Abstract
J Clin Hypertens (Greenwich). © 2010 Wiley Periodicals, Inc.
The study of genes and mechanisms associated with hypertension is hampered by the heterogeneity of hypertensive patients. Refining the definition of hypertension is a potential means of improving the clarity of mechanistic studies, but the lack of intermediate phenotypes hinders the assessment of causal relationships. Looking at younger individuals and hemodynamic subsets of hypertension is one such refinement. The authors argue that the separate analysis of patients with isolated diastolic hypertension, predominantly diastolic hypertension, and isolated systolic hypertension in the young in combination with common biomarkers may be an initial step to decrease heterogeneity within patient subsets, thus providing new avenues for genetic and pathophysiological studies.
Hypertension (HTN) is the most common risk factor for cardiovascular morbidity and mortality. 1 , 2 , 3 Despite extensive research, its etiology and genetic determinants remain elusive. Potential reasons for this inability to determine causality include epigenetic phenomena (DNA methylation, and post‐translational histone modifications), 4 gene‐environment interactions (such as obesity, salt intake, and exercise), lack of adequate control groups, and maybe even the fact that genetic influence of HTN may not be as important as previously proposed. Another option is that many common genetic variants, each with a small contribution, participate in maintaining blood pressure (BP) levels, but as noted further in this article, recent reports question this concept. More likely is the possibility that HTN is due to numerous distinct genes. Many different knockout experiments have shown increased BP in animals and support this concept. If this is the case, finding intermediate phenotypes becomes imperative. “Lumping” all hypertensives together and searching for a common cause will continue to be an unrewarding task. While this is not a new idea, the lack of articles reporting this approach is surprising. We propose a simple approach toward subdividing hypertensive phenotypes that may help elucidate genetics and pathophysiology further.
The Need for the Identification of More Specific Phenotypes in HTN
In recent years the search for homogeneous phenotypes within HTN has furthered our understanding of monogenic Mendelian HTN. 5 Initially focusing on severe familiar HTN and hypokalemia, Lifton and colleagues have found genes that can cause elevated and decreased BP. We now understand why some patients previously classified as having “essential HTN” with hypokalemia have different pathophysiologic mechanisms than those with just plain elevation of BP, but it took insightful vision and hard work to prove that these former “essential hypertensives” were unique. Unfortunately, the phenotypes and genes encountered so far only apply to a small fraction of the millions of hypertensive patients worldwide. 5 This led to the thought that finding common genetic variants would solve the problem, and the genome wide association study (GWAS) approach was developed. The 2 largest current studies have identified several loci associated with HTN, 6 , 7 but other important GWAS failed to do so, 8 and there is always concern about the reproducibility of results. 9 An interpretation of recent findings was that the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)6 and the Global Blood Pressure Genetics (Global BPgen)7 were able to identify, despite some significant inconsistencies, genetic loci that accounted for only about 2% of the genetic factors believed to influence BP. The loci were associated with an estimated effect on BP of 1 mm Hg or less. It is likely that many other loci exerting even smaller effects (<0.5 mm Hg) exist. 10 These studies highlight the limited likelihood that any significant findings will be further obtained with this methodology.
How can we subdivide essential hypertensive patients into more homogenous subgroups? Phenotypic biomarkers can help in this distinction because they integrate multiple genetic and nongenetic influences. Several potential intermediate phenotypes have recently emerged, including common laboratory biomarkers such as hyperuricemia, or a combination of biomarkers and clinical features such as in the metabolic syndrome. An exciting recent publication demonstrated that allopurinol decreased BP in adolescents with hyperuricemia. 11 Not all adolescents have hyperuricemia and surely these results will have to be confirmed in larger studies, but this is an example of how looking at the correct subgroup may help understand mechanisms and appropriate therapy. It will be interesting if a drug typically without antihypertensive action, like allopurinol, turns out to be appropriate BP‐lowering therapy in some hyperuricemic patients.
An example of “teasing out” complex traits with well established phenotypes was recently published. Using linkage analysis Mani and associates found a gene (LRP6) that associates with early coronary artery disease, metabolic syndrome, and osteoporosis in affected family members. 12 All these patients had “garden variety” metabolic syndrome: their diabetes mellitus and HTN appeared at midlife, cholesterol values were not as elevated as seen in heterozygous loss of function of the low‐density lipoprotein receptor, but they stood out because of coronary artery disease before age 50 in men and 55 in women, and osteoporosis. Impressive is the fact that unaffected members did not have metabolic syndrome despite similar lifelong habits. Analysis of the metabolic syndrome indicates that it probably represents a more homogenous subgroup than the universe of essential hypertensives, but the definition is still a “work in progress” that includes individuals with different components of the syndrome, even the absence of elevated BP. 13 , 14 Thus, it is likely that these patients will have many genes and mechanisms involved in their HTN. 15 This example epitomizes the need to search for more specific hypertensive phenotypes, because in clinical practice apparently unrelated associations of HTN with kidney stones, early atherosclerosis, osteoporosis, and gastritis, to mention a few, are commonly encountered.
Research on this approach should not stop short of completion, as happened in the past with the evaluation of plasma renin activity in HTN. The division of all hypertensive patients into high, normal, and low renin status was initially perceived as the holy grail in HTN, but fell out of favor as research failed to demonstrate that it led to significant advances in the understanding of pathophysiology and treatment. Current view is that each of these categories probably harbors many different subcategories (increased volume status and low renin can be achieved by various renal tubular mechanisms), but this complexity should not hinder the importance of renin or other biomarkers in defining phenotypes.
Pulse Pressure as a Potentially Relevant Phenotypical Trait in HTN
We propose that the characterization of phenotypes should take age and pulse pressure (PP) patterns into consideration. Why should a 30 year‐old patient with a BP of 130/100 mm Hg have the same genetic background and physiology as a 70 year‐old recently diagnosed as hypertensive with a BP of 160/80 mm Hg? Our current definition of HTN as systolic BP >140 mm Hg and/or diastolic BP >90 mm Hg 16 has led multiple candidate gene and genome wide scans to analyze these patients together. We must realize that this definition comes from clinical trials and its continued use for understanding mechanisms in HTN is unlikely to be helpful. Along the same lines, why would a 17 year‐old with a BP of 156/84 mm Hg and a 24 year‐old with a BP of 130/100 mm Hg have the same genes and the same physiology to explain their newly diagnosed HTN? Therefore, we will focus our discussion on the isolated systolic HTN (ISH) and isolated diastolic HTN (IDH) subtypes in young patients.
Isolated Systolic HTN
ISH is commonly recognized because this “PP phenotype” has increased cardiovascular risk 17 , 18 , 19 and drug treatment can diminish it. 20 , 21 , 22 These patients are arbitrarily defined as having systolic BP >140 mm Hg and diastolic BP <90 mm Hg. They tend to be older and most have advanced cardiovascular disease. Given their typically older age and longer exposure to the “environment,” this phenotype is quite heterogeneous and generally less suited for genetic studies. Despite this presumed limitation, a metaanalysis of several GWAS of HTN and elevated PP revealed 5 chromosomal regions with evidence for linkage with PP. 23 In fact, the Framingham Study demonstrated that an ISH phenotype in the elderly can be preceded by systo‐diastolic HTN (SDH) and IDH, but is more likely to evolve from normal or high‐normal BP. 24 This finding suggests that ISH is not always the final step of “burnt‐out” high BP, 25 and we speculate that patients who first become hypertensive as “de novo” ISH may have different genes and mechanisms than those that end up with ISH after many years of SDH.
A distinct “wide PP HTN phenotype” is found in young adults with ISH. These individuals were initially characterized as tall males with elevated peripheral BP and normal central aortic pressures. 26 , 27 It was speculated that the reason for the dichotomy between central and peripheral BP was an exaggeration of PP amplification by enhanced arterial elasticity. Therefore the pulse waveform is of normal characteristics in the periphery, but with a taller peak, whereas the central pulse waveform is entirely normal. This hemodynamic pattern induced many authors to name this entity “pseudo‐systolic HTN” or “spurious systolic HTN.” 27 However, the ENIGMA study recently disputed this concept and demonstrated that the PP amplification is normal in these individuals (Table). 28 These authors claim that ISH is the most common form of HTN in young adults, and some investigators have recognized this condition as a common phenotype, 29 though others have found more IDH than ISH. 30 Some reports have speculated that the increased PP pattern in young patients with ISH is due to elevated stroke volume with normal peripheral vascular resistance, 31 but the ENIGMA investigators demonstrated that this entity is heterogeneous. Some young adults with ISH have increased stroke volume while others have increased stroke volume and aortic pulse wave velocity. In fact, they have conjectured that these individuals may later evolve into a more predominantly diastolic pattern of high BP. 28 Another subgroup, approximately 20% of young ISH hypertensives, have normal stroke volume and increased pulse wave velocity, clearly mimicking the hemodynamic pattern of older ISH patients and may represent an earlier presentation of elevated BP due to a stiff aorta. 28 This finding casts doubt on the contention that all ISH in young individuals is benign. Also, it should be noted that Framingham data showed an inverse relationship between brachial PP and coronary events in men <40 years of age. 32 Furthermore, it is not known whether these patients remain with ISH, or if the decline of ISH in young patients is because their PP pattern normalizes or evolves into a more diastolic HTN. 30 , 33 ENIGMA and the Hypertension and Ambulatory Recording Venetia Study (HARVEST) are prospective cohorts that may help answer this question. Without long term studies, the indication for pharmacological therapy is still a matter of debate.
Table.
Demographics and Blood Pressure Indices in Normotensive, Isolated Systolic Hypertension (ISH), and Essential Hypertension (EH) Patients
| Parameter | Normotensive (n=722) | ISH (n=93) | EH (n=42) |
|---|---|---|---|
| Age, y | 20±3 | 20±3 | 20±3 |
| Male/female | 330/392 | 85/8 | 23/19 |
| Height, m | 1.71±0.09 | 1.79±0.07a | 1.70±0.09a,b |
| Weight, kg | 68±13 | 82±14a | 75±17a |
| BMI, kg/m 2 | 23.1±3.6 | 25.7±4.1a | 25.8±4.6a |
| PP amplificationc | 1.69±0.14 | 1.72±0.11 | 1.63±0.2a,b |
| CO, L/minc | 6.9±1.9 | 8.1±1.9a | 6.8±1.7b |
| SV (mL)c | 83±21 | 93±24a | 78±18b |
| PVR, dynes/sc | 12.6±4.6 | 12.5±3.4 | 15.9±4.3a,b |
Data are mean±SD (n=857). Patients with high‐normal blood pressure were excluded (n=151). a P<.01 vs normotensives. b P<.01, EH vs ISH. cIndicates data corrected for mean arterial pressure and sex. Table compiled from data from reference 28. Abbreviations: BMI, body mass index; CO, cardiac output; PP, pulse pressure; PVR, peripheral vascular resistance; SV, stroke volume.
This is a good example of a not generally recognized intermediate phenotype that has not been studied properly. If each ISH subgroup is carefully selected by hemodynamic parameters and different biomarkers, a candidate gene approach or similar will have a greater likelihood of genetic and pathophysiologic yield as long as an adequate sample size of phenotypically similar individuals are studied.
Isolated Diastolic (and Predominantly Diastolic) HTN
IDH has taken longer to be recognized than “classical” ISH, as early reports described this BP profile as being associated with very low cardiovascular risk. 34 , 35 , 36 , 37 This is not surprising as patients with IDH tend to be younger than SDH and ISH and their BP category is usually stage I. 24 , 33 However, this group has become a focus of attention because cardiovascular risk is mostly associated with diastolic BP until age 55. 24 , 38 , 39 A Chinese report demonstrates that, although less than ISH and SDH, patients with IDH have higher rates of cardiovascular disease than normotensive individuals. 40 Patients with IDH are usually young adults and studies uniformly show increased small vessel resistance. 28 It has been postulated that IDH can progress and most patients with SDH have had IDH in their early hypertensive years. 24 An analysis of the Framingham Study demonstrated that patients with IDH at baseline had a seven‐fold increase in the 10‐year hazard of developing SDH as compared to individuals who were normotensive at inception. In addition, the younger the patient stratum analyzed, the higher the predominance of IDH as the most common subtype, 33 and IDH patients were on average 2–9 years younger than SDH patients. 24 , 34 , 41 , 42 It is thus plausible that IDH and SDH may represent the same pathophysiologic process at different time points in a patient’s clinical course (Figure). Because patients with SDH can have narrow, normal, or wide PP, we believe that patients with narrow PP may have similar physiology as IDH. We have named this subgroup with narrow PP “predominantly diastolic HTN” (PDH), a group of patients where diastolic BP is the most relevant alteration, either in absolute terms or in terms that integrate the degree of elevation of diastolic BP and PP. 43 Along the same lines, Blank and associates arbitrarily defined this group as having a PP/diastolic BP ratio <0.45. 34 This number was arbitrarily chosen based on what the observed ratio would be for a hypothetical patient with a high diastolic BP (90 mm Hg) and a “normal” systolic BP (130 mm Hg; PP=40 mm Hg, PP/diastolic BP ratio ∼0.45).
Figure.
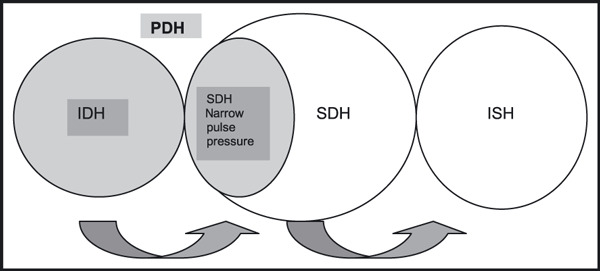
Predominantly diastolic hypertension (PDH) encompasses isolated diastolic hypertension (IDH) and systo‐diastolic hypertension (SDH) with a narrow pulse pressure. Arrows on the bottom demonstrate how progression of disease can occur (from IDH to SDH and then isolated systolic hypertension [ISH]), but patients may become hypertensive at any stage and also skip or reverse categories.
We speculate that patients with PDH share similar hemodynamic patterns and are a more homogeneous subtype of HTN. Unfortunately, we are not aware of any studies that have addressed this issue. It must be stressed that this is not the same subgroup of “borderline” hypertensives described in the past. Borderline hypertensives have been defined as patients who had at least one diastolic BP with a value of 90 mm Hg or more and at least another value of less than 90 mm Hg on 3 casual BP determinations, 44 or who had BP intermittently above 150/90 mm Hg, with average readings above 150/90 mm Hg but below 160/100 mm Hg. 45 Also, these patients differ from the group studied by the Bergen Study. These were young patients with mild HTN and mean BP 152/92 mm Hg and 160/99 mm Hg at the initial visit. 46
Jimenez and colleagues have recently described a preliminary association between PDH and the angiotensin‐converting enzyme DD polymorphism in a small number of untreated patients with PDH. 43 Whether this is a true finding or not will have to be tested in a larger number of patients, but similar observations were reported in the Framingham cohort, wherein an association of DD genotype and diastolic BP was observed in men. 47 The definition of PDH is an attempt at finding more homogeneous phenotypes within a large number of patients with essential HTN. IDH prevalence is as high as 23% in some studies 48 and if patients with SDH who have narrow PP are added to this group, it may account for 30% to 40% of essential HTN. It also opens a series of other questions. Are there other subgroups within it? There are several mechanisms that can increase diastolic BP, so are blood vessel ion channels involved? Is the renin‐angiotensin system or any other vasopressor hormone system involved? Do they have a higher prevalence of metabolic syndrome 48 or increased body mass index 49 as recent publications have demonstrated (though not confirmed by others)? 30 , 40 In our cohort we found a low average body mass index challenging the fact that IDH is seen in obese patients with metabolic syndrome. 43 We look forward to further studies in the IDH field. Again, despite heterogeneity within this group, judicious IDH subset subdivision is expected to increase the chance of success in genetic association studies.
“Hyperdynamic” HTN
Another hypertensive phenotype described in young individuals many years ago is the “hyperkinetic” or “hyperdynamic” state. 50 , 51 , 52 , 53 , 54 This hypertensive pattern has been linked to excessive activation of the sympathetic nervous system 45 and sometimes in combination with obesity, 55 although activation of the sympathetic nervous system as a cause of HTN in obese patients has many caveats. 56 These patients may have clinical signs and symptoms of sympathetic overactivity (tachycardia, warm skin, sweaty palms and soles, palpitations, etc.). 50 These previous studies claim that this would be an early clinical state that later develops into a more classical pattern of essential HTN. 51 , 52 , 53 , 57 In the ENIGMA study, some of the patients with ISH fit this pattern of increased stroke volume alone or in association with increased pulse wave velocity. 28 Both borderline hypertensive groups described by Safar and colleagues 44 (normal and high cardiac index patients) had ISH based on reported BP determinations. In our clinical experience, we have observed young hyperdynamic patients who have diastolic predominance. The above reaffirms the concept that young patients with ISH and PDH are most likely heterogeneous hypertensive groups and that hyperdynamic subtypes may be seen in each of these phenotypes, not necessarily having the same genetics and pathophysiology.
It is of interest that Widimsky and associates 50 recognized that 70% of young patients with HTN studied had increased cardiac output. They found that more than half had stroke volume elevation without tachycardia, and less often tachycardia with an increase in stroke volume.
Per Lund‐Johansen was among the pioneers that studied the hemodynamics of early mild essential HTN in young patients. His group and others found that hypertensive patients had a 15% higher cardiac index and heart rate with “normal” peripheral vascular resistance, although some have interpreted these “normal” values as inappropriately high for the observed high cardiac index. 46 The theory at the time was that the tissue over‐perfusion led to peripheral vasoconstriction and when cardiac output tended to normalize, high BP was maintained by increased peripheral resistance. 46 But even many years ago, controversy existed because not all borderline hyperkinetic patients displayed these characteristics. 58 Hyperdynamic hypertensive patients have been treated intuitively and experimentally with β‐blockers, 45 successfully controlling BP and heart rate and/ or palpitations, but no long‐term data are available.
In summary, HTN research should progress toward a fresh look at new onset HTN in young people now that we have more insight about how heterogenous these intermediate phenotypes can be. This is likely to be the path that will lead us to a better understanding of HTN. Since younger patients have potentially less environmental influence on their BP (and are therefore better suited for genetic and/or mechanistic protocols), an intensified attempt at defining phenotypes in this age group is essential. Among young hypertensives, we believe ISH and PDH stand out as interesting phenotypes that need further characterization and subdivision.
Disclosure: This work has not been published in its current form or a substantially similar form (in print or electronically, including on a Web site), has not been accepted for publication elsewhere, and is not under consideration by another publication. The authors have no conflicts of interest to disclose.
References
- 1. Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. [DOI] [PubMed] [Google Scholar]
- 2. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. [DOI] [PubMed] [Google Scholar]
- 3. Primatesta P, Brookes M, Poulter NR. Improved hypertension management and control: results from the health survey for England 1998. Hypertension. 2001;38:827–832. [PubMed] [Google Scholar]
- 4. Schones DE, Zhao K. Genome‐wide approaches to studying chromatin modifications. Nat Rev Genet. 2008;9:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. [DOI] [PubMed] [Google Scholar]
- 6. Levy D, Ehret GB, Rice K, et al. Genome‐wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newton‐Cheh C, Johnson T, Gateva V, et al. Genome‐wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wellcome Trust Case Control Consortium . Genome‐wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NCI‐NHGRI Working Group on Replication in Association Studies . Replicating genotype‐phenotype associations. Nature. 2007;447:655–660. [DOI] [PubMed] [Google Scholar]
- 10. Harrap S. Blood pressure genetics: time to focus. J Am Soc Hypertens. 2009;3:231–237. [DOI] [PubMed] [Google Scholar]
- 11. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Diabetes Federation . The IDF consensus worldwide definition of the metabolic syndrome. Lancet. 2005;366:1059–1062. 16182882 [Google Scholar]
- 14. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 15. Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. [DOI] [PubMed] [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 17. Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation. 1999;100:354–360. [DOI] [PubMed] [Google Scholar]
- 18. Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long‐term cardiovascular mortality in a french male population. Hypertension. 1997;30:1410–1415. [DOI] [PubMed] [Google Scholar]
- 19. Domanski M, Mitchell G, Pfeffer M, et al. Pulse pressure and cardiovascular disease‐related mortality: follow‐up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 2002;287:2677–2683. [DOI] [PubMed] [Google Scholar]
- 20. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 21. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (SYST‐EUR) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 22. Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst‐China) Collaborative Group. J Hypertens. 1998;16:1823–1829. [DOI] [PubMed] [Google Scholar]
- 23. Zintzaras E, Kitsios G, Kent D, et al. Genome‐wide scans meta‐analysis for pulse pressure. Hypertension. 2007;50:557–564. [DOI] [PubMed] [Google Scholar]
- 24. Franklin SS, Pio JR, Wong ND, et al. Predictors of new‐onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation. 2005;111:1121–1127. [DOI] [PubMed] [Google Scholar]
- 25. Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 26. O’Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth. Vasc Med. 2000;5:141–145. [DOI] [PubMed] [Google Scholar]
- 27. Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens. 2003;16:229–232. [DOI] [PubMed] [Google Scholar]
- 28. McEniery CM, Yasmin, Wallace S, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. [DOI] [PubMed] [Google Scholar]
- 29. Mallion JM, Hamici L, Chatellier G, et al. Isolated systolic hypertension: data on a cohort of young subjects from a French working population (IHPAF). J Hum Hypertens. 2003;17:93–100. [DOI] [PubMed] [Google Scholar]
- 30. Saladini F, Dorigatti F, Santonastaso M, et al. Natural history of hypertension subtypes in young and middle‐age adults. Am J Hypertens. 2009;22:531–537. [DOI] [PubMed] [Google Scholar]
- 31. Galarza CR, Alfie J, Waisman GD, et al. Diastolic pressure underestimates age‐related hemodynamic impairment. Hypertension. 1997;30:809–816. [DOI] [PubMed] [Google Scholar]
- 32. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 33. Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle‐aged and elderly US hypertensives: analysis based on National Health And Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 34. Blank SG, Mann SJ, James GD, et al. Isolated elevation of diastolic blood pressure. Real or artifactual? Hypertension. 1995;26:383–389. [DOI] [PubMed] [Google Scholar]
- 35. Pickering TG. Isolated diastolic hypertension. J Clin Hypertens (Greenwich). 2003;5:411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hozawa A, Ohkubo T, Nagai K, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self‐measurement of blood pressure at home: the Ohasama Study. Arch Intern Med. 2000;160:3301–3306. [DOI] [PubMed] [Google Scholar]
- 37. Strandberg T, Salomaa V, Vanhanen H, et al. Isolated diastolic hypertension, pulse pressure, and mean arterial pressure as predictors of mortality during a follow‐up of up to 32 years. J Hypertens. 2002;20:399–404. [DOI] [PubMed] [Google Scholar]
- 38. Sesso HD, Stampfer MJ, Rosner B, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801–807. [DOI] [PubMed] [Google Scholar]
- 39. Glynn RJ, L’Italien GJ, Sesso HD, et al. Development of predictive models for long‐term cardiovascular risk associated with systolic and diastolic blood pressure. Hypertension. 2002;39:105–110. [DOI] [PubMed] [Google Scholar]
- 40. Kelly TN, Gu D, Chen J, et al. Hypertension subtype and risk of cardiovascular disease in Chinese adults. Circulation. 2008;118:1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fang J, Madhavan S, Cohen H, et al. Isolated diastolic hypertension: a favorable finding among young and middle‐aged hypertensive subjects. Hypertension. 1995;26:377–382. [DOI] [PubMed] [Google Scholar]
- 42. Nielsen WB, Lindenstrom E, Vestbo J, et al. Is diastolic hypertension an independent risk factor for stroke in the presence of normal systolic blood pressure in the middle‐aged and elderly? Am J Hypertens. 1997;10:634–639. [DOI] [PubMed] [Google Scholar]
- 43. Jimenez PM, Conde C, Casanegra A, et al. Association of ACE genotype and predominantly diastolic hypertension: a preliminary study. J Renin Angiotensin Aldosterone Syst. 2007;8:42–44. [DOI] [PubMed] [Google Scholar]
- 44. Safar ME, Weiss YA, Levenson JA, et al. Hemodynamic study of 85 patients with borderline hypertension. Am J Cardiol. 1973;31:315–319. [DOI] [PubMed] [Google Scholar]
- 45. Julius S, Esler M. Autonomic nervous cardiovascular regulation in borderline hypertension. Am J Cardiol. 1975;36:685–696. [DOI] [PubMed] [Google Scholar]
- 46. Lund‐Johansen P. Haemodymnamics in early essential hypertension‐‐still an area of controversy. J Hypertens. 1983;1:209–213. [DOI] [PubMed] [Google Scholar]
- 47. O’Donnell CJ, Lindpaintner K, Larson MG, et al. Evidence for association and genetic linkage of the angiotensin‐converting enzyme locus with hypertension and blood pressure in men but not women in the Framingham Heart Study. Circulation. 1998;97:1766–1772. [DOI] [PubMed] [Google Scholar]
- 48. Franklin SS, Barboza MG, Pio JR, et al. Blood pressure categories, hypertensive subtypes, and the metabolic syndrome. J Hypertens. 2006;24:2009–2016. [DOI] [PubMed] [Google Scholar]
- 49. Wu H, Xu J, Zhuo L, et al. Comparison of risk factors associated with hypertension subtypes by classification tree method in Tongshan County of Jiangsu Province, China. Am J Hypertens. 2009;22:1287–1294.Epub 2009 Oct 1. [DOI] [PubMed] [Google Scholar]
- 50. Widimsky J, Fejfarova M, Fejfar Z. Changes of cardiac output in hypertensive disease. Cardiologia. 1957;31:381–389. [DOI] [PubMed] [Google Scholar]
- 51. Julius S, Pascual AV, London R. Role of parasympathetic inhibition in the hyperkinetic type of borderline hypertension. Circulation. 1971;44:413–418. [DOI] [PubMed] [Google Scholar]
- 52. Messerli FH, Frohlich ED, Suarez DH, et al. Borderline hypertension: relationship between age, hemodynamics and circulating catecholamines. Circulation. 1981;64:760–764. [DOI] [PubMed] [Google Scholar]
- 53. Julius S, Krause L, Schork NJ, et al. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens. 1991;9:77–84. [DOI] [PubMed] [Google Scholar]
- 54. Jiang X, Srinivasan SR, Urbina E, et al. Hyperdynamic circulation and cardiovascular risk in children and adolescents. The Bogalusa Heart Study. Circulation. 1995;91:1101–1106. [DOI] [PubMed] [Google Scholar]
- 55. Sorof JM, Poffenbarger T, Franco K, et al. Isolated systolic hypertension, obesity, and hyperkinetic hemodynamic states in children. J Pediatr. 2002;140:660–666. [DOI] [PubMed] [Google Scholar]
- 56. Esler M, Straznicky N, Eikelis N, et al. Mechanisms of sympathetic activation in obesity‐related hypertension. Hypertension. 2006;48:787–796. [DOI] [PubMed] [Google Scholar]
- 57. Fouad FM, Tarazi RC, Dustan HP, et al. Hemodynamics of essential hypertension in young subjects. Am Heart J. 1978;96:646–654. [DOI] [PubMed] [Google Scholar]
- 58. Lund Johansen P. Essential hypertension: hemodynamic and therapeutic changes over 20 years. J Cardiovasc Pharmacol. 1991;18:S1–S7. [PubMed] [Google Scholar]


