Abstract
J Clin Hypertens (Greenwich). 2010;12:678–686. ©2010 Wiley Periodicals, Inc.
Hypertension treatment commonly requires multiple agents to achieve target blood pressure (BP). β‐Blockers and angiotensin‐converting enzyme inhibitors (ACEIs) are commonly co‐prescribed in clinical practice although few data are available that test their additivity on BP lowering. The efficacy and safety of once‐daily extended‐release carvedilol (carvedilol CR) combined with the ACEI lisinopril in a double‐blind, randomized, factorial design study were studied. Patients (N=656) with stage 1 or 2 hypertension were randomized evenly to 1 of 15 groups for 6 weeks: carvedilol CR monotherapy 20 mg, 40 mg, or 80 mg/d; lisinopril monotherapy 10 mg, 20 mg, or 40 mg/d; or 1 of 9 combinations of carvedilol CR plus lisinopril initiated simultaneously. Primary efficacy measures (assessed by ambulatory BP monitoring [ABPM]) were change from baseline in 24‐hour mean diastolic BP (DBP) and in trough (20–24 hours) DBP. Continuous efficacy variables were assessed using analysis of covariance. Whether any combination dose was superior to its monotherapy components was assessed using the Hung AVE procedure. Despite the presence of additional BP lowering observed with most of the combinations compared with their monotherapy components, the Hung AVE test was not significant for either primary efficacy measures. Post hoc analyses of the high‐dose combination groups (carvedilol CR/lisinopril regimens of 80/10 mg, 80/20 mg, 80/40 mg, 20/40 mg, and 40/40 mg) showed a significant treatment difference compared with both carvedilol CR 80 mg and lisinopril 40 mg for 24‐hour mean DBP but not for trough DBP. With the exception of dizziness, individual adverse events did not increase with ascending doses or combinations. The superiority of initiating combination treatment with carvedilol CR and lisinopril compared with the monotherapy components was not demonstrated with the ABPM measurements. Nonetheless, the post hoc assessment combining all high‐dose groups did produce significant 24‐hour mean BP reduction when compared with the high‐dose monotherapy groups. The tolerability profile of initiating combination therapy was generally comparable to the initiation of treatment with monotherapy.
The majority of patients with hypertension require at least 2 antihypertensive medications to reduce their blood pressure (BP) to current guideline‐recommended goals. 1 , 2 Most of the major antihypertensive classes have been studied in combination, 3 , 4 , 5 , 6 resulting in the availability of several fixed‐dose combination options for antihypertensive therapy. The combination of a renin‐angiotensin system (RAS) blocker and a β‐blocker has not been extensively studied 7 , 8 , 9 and currently no such fixed combination is available for patients. A common assumption is that this combination does not provide a strong rationale for an additive BP effect, since both classes use a common mechanism of action involving renin inhibition. 10 , 11 , 12 , 13 In the Glycemic Effects in Diabetes Mellitus: Carvedilol‐Metoprolol Comparison in Hypertensives (GEMINI) trial, the effects of carvedilol and metoprolol added to RAS blockers in patients with hypertension and diabetes were assessed. When either β‐blocker was added to existing RAS inhibitor therapy, additional BP lowering was achieved, even when combined at less than maximal doses. 14 Therefore, the Coreg and Lisinopril Combination Therapy in Hypertensive Subjects (COSMOS) trial was designed to evaluate whether additional BP effects, superior to monotherapy, could be achieved when carvedilol extended‐release (CR) and lisinopril were initiated together and titrated across a full range of dose combinations.
Methods
Study Population
Eligible patients included men and nonpregnant women aged 18 years and older with stage 1 or 2 hypertension who, at screening, had either a documented history of hypertension and were taking 2 antihypertensive medications with a mean sitting diastolic BP (sDBP) <90 mm Hg (or for diabetic patients, a mean sDBP <80 mm Hg) or were taking 1 antihypertensive medication with a mean sDBP ≤109 mm Hg and could be safely withdrawn from all antihypertensive medication or were newly diagnosed with a mean sDBP ≥95 and ≤109 (or for diabetic patients, a mean sDBP ≥85 and ≤109). The main criteria for exclusion were treatment with ≥3 antihypertensive medications, any known contraindications to angiotensin‐converting enzyme (ACE) inhibitors, α‐ or β‐blocker treatment, hyperkalemia or history of hyperkalemia, female of childbearing potential, secondary or malignant hypertension, mean sitting systolic BP (sSBP) ≥180 mm Hg, advanced hypertensive retinopathy (Keith Wagner grade IV), type 1 diabetes mellitus (DM), or type 2 DM with a hemoglobin A1c≥9% at screening.
Study Design
The COSMOS study was a randomized, double‐blind, factorial design trial that was conducted at 172 clinical sites in the United States between 2006 and 2008. Protocol and written informed consents were reviewed and approved by institutional review boards prior to participant enrollment into the study. After a single‐blind 2‐week run‐in period, patients were randomized to 1 of 15 arms (carvedilol CR at once‐daily oral doses of 20 mg, 40 mg, and 80 mg; lisinopril at 10 mg, 20 mg, and 40 mg; and the 9 potential combinations of those doses) for 6 weeks of treatment. Testing this number of combinations was intended to provide support for a US Food and Drug Administration (FDA) registration of the combination product. In the combination arms, all patients were started simultaneously on carvedilol CR 20 mg and lisinopril 10 mg. Study medication was uptitrated every 2 weeks (or weekly if deemed necessary by the investigator) until the randomized or highest‐tolerated dose level was reached. Patients could be uptitrated to the randomized dose after they had been taking the lower dose for at least 2 weeks and their mean sSBP was not <120 mm Hg and/or their mean sDBP was not <70 mm Hg. However, if a patient had symptoms of orthostatic hypotension, they were not to be uptitrated regardless of BP. Following the treatment phase, patients were down‐titrated from study medication for up to 2 weeks.
End Points
COSMOS had two primary efficacy measures assessed by ambulatory BP monitoring (ABPM): change from baseline in 24‐hour mean DBP and change from baseline in trough (20–24 hour) DBP. DBP was chosen as the primary end point based on discussions with the FDA to obtain an end point that met their registration standards. Key secondary objectives were to evaluate changes from baseline in 24‐hour mean SBP and in trough SBP by ABPM and the dose‐response relationship between incremental doses of carvedilol CR and lisinopril and mean 24‐hour ABPM DBP (data not shown).
Statistical Methods
Continuous efficacy variables were analyzed by parametric analysis of covariance (ANCOVA), adjusting for treatment, center, and corresponding baseline BP. The two primary efficacy variables were tested in a hierarchical fashion. The first test corresponded with the assessment of 24‐hour mean DBP and the second test to trough DBP. If the first null hypothesis was rejected at the .05 significance level, then the second hypothesis was to be tested at the .05 significance level.
Primary inference was based on the Hung AVE procedure, 15 which is an omnibus test to investigate the existence of at least one combination dose that outperforms its components. If the Hung AVE statistic was significant, it would imply that there was at least one combination regimen that outperformed its monotherapy counterparts. No treatment groups were combined for this analysis. Pairwise comparisons of individual combination arms to their respective monotherapy components were not performed.
The following subgroups were prespecified: sex, race (Caucasian, African American, other), age (<65 years and ≥65 years), body mass index (<27 and ≥27), diabetes at baseline (yes or no), stage of hypertension at baseline, and estimated glomerular filtration rate (<60 vs ≥60 mL/min per 1.73 m2). In the subgroup analyses, the pooled data from all combination groups were compared with the pooled data from each monotherapy group. Separate analyses were performed for each subgroup variable. The ANCOVA model contained effects for treatment, center, baseline, subgroup, and treatment by subgroup interaction. A prespecified post hoc high‐dose analysis was performed comparing the pooled high‐dose combination groups with each of the high‐dose monotherapy groups. These post hoc analyses were based on an ANCOVA model with effects for treatment, center, corresponding baseline BP, hypertension stage, and race.
Treatment‐related interactions were tested at the .1 level of significance. Baseline BP was defined as the last‐available pre‐dose assessment prior to randomization. Missing on‐therapy values were estimated by the last (on‐therapy) observation available. Efficacy‐evaluable patients comprised all randomized participants with efficacy data after a minimum of 2 weeks on treatment. Multiplicity adjustments to the type I error were performed only for the primary efficacy analyses. All safety data were presented by descriptive statistics.
Results
Patient Disposition and Baseline Characteristics
The COSMOS study enrolled 654 patients. Overall, 551 patients (84%) completed the 6 weeks of treatment. The most common reason for patient withdrawal from the study was an adverse event (28 patients; 4% of those randomized). Table I summarizes the other reasons for patient withdrawal. The demographic characteristics of the study patients are shown in Table II. The baseline (mean, SD) for 24‐hour DBP and trough DBP by ABPM were 92.4 and 5.98 mm Hg and 90.8 and 8.94 mm Hg, respectively. Individuals with diabetes represented 24% of the population. The baseline severity profile of the study population represents the average patient with primary uncontrolled hypertension. Twenty five percent of the participants were treatment‐naïve, 35% were taking 1 antihypertensive medication, 35% were taking 2 antihypertensive medications, and 2% were taking ≥3. These 13 people were taking 2 antihypertensive medications but were receiving medications that had antihypertensive effects and were given for other conditions (eg, glaucoma and angina). Use of ACE inhibitors and angiotensin receptor blockers (ARBs) combined (48%) was predominant, followed by—in decreasing order of frequency—diuretics (21%), calcium channel blockers (11%), and β‐blockers (11%).
Table I.
Patient Disposition
| Lisinopril Monotherapy (n=130) | Carvedilol CR Monotherapy (n=131) | Carvedilol CR + Lisinopril Combination Therapy (n=393) | Total (N=654) | |
|---|---|---|---|---|
| Withdrawn, No. (%) | 19 (15) | 26 (20) | 58 (15) | 103 (16) |
| Primary reason for withdrawal, No. (%) | ||||
| Adverse event | 3 (2) | 7 (5) | 18 (5) | 28 (4)a |
| Lost to follow‐up | 0 | 4 (3) | 5 (1) | 9 (1) |
| Protocol violation | 6 (5) | 5 (4) | 4 (1) | 15 (2) |
| Patient decision to withdraw | 3 (2) | 7 (5) | 14 (4) | 24 (4) |
| Lack of efficacy | 5 (4) | 2 (2) | 2 (<1) | 9 (1) |
| Other | 2 (2) | 1 (<1) | 15 (4) | 18 (3) |
aBased on the end‐of‐study status clinical report form. Three additional participants had at least 1 adverse event with an action of “withdrew IP”; however, they each had a study conclusion status of “not withdrawn” from the study. They are included in the section that describes patients with adverse events leading to withdrawal of the investigational product.
Table II.
Demographics and Selected Clinical Characteristics at Baseline
| Lisinopril Monotherapy (n=130) | Carvedilol CR Monotherapy (n=131) | Carvedilol CR + Lisinopril Combination Therapy (n=393) | Total (N=654) | |
|---|---|---|---|---|
| Age, mean ± SD, y | 52.2±10.6 | 53.9±9.6 | 53.2±9.7 | 53.1±9.9 |
| Age, No. (%) | ||||
| <65 y | 118 (91) | 111 (85) | 347 (88) | 576 (88) |
| ≥65 y | 12 (9) | 20 (15) | 46 (12) | 78 (12) |
| Sex, No. (%) | ||||
| Female | 49 (38) | 51 (39) | 132 (34) | 232 (35) |
| Male | 81 (62) | 80 (61) | 261 (66) | 422 (65) |
| Race, No. (%) | ||||
| White | 85 (65) | 88 (68) | 244 (62) | 417 (64) |
| African American | 40 (31) | 33 (25) | 121 (31) | 194 (30) |
| Other | 5 (4) | 8 (6) | 23 (6) | 36 (6) |
| Diabetes status at baseline, No. (%) | ||||
| No | 96 (74) | 105 (80) | 296 (75) | 497 (76) |
| Yes | 34 (26) | 26 (20) | 97 (25) | 157 (24) |
| Disease history, No. (%) | 130 | 131 | 393 | 654 |
| Treatment‐naïve | 37 (28) | 40 (31) | 106 (27) | 183 (28) |
| Controlled with 1 drug | 36 (28) | 47 (36) | 149 (38) | 232 (35) |
| Controlled with 2 drugs | 55 (42) | 42 (32) | 129 (33) | 226 (35) |
| Controlled with ≥3 drugs | 2 (2%) | 2 (2) | 9 (2) | 13 (2) |
| Baseline sitting SBP, mean ± SD, mm Hg | 149.0±11.6 | 149.9±12.5 | 147.2±12.4 | 148.1±12.3 |
| Baseline sitting DBP, mean ± SD, mm Hg | 95.3±7.8 | 96.2±6.9 | 95.5±7.1 | 95.6±7.2 |
| Stage of hypertension, No. (%) | 130 | 131 | 393 | 654 |
| Stage I | 64 (49) | 61 (47) | 195 (50) | 320 (49) |
| Stage II | 55 (42) | 59 (45) | 161 (41) | 275 (42) |
| Other | 11 (8) | 11 (8) | 37 (9) | 59 (9) |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Effects on DBP
Of the 654 participants enrolled, 619 were considered evaluable for the intent‐to‐treat population and 493 were evaluable for the primary ABPM analyses. To be evaluable for the primary ABPM analyses, patients had to have both a baseline and at least one on‐therapy ABPM assessment and have taken at least 2 weeks of study medication. Reductions with monotherapy from baseline in 24‐hour mean DPB ranged between approximately 4 mm Hg and 7 mm Hg for the model‐adjusted change, while, for the combination arms, these reductions ranged from approximately 7 mm Hg to 11 mm Hg (Table III). For trough DBP by 24‐hour ABPM, the reductions with monotherapy ranged from approximately 2 mm Hg to 8 mm Hg, and for the combination arms, ranged from approximately 5 mm Hg to 8 mm Hg. Figure 1 displays the change in baseline for the primary analyses for the high‐dose monotherapy groups, the highest combination group (carvedilol CR 80 mg/lisinopril 40 mg), and the two groups in which the highest doses of one monotherapy was combined with lower doses of the other agent. In the primary analyses, it was not possible to detect a combination arm that was significantly better than both its monotherapy components (Table IV).
Table III.
Summary of Hung AVE Test Results for the Co‐primary End Points
| Ambulatory BP Monitoring | Variable | Hung AVE Test Statistica | P Value |
|---|---|---|---|
| Diastolic BP, mm Hg | 24‐hour mean | −0.41499 | >.05 (NS)b |
| Trough (20–24 hours) | −0.00485 | Not applicablec |
Abbreviation: BP, blood pressure. aTests an average of the minimum gains (in response) for the 9 combination cells over the corresponding monotherapies. bThe nonsignificant (NS) P value implies that it was not possible to detect a fixed‐dose combination, which was more efficacious than both its monotherapy components as initial therapy in the treatment of hypertension. c P value was not suitable for inference because the previous P value for 24‐hour mean was NS.
Figure 1.
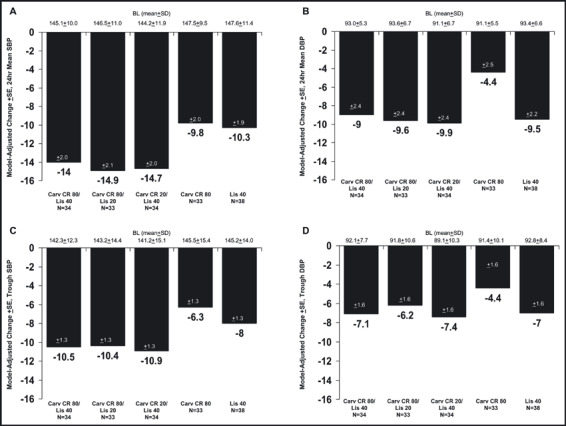
Model‐adjusted changes in blood pressure (mm Hg) measured by ambulatory blood pressure monitoring (efficacy population, last‐observation‐carried‐forward). Panel A, change from baseline (BL) in 24‐hour mean systolic blood pressure (SBP); Panel B, change from baseline in 24‐hour mean diastolic blood pressure (DBP); Panel C, change from baseline in trough SBP; Panel D, change from baseline in trough DBP. SD indicates standard deviation; SE, standard error of the mean; Carv CR, extended‐release carvedilol; Lis, lisinopril.
Table IV.
Change from Baseline in 24‐Hour Mean BP and Trough (20–24 hours) BP Measured by 24‐Hour ABPM (Efficacy Population With Last‐Observation‐Carried‐Forward)
| Treatment Group, No. | No.a | 24‐H Mean SBP/DBP by 24‐H ABPM | Trough SBP/DBP by 24‐H ABPM | ||
|---|---|---|---|---|---|
| Baseline, mean ± SD | Model‐Adjusted Change,b mean ± SE | Baseline, mean ± SD | Model‐Adjusted Change,b mean ± SE | ||
| Lis 10 | 29/29 | 145.6±11.1/93.9±5.1 | −7.5±2.1/−7.7±2.5 | 143.9±14.0/93.1±7.4 | −4.0±1.4/−4.2±1.7 |
| Lis 20 | 30/29 | 146.1±11.9/90.6±6.0 | −11.8±2.2/−7.6±2.6 | 140.0±11.9/88.3±7.2 | −7.4±1.4/−6.1±1.7 |
| Lis 40 | 38/38 | 147.6±11.4/93.4±6.6 | −10.3±1.9/−9.5±2.2 | 143.3±14.4/91.2±8.1 | −7.3±1.2/−8.1±1.5 |
| Carv CR 20 | 27/27 | 149.3±9.3/94.4±5.4 | −7.7±2.4/−1.7±2.8 | 146.9±13.2/94.9±9.0 | −5.5±1.5/−2.4±1.9 |
| Carv CR 40 | 34/34 | 146.8±10.6/93.4±6.4 | −9.8±2.0/−7.7±2.4 | 145.2±14.0/92.8±8.4 | −8.0±1.3/−7.0±1.6 |
| Carv CR 80 | 33/33 | 147.5±9.5/91.9±5.5 | −9.8±2.0/−4.4±2.5 | 145.5±15.4/91.4±10.1 | −6.3±1.3/−4.4±1.6 |
| Carv CR 20/Lis 10 | 35/33 | 143.6±10.4/92.4±6.0 | −13.5±2.0/−8.7±2.4 | 137.6±12.8/89.5±9.0 | −9.6±1.3/−6.0±1.6 |
| Carv CR 20/Lis 20 | 37/35 | 145.0±10.7/92.9±4.6 | −10.4±1.9/−7.5±2.3 | 141.2±13.7/90.6±8.2 | −7.4±1.2/−5.2±1.5 |
| Carv CR 20/Lis 40 | 34/34 | 144.2±11.9/91.1±6.7 | −14.7±2.0/−9.9±2.4 | 141.2±15.1/89.1±10.3 | −10.9±1.3/−7.4±1.6 |
| Carv CR 40/Lis 10 | 32/29 | 143.6±9.1/91.0±5.6 | −12.8±2.1/−10.7±2.6 | 140.4±10.4/89.3±7.9 | −9.7±1.3/−8.2±1.7 |
| Carv CR 40/Lis 20 | 35/33 | 142.5±10.4/91.4±5.8 | −14.2±2.0/−10.2±2.4 | 139.6±13.0/90.4±8.5 | −10.4±1.3/−8.2±1.6 |
| Carv CR 40/Lis 40 | 34/33 | 145.6±9.9/91.5±6.7 | −13.1±2.0/−6.4±2.4 | 138.3±14.5/87.4±11.6 | −9.6±1.3/−5.3±1.6 |
| Carv CR 80/Lis 10 | 28/28 | 146.2±13.1/91.5±6.2 | −16.1±2.2/−9.0±2.6 | 144.0±16.4/90.4±7.8 | −11.0±1.4/−5.7±1.7 |
| Carv CR 80/Lis 20 | 33/33 | 146.5±11.0/93.6±6.7 | −14.9±2.1/−9.6±2.4 | 143.2±14.4/91.8±10.6 | −10.4±1.3/−6.2±1.6 |
| Carv CR 80/Lis 40 | 34/34 | 145.1±10.0/93.0±5.3 | −14.0±2.0/−9.0±2.4 | 142.3±12.3/92.1±7.7 | −10.5±1.3/−7.1±1.6 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; Carv CR, carvedilol extended‐release; DBP, diastolic blood pressure; Lis, lisinopril; SBP, systolic blood pressure. aNumber of patients with a value at baseline and at end point for that measure. bBased on analysis of covariance: change=baseline+center+treatment.
Post hoc analyses of the mean change from baseline in 24‐hour mean DBP by ABPM and trough DBP by ABPM compared the combined data from participants who were randomized to 1 of the 5 combination arms that included either 80 mg of carvedilol CR or 40 mg of lisinopril (“high‐dose combination”) with high‐dose monotherapy, ie, 80 mg of carvedilol CR or 40 mg of lisinopril. Hypertension stage and race were included as covariates in these analyses because the significance of these effects had been demonstrated in the subgroup analyses of the primary efficacy variables (results not shown). Therefore, the post hoc analyses were based on an ANCOVA model with effects for treatment, center, corresponding baseline BP, and these two covariates. This analysis showed a statistically significant reduction in 24‐hour mean DBP by ABPM for the high‐dose combination group compared with monotherapy with carvedilol 80 mg or lisinopril 40 mg (Figure 2). The analysis performed for trough DBP by ABPM did not show a significant difference between the groups. The inability to demonstrate a significant difference at trough remains incompletely explained. It is likely multifactorial in nature, involving the small size of the treatment groups, leading to potential differences in important baseline characteristics, differences in the inclusion criteria as noted below, and increased variability than predicted in the trough DBP analyses.
Figure 2.
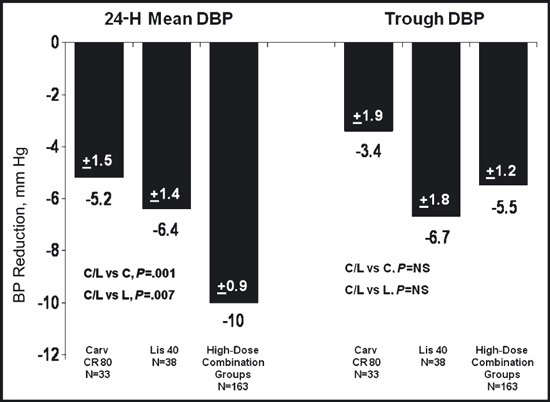
Model‐adjusted changes in blood pressure (mm Hg) measured by ambulatory blood pressure monitoring (efficacy population, last‐observation‐carried‐forward) comparing high‐dose monotherapy groups with the combined group who received either extended‐release carvedilol/lisonopril (Carv CR/Lis) 80/10 mg, 80/20 mg, 80/40 mg, 20/40 mg, and 40/40 mg. C indicates carvedilol; L, lisinopril; NS, not significant.
In an attempt to better understand the unexpected results of the trough analyses, an additional post hoc analysis was performed in which patients were only included if they met the same BP entry criteria that was used for the carvedilol CR registration study. 16 , 17 Specifically, patients were to meet qualifying BP criteria at both the baseline visit and the ABPM qualifying visit and to have a DBP >95 mm Hg. When these modified inclusion criteria were applied to the COSMOS database, the ABPM analyses showed similar results to the results described above.
Safety and Tolerability
No major safety concerns were observed during the course of the study. Overall, 52% of patients reported at least one treatment‐emergent adverse event (AE). The 5 most common treatment‐emergent AEs in the trial overall were headache (9%), dizziness (7%), cough (6%), fatigue (6%), and nausea (4%) (Table V). There were no notable differences among the carvedilol CR monotherapy group, lisinopril monotherapy group, and the combination of all the carvedilol CR/lisinopril groups. With the exception of dizziness, the incidence of individual AEs did not appear to increase with ascending doses. For carvedilol CR 20 mg/lisinopril 10 mg, carvedilol CR 20 mg/lisinopril 20 mg, and carvedilol CR 20 mg/lisinopril 40 mg, the incidence of dizziness increased from 2% to 7% to 14%, respectively. Dizziness did not appear to increase with dose across other combination treatment groups. There was a trend noted for increased fatigue reported for the combination groups containing carvedilol CR 40 mg and 80 mg, with carvedilol CR 80 mg/lisinopril 20 mg having the highest proportion of patients with fatigue (16%).
Table V.
Adverse Events
| Adverse Event, No. (%) | Lisinopril Monotherapy (n=129) | Carvedilol CR Monotherapy (n=130) | Carvedilol CR + Lisinopril Combination Therapy (n=391) | Total (N=650) |
|---|---|---|---|---|
| Headache | 12 (9%) | 16 (12%) | 31 (8%) | 59 (9%) |
| Dizziness | 9 (7%) | 10 (8%) | 27 (7%) | 46 (7%) |
| Cough | 10 (8%) | 1 (<1%) | 27 (7%) | 38 (6%) |
| Fatigue | 5 (4%) | 6 (5%) | 25 (6%) | 36 (6%) |
| Nausea | 5 (4%) | 5 (4%) | 13 (3%) | 23 (4%) |
| Diarrhea | 3 (2%) | 1 (<1%) | 17 (4%) | 21 (3%) |
| Upper respiratory tract infection | 5 (4%) | 0 | 14 (4%) | 19 (3%) |
| Back pain | 1 (<1%) | 4 (3%) | 11 (3%) | 16 (2%) |
| Nasopharyngitis | 2 (2%) | 2 (2%) | 11 (3%) | 15 (2%) |
| Influenza | 1 (<1%) | 0 | 10 (3%) | 11 (2%) |
| Sinus congestion | 1 (<1%) | 2 (2%) | 5 (1%) | 8 (1%) |
| Sinusitis | 0 | 2 (2%) | 5 (1%) | 7 (1%) |
| Myalgia | 3 (2%) | 0 | 4 (1%) | 7 (1%) |
| Dyspnea | 1 (<1%) | 1 (<1%) | 5 (1%) | 7 (1%) |
| Vertigo | 0 | 0 | 3 (<1%) | 3 (<1%) |
There were 16% (105 of 650) of patients overall who did not meet their target dose level. Although the majority of patients who did not reach their target dose level were in the higher dose groups, a review of these patients concluded that the reason they did not reach their target dose was mainly due to having met the criteria specified in the protocol to limit uptitration, rather than other safety‐related reasons or age.
There were no on‐therapy or post‐treatment deaths; one patient died due to a myocardial infarction on the first day of the placebo run‐in phase. Serious nonfatal AEs were reported for 2% of patients overall. No pattern for withdrawal due to AEs was observed for the monotherapy (3% and 6% for the lisinopril and carvedilol CR, respectively) or the combination therapy groups (5%). The highest proportion of patients with any on‐therapy AE leading to withdrawal was 12% (5 of 43) in the carvedilol CR 20‐mg group. The individual events leading to withdrawal were reported by only single patients in any of the treatment groups.
Discussion
COSMOS is the first study to evaluate the BP‐lowering effects of the combination of a vasodilating β‐blocker and an ACE inhibitor and, in this trial, the combination was initiated simultaneously in patients with stage 1 or 2 hypertension. The study evaluated all approved once‐daily doses of carvedilol CR (20 mg, 40 mg, and 80 mg), 3 of the commonly used approved doses of lisinopril for hypertension (10 mg, 20 mg, and 40 mg), and all of the possible combinations of these doses. The trial’s primary analyses did not demonstrate the superiority of at least one combination over both of its monotherapy components for the primary efficacy variables (mean 24‐hour DBP or trough DBP by ABPM). Nevertheless, the study did demonstrate a meaningful additive effect of carvedilol CR and lisinopril in reducing 24‐hour mean DBP from baseline when the 5 combinations that included the highest doses of carvedilol CR or lisinopril were combined and compared with the high‐dose monotherapy regimens.
Do the results of COSMOS further validate the commonly held position that combining agents with β‐blockade and RAS blockade in the treatment of hypertension does not produce significant further effect? The primary analyses of COSMOS were designed to identify, in the aggregate, whether any combination among the 9 tested would be superior to the monotherapy component, thus it was underpowered for evaluating individual group comparisons. Although the BP reductions observed were of at least marginal clinical relevance, the inability to demonstrate a statistically significant difference in BP reduction remains incompletely explained.
There are a number of potential factors related to the study design and conduct that may have influenced the results. Given the size of the trial, each arm evaluated 35 to 45 patients. No stratifications for demographic features were performed and some differences were noted among the regimens in the proportion of patients with demographic features that might have influenced their response to antihypertensive drugs (eg, age, race, hypertension stage). This trial included patients with either stage 1 or stage 2 hypertension, which may have led to the enrollment of a population close enough to target BP that the difference achieved in initiating combination therapy vs monotherapy could not be appreciated. A greater variability in the BP effect of the monotherapy regimens was noted than the variability assumed for sample size computations, and there was also greater variability than had been predicted in the trough ABPM results. This variability is particularly notable for the lower doses of lisinopril, as reflected in calculated trough to peak ratios approaching or exceeding 100%.
Previous and recent data have suggested that the addition of carvedilol to lisinopril produces significant BP reduction. In the GEMINI trial, where investigator‐determined doses and agent selection of ACE inhibitors or ARBs were used, lisinopril was the ACE inhibitor used most frequently. Titrated doses of carvedilol added to the ACE inhibitor noted additivity in reduction from baseline in office BP. 17 The results of one additional trial in which carvedilol CR was added to lisinopril has recently been reported. In the Comparable Blood Pressure Control and Left Ventricular Mass Reduction (CLEVER) trial, patients whose BP was uncontrolled on lisinopril 20 mg were randomized to receive either further titration with lisinopril or the addition of atenolol or carvedilol CR and titrated to target BP. 18 After 12 months there was a significant reduction in BP in all 3 groups as compared with baseline (carvedilol CR/lisinopril [N=83]: −19/−10 mm Hg; atenolol/lisinopril [N=92]: −20/−13 mm Hg; lisinopril [N=89]: −21/−11 mm Hg). These results taken together with the earlier GEMINI results continue to suggest the ability to achieve meaningful BP reduction with this combination. It is therefore possible that the results of COSMOS reflect the lesser benefit that is achieved when the 2 drugs are initiated at the same time in patients with hypertension (particularly in stage 1) combined with the protocol design factors noted above.
The benefit of combination therapy in improving compliance and adherence with drug therapy is recognized and is particularly important in high‐risk groups, such as patients with concomitant type 2 diabetes. The combination of β‐blockers, particularly vasodilating β‐blockers and ACE inhibitors, will continue to be used in clinical practice because of the prevalence of high‐risk comorbid conditions including coronary disease and heart failure. It is unlikely that this combination will undergo further formal clinical trial testing in hypertension and it will be important for the practitioner to evaluate all available data and individual patient response in determining the potential value of combining these drugs.
Conclusions
The superiority of initiating combination treatment with carvedilol CR and lisinopril compared with the monotherapy components was not demonstrated with the ABPM measurements. Nonetheless, the post hoc assessment combining all high‐dose groups did produce significant 24‐hour mean BP reduction when compared with the high‐dose monotherapy groups. The tolerability profile of initiating combination therapy was generally comparable to the initiation of treatment with monotherapy.
Disclosure: Drs Bakris and Weber designed this study with the help of the other authors. Drs Bakris and Weber are consultants for GlaxoSmithKline (GSK). Drs Ilenger, Lukas, and Ordronneau are employees of GSK. The COSMOS trial is registered at www.clinicaltrials.gov, study number NCT00347360.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Bakris GL. An approach to achieving recommended blood pressure goals in diabetic patients. Arch Intern Med. 2001;161:2661–2667. [DOI] [PubMed] [Google Scholar]
- 3. Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benzazeprilHCl versus comparable component‐based therapy. Chem Herit. 2003;9:324–332. [DOI] [PubMed] [Google Scholar]
- 4. Jamerson K, Weber MA, Bakris GL, et al.; ACCOMPLISH Trial Investigators . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008. ;359:2417–2428 [DOI] [PubMed] [Google Scholar]
- 5. Weinberger MH. Blood pressure and metabolic responses to hydrochlorothiazide, captopril, and the combination in black and white mild‐to‐moderate hypertensive patients. J Cardiovasc Pharmacol. 1985;7(suppl 1):S22–S55. [DOI] [PubMed] [Google Scholar]
- 6. Cappuccio FP, Markandu ND, Tucker FA, et al. Does a diuretic cause a further fall in blood pressure in hypertensive patients already on nifedipine? J Clin Hypertens. 1986;4:346–353. [PubMed] [Google Scholar]
- 7. Soininen K, Gerlin‐Piira L, Suihkonen J, et al. A study of the effects of lisinopril when used in addition to atenolol. J Hum Hypertens. 1992;6:321–324. [PubMed] [Google Scholar]
- 8. Huttunen M, Lampainen E, Lilja M, et al. Which anti‐hypertensive to add to a beta‐blocker: ACE inhibitor or diuretic? J Hum Hypertens. 1992;6:121–125. [PubMed] [Google Scholar]
- 9. Bursztyn M, Gavras I, Gourley L, et al. Effect of combination therapy with atenolol and the angiotensin‐converting enzyme inhibitor benazepril. Clin Ther. 1994;16:429–436. [PubMed] [Google Scholar]
- 10. Wing LM, Chalmers JP, West MJ, et al. Treatment of hypertension with enalapril and hydrochlorothiazide or enalapril and atenolol: contrasts in hypotensive interactions. J Hypertens. 1987;5:S603–S606. [PubMed] [Google Scholar]
- 11. Drayer JIM, Weber MA, Lipson JL, et al. Differential effects of diuresis and betaadrenoreceptor blockade during angiotensin converting enzyme inhibition in patients with severe hypertension. J Clin Pharmacol. 1982;22:179–186. [DOI] [PubMed] [Google Scholar]
- 12. Staessen J, Fagard R, Lijnen P, et al. Double‐blind comparison between propranolol and bendroflumethiazide in captopril‐treated resistant hypertensive patients. Am Heart J. 1983;106:321–328. [DOI] [PubMed] [Google Scholar]
- 13. Ruddy MC, Bialy GB, Kostis JB. Intrinsic sympathomimetic activity counteracts beta‐blocker inhibition of renin activation. Angiology. 1989;40:45–50. [DOI] [PubMed] [Google Scholar]
- 14. Wright JT Jr, Bakris GL, Bell DS, et al. Lowering blood pressure with beta‐blockers in combination with other renin‐angiotensin system blockers in patients with hypertension and type 2 diabetes: results from the GEMINI Trial. J Clin Hypertens (Greenwich). 2007;9:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hung HM, Chi GYH, Lipicky RJ. Testing for the existence of a desirable dose combination. Biometrics. 1993;49:85–94. [PubMed] [Google Scholar]
- 16. Weber MA, Bakris GL, Tarka EA, et al. Efficacy of a once‐daily formulation of carvedilol for the treatment of hypertension. J Clin Hypertens (Greenwich). 2006;8:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber MA, Sica DA, Tarka EA, et al. Controlled‐release carvedilol in the treatment of essential hypertension. Am J Cardiol. 2006;7A:32L–38L. [DOI] [PubMed] [Google Scholar]
- 18. Miller A, Reichek N, St. John Sutton M, et al. Late‐breaking abstracts presented at the ASH 24th Annual Scientific Meeting and Exposition, May 6–9, 2009, San Francisco, CA. Comparable blood pressure control and LV mass reduction in patients with hypertension and LVH with ACE inhibitor alone or combined with B‐blockade. J Clin Hypertens. 2009;11:396. [Google Scholar]


