receiving chlorthalidone 50 mg/d had numerically superior blood pressure reductions (weighted average, −21.4/−12.6 mm Hg) compared with either hydrochlorothiazide 50 mg/d (−10.6/−5.0 mm Hg) or hydrochlorothiazide 100 mg/d (−20.4/−16.5 mm Hg).
More recently, Ernst and colleagues 30 compared hydrochlorothiazide (initially 25 mg/d, force‐titrated to 50 mg/d) with chlorthalidone (initially 12.5 mg/d, force‐titrated to 25 mg/d) in 30 hypertensive patients. Although originally designed as a crossover study, there was a large carryover effect in the group originally assigned to chlorthalidone (even after a 4‐week washout period), so the data were analyzed as a parallel‐group study. The primary end point was change in 24‐hour mean blood pressure based on ambulatory blood pressure monitoring at 8 weeks compared with baseline. Nighttime mean blood pressure and office systolic blood pressure levels at 2‐week intervals were secondary end points. The results are shown in Figure 2. It should not be surprising that the nocturnal fall in blood pressure was greater with chlorthalidone, given its much longer serum elimination half‐life (∼70 vs ∼8 hours) and duration of action. 31 These data suggest that, even at half the daily dose, chlorthalidone provides quicker and more sustained blood pressure reduction than hydrochlorothiazide, especially at the low doses recommended today.
Figure 2.
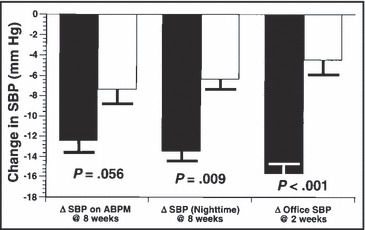
Summary of the results of the randomized, head‐to‐head comparison of low‐dose chlorthalidone (12.5 mg/d initially, force‐titrated to 25 mg/d, black bars) vs hydrochlorothiazide (25 mg/d initially, force‐titrated to 50 mg/d, open bars) in 30 hypertensive patients. The primary end point (change in 24‐hour systolic blood pressure [SBP] from baseline at 8 weeks by ambulatory blood pressure monitoring [ABPM]) barely missed statistical significance, but the change in average nighttime SBP and the change in office SBP at 2 weeks both were significantly greater with chlorthalidone than with twice the daily dose of hydrochlorothiazide. Data are from Ernst et al, 2006. 30
Supporting evidence in favor of chlorthalidone’s better blood pressure–lowering efficacy comes from an unblinded consecutive series of 19 hypertensive patients taking hydrochlorothiazide who were switched to the same dose of open‐label chlorthalidone. 32 When reassessed after about a month, these patients experienced a 12‐mm Hg reduction in systolic blood pressure (P<.03) on average and no difficulties with hypokalemia. This was most likely due to the low doses used and the fact that all were taking either an ACE inhibitor or an ARB.
Chlorthalidone Has More Extensive and Better Outcomes Data Than Hydrochlorothiazide
Both chlorthalidone and hydrochlorothiazide have been used in outcomes‐based clinical trials (Table), but their performance records are different. Evidence suggests that patients treated with chlorthalidone have better outcomes than those treated with a hydrochlorothiazide‐based regimen.
Table.
Summary of Results of Outcomes‐Based Clinical Trials Involving Chlorthalidone or Hydrochlorothiazide
| Chlorthalidone | Hydrochlorothiazide |
|---|---|
| Hypertension Detection and Follow‐Up Program 33 (HDFP, better than “referred care”) | Veterans Affairs Cooperative Study Group on Antihypertensive Agents 34 (better than placebo, with help) |
| Multiple Risk Factor Intervention Trial 35 (MRFIT, better than hydrochlorothiazide) | Multiple Risk Factor Intervention Trial 35 (MRFIT, inferior to, and replaced by, chlorthalidone) |
| Systolic Hypertension in the Elderly Program 15 (SHEP, better than placebo) | Heart Attack Primary Prevention in Hypertension 36 (better than 2 β‐blockers) |
| Treatment of Mild Hypertension Study 37 (TOMHS, no outcomes data available) | Metoprolol Atherosclerosis Prevention in Hypertensives 38 (MAPPHY, inferior to metoprolol) |
| Verapamil Hypertension Atherosclerosis Study 39 (VHAS, similar to verapamil) | Medical Research Council Trial in the Elderly 40 (MRC‐E, better than placebo and atenolol) |
| Antihypertensive and Lipid‐Lowering to Prevent Heart Attack Trial 5 , 6 (ALLHAT, better than doxazosin, lisinopril, and amlodipine for heart failure) | Multicenter Isradipine Diuretic Atherosclerosis Study 41 (MIDAS, similar to isradipine) International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment 42 (INSIGHT, similar to nifedipine) |
| Post‐stroke Antihypertensive Treatment Study 43 (PATS, better than placebo) | |
| Controlled Onset Verapamil Investigation of Cardiovascular Endpoints 44 (CONVINCE, similar to verapamil in subgroup analysis) | |
| Second Australian National Blood Pressure trial 45 (ANBP‐2, similar to enalapril, but worse in men) |
A network meta‐analysis of outcomes studies in hypertension provided an indirect comparison of the risk for several cardiovascular end points with chlorthalidone compared with other types of thiazide‐like diuretics and concluded that there were no significant differences between the two. 46 However, this analysis did not include the only study in which 8012 hypertensive men in the Multiple Risk Factor Intervention Trial (MRFIT) were given either chlorthalidone or hydrochlorothiazide, based on the choice of the principal investigator at each study site. Although the assignment of the diuretic in this study was not randomized, the outcomes were compared at each site with the control group (randomized to usual care [UC] in each community). During the first half of 1980, the steering committee of MRFIT recommended that all special intervention (SI) participants should be switched from hydrochlorothiazide to chlorthalidone (at ≤50 mg/d). This decision was based on several lines of evidence comparing both coronary heart disease and all‐cause mortality experience across the 22 participating clinics, all of which pointed to less benefit in patients taking hydrochlorothiazide compared with chlorthalidone. These data have not yet been published in full form. 35 , 47 , 48 When the clinics were divided into tertiles regarding the relative use of the 2 diuretics (before switching), there was a significant decrease in both end points in the SI and the UC groups (as well as the cohort as a whole) as the use of hydrochlorothiazide decreased. This was seen only in hypertensive men (G. E. Bartsch, written communication, June 1986). Further analyses were performed and reported after completion of the trial. 35 The major findings were that in the 9 clinics that predominantly prescribed hydrochlorothiazide, the SI group had a 44%excess risk of death from coronary heart disease compared with their randomized UC groups, whereas the 6 clinics that predominantly prescribed chlorthalidone had a 58%reduction in risk compared with their UC controls. 35 , 47 In addition, all‐cause mortality in the SI groups in the clinics that prescribed hydrochlorothiazide was increased (by 16%), compared to a 41%decrease in all‐cause mortality in the 6 clinics that prescribed mostly chlorthalidone. During the 5 years of follow‐up after the switch from hydrochlorothiazide to chlorthalidone, coronary heart disease mortality in the 9 clinics decreased by 28%, compared with their UC groups (P=.04 for comparison of percentage differences in coronary heart disease mortality rates for the 2 time periods). After the switch was accomplished, a similar 26% reduction was also seen in all‐cause mortality (compared with the UC controls, P=.06). 35 It has been difficult to understand why a difference in mortality in MRFIT between the SI and UC groups was not seen during the initial years of funded follow‐up but appeared much later. The switch in diuretic may have played a role, because after 1980 almost all SI participants received chlorthalidone. However, most UC participants received hydrochlorothiazide from their community‐based physicians because these physicians were not aware of the protocol change or the reasons behind it.
Adverse Effects of Diuretics
Many of the adverse effects of diuretics are dose‐dependent (eg, possible hypotension, volume depletion, polyuria, hypokalemia, hypomagnesemia, and hyponatremia). Hypercalcemia may be dose‐related with thiazide‐like diuretics, but hypocalcemia is more common with loop diuretics. Erectile dysfunction may be more common with diuretics than with other commonly‐used antihypertensive drugs, but the inhibitors of phosphodiesterase‐5 are usually quite effective in alleviating this possible adverse effect. Metabolic effects of diuretics (including increased insulin resistance and other components of the metabolic syndrome) are relatively widely recognized, but their long‐term consequences (if any) are still quite controversial. Although increased cardiovascular risk has been noted after 5 to 28 years of follow‐up among individuals in whom diabetes developed during follow‐up, 49 , 50 , 51 , 52 , 53 2 important clinical trials with chlorthalidone (SHEP and ALLHAT) reported no excess cardiovascular risk despite these metabolic changes. 6 , 54 , 55 Of greatest concern to many is the increased risk of incident diabetes, which was higher with initial diuretic therapy compared with an ACE inhibitor or an ARB in a recent network meta‐analysis of antihypertensive drugs. 56 In a sensitivity analysis, the risk of incident diabetes with chlorthalidone (compared with placebo) was 1.25 (95% CI, 1.00–1.55; P=.055), whereas it was 1.48 (95% CI, 1.16–1.90; P=.002) with non‐chlorthalidone thiazide‐type diuretics. 56 Although this difference was not statistically significant (in an indirect comparison), it may be one more reason to favor chlorthalidone over hydrochlorothiazide. Similarly, the increase in serum cholesterol and uric acid concentrations typically seen with thiazide diuretics has been greatly reduced by using lower doses and has not been linked to increased cardiovascular risk in follow‐up of randomized clinical trials. Most such studies last only 5 or so years, however, and longer follow‐up may be needed to discern such an effect. 52
Contraindications, Warnings, and Drug Interactions With Diuretics
Thiazide‐like and thiazide‐type diuretics are contraindicated in patients with anuria and those with absolute contraindications to sulfa drugs 20 and are typically not very effective at low doses if the estimated glomerular filtration rate is <40 mL/min/1.73 m2. Similarly, loop diuretics are contraindicated in patients with anuria, hepatic coma, and severe electrolyte derangements. Potassium‐sparing diuretics (including triamterene, amiloride, and spironolactone) are contraindicated in patients with hyperkalemia or those with stage 4 or 5 chronic kidney disease. In addition, hyperkalemia (and death related to it) with these drugs is a concern when they are given with salt substitutes, ACE inhibitors, ARBs, high‐potassium foods, and nonsteroidal anti‐inflammatory drugs.
All diuretics have the possibility of interacting with digoxin, lithium, and nonsteroidal anti‐inflammatory drugs. Alcohol and central nervous system depressants increase the risk of orthostatic hypotension with coadministered diuretics. Corticosteroids, corticotropin, and amphotericin B increase the risk of hypokalemia when administered with diuretics. On the positive side, diuretics increase the blood pressure–lowering efficacy of essentially every other class of antihypertensive drug. Thiazide‐type and thiazide‐like diuretics can potentiate the nondepolarizing muscle relaxants, antagonize norepinephrine, and interfere with parathyroid blood testing. Eplerenone has significant interactions with drugs that are metabolized by the CYP3A4 system (eg, the azole antifungal agents). However, placebo‐controlled studies have shown that all of these adverse effects are uncommon and are generally outweighed by the benefits of diuretics, which can reduce pharyngeal edema in patients with obstructive sleep apnea. 57
Conclusions
This brief review has attempted to remind the reader that diuretics are extremely useful antihypertensive drugs, particularly for individuals with chronic kidney disease (in whom loop diuretics are often more useful), 22 diabetes (because of their lower blood pressure target), 8 and those at high risk for heart failure. 2 Generally, furosemide, given twice daily for the total daily dose that is the sum of the patient’s age plus blood urea nitrogen concentration, or chlorthalidone (starting at 12.5 mg/d, a dose not approved by the US Food and Drug Administration) can be recommended. Although available in fewer fixed‐dose combinations, chlorthalidone has better blood pressure–lowering efficacy than the much more commonly prescribed hydrochlorothiazide, more and better results in long‐term outcomes‐based clinical trials, a longer duration of action, and perhaps a lower risk of new‐onset diabetes. Perhaps if physicians were not allowed to use the abbreviation “HCTZ,” they would be more likely to avoid hydrochlorothiazide and would write “chlorthalidone” instead, since it has 5 fewer letters.
Disclosure: The writing of this manuscript was not supported by any entity. Dr Elliott has received research grants from the National Institutes of Health and Pfizer Inc; has served as a consultant to and participated in Speakers' Bureaus for Novartis, Pfizer Inc, Bristol‐Myers Squibb/Sanofi‐Synthelabo, and AstraZeneca, LP; and has received other support from Elsevier. Dr Grimm has received research grants and served as a consultant to Pfizer Inc, Novartis, Merck, and Takeda
References
- 1. Whitworth JA, for the World Health Organization, International Society of Hypertension Writing Group . 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 2. National Institute for Health and Clinical Excellence . Hypertension: Management of Hypertension in Adults in Primary Care, a Partial Update of NICE Clinical Guideline 18; pp. 1–45, issued 28 June, 2006. http://www.nice.org.uk/nicemedia/pdf.CG034NICEguideline.pdf. Accessed October 16, 2008. [Google Scholar]
- 3. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension. Task force for the management of arterial hypertension of the European Society of hypertension and the European Society of cardiology. J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 4. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 5. The ALLHAT Collaborative Research Group . Major cardiovascular events in hypertensive patients randomized to doxazosin vs. chlorthalidone: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 6. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic: the antihypertensive and lipid lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 7. Elliott WJ. Systemic hypertension. Curr Prob Cardiol. 2007;32:201–259. [DOI] [PubMed] [Google Scholar]
- 8. Standards of Medical Care in Diabetes – 2007 (Technical Review). Diabetes Care. 2007;30(Suppl1): S4–S41. [DOI] [PubMed] [Google Scholar]
- 9. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin converting enzyme inhibition in diabetic nephropathy. N Engl J Med. 1993;323:1456–1462. [DOI] [PubMed] [Google Scholar]
- 10. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to Type 2 diabetes. Collaborative Study Group. N Engl J Med. 2001; 345:851–860. [DOI] [PubMed] [Google Scholar]
- 11. Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with Type 2 diabetes and nephropathy. Reduction of endpoints in non‐insulin dependent diabetes mellitus with the angiotensin II antagonist losartan (RENAAL) Study Group. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 12. Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin‐converting enzyme inhibition: a patient‐level meta‐analysis. Ann Intern Med. 2003; 139:244–252. [DOI] [PubMed] [Google Scholar]
- 13. Rahman M, Pressel S, Davis BR, et al. Renal outcomes in high‐risk hypertensive patients treated with an angiotensin‐converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). Arch Intern Med. 2005;165:936–946. [DOI] [PubMed] [Google Scholar]
- 14. Rahman M, Pressel S, Davis BR, et al. Cardiovascular outcomes in high‐risk hypertensive patients stratified by baseline glomerular filtration rate. Ann Intern Med. 2006;144:172–180. [DOI] [PubMed] [Google Scholar]
- 15. The SHEP Cooperative Study Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 16. Staessen JA, Fagard R, Thijs L, et al. Morbidity and mortality in the placebo‐controlled European Trial on Isolated Systolic Hypertension in the Elderly. Lancet. 1997; 360:757–764. [Google Scholar]
- 17. Liu L, Wang J, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. J Hypertens. 1998;16:1823–1829. [DOI] [PubMed] [Google Scholar]
- 18. Moser M, Setaro JF. Resistant or difficult‐to‐control hypertension. N Engl J Med. 2006;355:385–392. [DOI] [PubMed] [Google Scholar]
- 19. PROGRESS Collaborative Group . Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. [DOI] [PubMed] [Google Scholar]
- 20. Wall GC, Gibner D, Craig S. Ethacrynic acid and the sulfa‐sensitive patient. Arch Intern Med. 2003;163:116–117. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Rocco MV, Anderson S, et al. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(suppl 1):S1–S290. [PubMed] [Google Scholar]
- 23. Knauf H, Mutschler E. Diuretic effectiveness of hydrochlorothiazide and furosemide alone and in combination in chronic renal failure. J Cardiovasc Pharmacol. 1995;26:394–400. [DOI] [PubMed] [Google Scholar]
- 24. Shem S. The House of God. New York, NY: Dell Publishing; 1979. [Google Scholar]
- 25. Top 200 generic drugs by units in 2006. http://www.drugtopics.com/drugtopics.modernmedicine/data/articlestandard/drugtopics/092007/407652/article.pdf. Accessed October 16, 2008.
- 26. Bowlus WE, Langford HG. A comparison of the antihypertensive effect of chlorthalidone and hydrochlorothiazide. Clin Pharmacol Ther. 1964;5:708–711. [DOI] [PubMed] [Google Scholar]
- 27. Finnerty FA Jr. A double‐blind study of chlorthalidone and hydrochlorothiazide in an outpatient population of moderate hypertensives. Angiology. 1976;27:738–744. [DOI] [PubMed] [Google Scholar]
- 28. Clark EC, Podolsky S, Thompson EJ. Double‐blind comparison of hydrochlorothiazide plus triamterene therapy versus chlorthalidone therapy in hypertension. South Med J. 1979;72:798–802. [DOI] [PubMed] [Google Scholar]
- 29. Van Soeren F. The antihypertensive and biochemical effects of hydrochlorothiazide/amiloride (Moduretic) versus chlorthalidone. J Int Med Res. 1980;8:132–135. [DOI] [PubMed] [Google Scholar]
- 30. Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. [DOI] [PubMed] [Google Scholar]
- 31. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone. Evidence supporting their interchangeability. Hypertension. 2004;43:4–9. [DOI] [PubMed] [Google Scholar]
- 32. Khosla N, Elliott WJ, Bakris GL. Are chlorthalidone and hydrochlorothiazide equivalent blood pressure‐lowering medications? J Clin Hypertens (Greenwich). 2005;7:354–356. [DOI] [PubMed] [Google Scholar]
- 33. Hypertension Detection and Follow‐up Cooperative Group . Persistence of reduction in blood pressure and mortality in participants in the Hypertension Detection and Follow‐Up Program. JAMA. 1988;259:2113–2122. [PubMed] [Google Scholar]
- 34. Veterans Administration Cooperative Study Group on Antihypertensive Agents . Effects of treatment on morbidity in hypertension: results in patients with diastolic blood pressure averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 35. Multiple Risk Factor Intervention Trial Research Group . Mortality after 10 1/2 years for hypertensive participants in the Multiple Risk Factor Intervention Trial. Circulation. 1990;82:1616–1628. [DOI] [PubMed] [Google Scholar]
- 36. Wilhelmsen L, Berglund G, Elmfeldt D, et al. Beta‐blockers versus diuretics in hypertensive men: main result from the HAPPHY trial. J Hypertension. 1987;5:560–572. [DOI] [PubMed] [Google Scholar]
- 37. Neaton JD, Grimm RH, Prineas RJ, et al. Treatment of Mild Hypertension Study: final results. JAMA. 1993;270:713–724. [PubMed] [Google Scholar]
- 38. Wikstrand J, Warnold I, Olsson G, et al. Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA. 1988; 259:1976–1982. [PubMed] [Google Scholar]
- 39. Agabiti‐Rosei E, Dal Palù C, Leonetti G, et al. Clinical results of the verapamil in hypertension and atherosclerosis study. The VHAS investigators. J Hypertens. 1997; 15:1337–1344. [DOI] [PubMed] [Google Scholar]
- 40. MRC Working Party . Medical Research Council Trial of Treatment of Hypertension in Older Adults: principal results. BMJ. 1992;304:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borhani NO, Mercuri M, Borhani PA, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS): a randomized trial. JAMA. 1996; 276:785–791. [PubMed] [Google Scholar]
- 42. Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: intervention as a goal in hypertension treatment (INSIGHT). Lancet. 2000;356:366–372. [DOI] [PubMed] [Google Scholar]
- 43. PATS Collaborating Group . Post‐stroke antihypertensive treatment study. A preliminary result. Chin Med J. 1995;108:710–717. [PubMed] [Google Scholar]
- 44. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) trial. JAMA. 2003;289:2073–2082. [DOI] [PubMed] [Google Scholar]
- 45. Wing LMH, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin‐converting‐enzyme inhibitors and diuretics for hypertension in the elderly. Second Australian National Blood Pressure Study Group. N Engl J Med. 2003;348:583–592. [DOI] [PubMed] [Google Scholar]
- 46. Psaty BM, Lumley T, Furberg CD. Meta‐analysis of health outcomes of chlorthalidone‐based vs nonchlorthalidone‐based low dose diuretic therapies. JAMA. 2004; 292:43–44. [DOI] [PubMed] [Google Scholar]
- 47. Bartsch G, Broste S, Grandits G, et al. Hydrochlorothiazide, chlorthalidone and mortality in the Multiple Risk Factor Intervention Trial [abstract]. Circulation. 1984; 70(suppl II):II‐1438. [Google Scholar]
- 48. Kolata G. Heart study produces a surprise result. Science. 1982;218:31–32. [DOI] [PubMed] [Google Scholar]
- 49. Verdecchia P, Reboldi G, Angeli F, et al. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004;43:963–969. [DOI] [PubMed] [Google Scholar]
- 50. Almgren T, Wilhelmsen L, Samuelsson O, et al. Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from a 28‐year follow‐up. J Hypertens. 2007;25:1311–1317. [DOI] [PubMed] [Google Scholar]
- 51. Aksnes TA, Kjeldsen SE, Rostrup M, et al. Impact of new‐onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial population. Hypertension. 2007;50:467–473. [DOI] [PubMed] [Google Scholar]
- 52. Fox CS, Sullivan L, D’Agostino RB Sr, et al. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004;27:704–708. [DOI] [PubMed] [Google Scholar]
- 53. Dunder K, Lind L, Zethelius B, et al. Increase in blood glucose concentration during antihypertensive treatment as a predictor of myocardial infarction: population‐based cohort study. BMJ. 2003;326:681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kostis JB, Wilson AC, Freudenberger RS, et al. Long‐term effect of diuretic‐based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005;95:29–35. [DOI] [PubMed] [Google Scholar]
- 55. Barzilay JI, Davis BR, Cutler JA, et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med. 2006;166:2191–2201. [DOI] [PubMed] [Google Scholar]
- 56. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet. 2007;369:201–207. erratum 1518. [DOI] [PubMed] [Google Scholar]
- 57. Biucca CB, Brussino L, Battistia A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–446. [DOI] [PubMed] [Google Scholar]


