Abstract
Dopamine is an endogenous natriuretic amine that contributes to the maintenance of sodium homeostasis. Deficiencies in the renal production of dopamine and the action of dopamine on renal tubular receptors have been observed in human hypertension and may contribute to salt sensitivity of blood pressure. Ethnic differences in the sodium‐to‐dopamine relationship may contribute to the higher prevalence of salt sensitivity in blacks. The authors assessed dopaminergic activity in two studies. In the first, daytime and nighttime excretion of sodium and dopamine were compared in 11 black and 17 white normotensive patients. No racial difference in the rate of sodium or dopamine excretion during either period was observed. In the second study, a graded infusion of the dopamine‐1 receptor agonist, fenoldopam, was performed in 14 black and 17 white normotensive patients. There was no racial difference in the natriuretic responses. Previously described lower rates of renal free water clearance and potassium excretion in blacks compared with whites were maintained during fenoldopam infusion, suggesting that dopamine is not a mediator of those differences. The authors conclude that there are no race‐related differences in dopamine excretion or activity in normotensive patients.
The increase in urinary dopamine (DA) excretion that results from increasing dietary sodium (Na) intake 1 , 2 , 3 , 4 or infusing Na intravenously 4 , 5 is part of a set of homeostatic responses that maintain overall Na balance. In some hypertensive patients, DA excretion fails to increase with Na loading, 6 and the defect seems to be associated with blood pressure (BP) responsiveness. Hypertensives whose BP does not change with increased dietary Na (non–salt sensitive [NSS]) have greater Na and DA excretion than those whose BP increases (salt sensitive [SS]). 7 , 8
An ethnic difference in DA‐dependent Na handling was suggested by the observation that West Africans do not have the expected positive relationship between urinary Na and DA excretion and do not show an increase in DA excretion with Na loading. 9 Lee provided further support for ethnic differences in a report on 5 populations, 2 of which failed to show a relationship between urinary DA and Na excretion. 10 Sowers and colleagues 11 demonstrated that urinary excretion of DA increases less in black hypertensives compared with normotensives and suggested that the generally smaller Na‐induced responses in urinary DA in blacks compared with whites might reflect the greater prevalence of SS in blacks. Finally, Damasceno and colleagues 12 demonstrated that an acute Na infusion provokes greater DA excretion in black NSS compared with SS patients.
Infusion of DA at low doses (≤3 μg/kg/min) suppresses both proximal and distal tubular Na reabsorption and promotes natriuresis and diuresis. 13 , 14 , 15 , 16 An ethnic difference in DA responsiveness was reported by Marinac and colleagues 17 who compared renal responses with low‐dose DA infusion in black and white normotensive patients and reported that Na excretion increased in whites but not in blacks. DA is an agonist for an entire family of DA receptors, so fenoldopam, a selective DA‐1 agonist, has been used to investigate the role of that receptor subtype. Like low‐dose DA, intravenous infusion of fenoldopam increases Na excretion in Na‐replete humans through both proximal and distal tubular actions. 18 , 19 To our knowledge there are no reports of racial differences in renal responses to fenoldopam, and we performed the studies reported here to address that issue.
Methods
Some of the methods for and data from these studies have been reported previously. 20 Both studies were approved by the University of Michigan’s institutional review board, and all participants gave written informed consent. Patients were healthy normotensive (seated auscultatory BP <140/90 mm Hg) black and white (non‐Hispanic) men and women, aged 18 to 50 years; race was self‐determined. A general health history was obtained and a physical examination performed to exclude other diseases including cancer, recent (within 6 months) stroke or myocardial infarction, diabetes, liver disease, chronic infections, and psychiatric disease of sufficient severity to interfere with a patient’s ability to adhere to the protocol. We screened for occult disease with a complete blood cell count, a thyroid‐stimulating hormone level, a comprehensive automated biochemical profile, and urinalysis. All values were required to be normal, including a serum creatinine (Cr) of <1.3 mg/dL for women and <1.5 mg/dL for men and a serum potassium (K) >3.5 mmol/L. Pregnancy was excluded by a rapid urine pregnancy test. Drug therapies that could affect renal tubular Na handling (eg, diuretics, nonsteroidal anti‐inflammatory drugs, caffeine, and theophylline) were not permitted.
Study 1
On day 1, patients who had previously agreed to participate in the study came to the outpatient research facility where the details of the protocol were reviewed, a medical history was obtained, and a physical examination was performed. An automated BP monitor (Spacelabs model 90207; Spacelabs, Inc, Redmond, WA) was placed and BP was recorded throughout the study period. Before leaving the facility, patients voided and were instructed to begin collecting all urine in 2 plastic jugs with 30 mL of 6 N HCl added to each jug. “Day” was considered the period from leaving the research facility until retiring to sleep, and “night” was the time from retiring through awakening, at which time patients collected their first morning void, completing the nighttime collection. The times of retiring and awakening were recorded. Patients were instructed to collect any voids during the night in the night jug. Blood was obtained when the patients returned the urine collections.
Urine volume and Na, K, Cr, osmolality (Osm), and DA concentrations were measured in both urine specimens. Serum and urine Na and K were measured by flame photometry and plasma Osm by freezing point depression. We measured urinary DA by high performance liquid chromatography with electrochemical detection. 21 Serum and urine Cr assays were performed using a modified Jaffe reaction. Osm was measured by freezing point depression.
Study 2
Patients consuming their usual diet came to an outpatient research facility at the University of Michigan Medical Center during the morning. Patients were asked to drink water prior to coming, as we wanted to ensure that they were well hydrated and could produce adequate urine volumes during the study. On arrival they were asked to drink 12 oz (360 mL) of water as rapidly as possible. An intravenous infusion of 5% glucose in water was begun and maintained throughout the study at a rate of 200 mL/h. Fenoldopam was intravenously infused for 3 hours in progressively increasing doses. Doses for the first three 30‐minute periods were 0.01, 0.05, and 0.10 μg/kg/min. For the last 90 minutes the dose was 0.20 μg/kg/min (Figure 1). BP and heart rate (HR) were monitored by automated oscillometry every 10 minutes and did not exceed prespecified limits of a systolic BP of 100 mm Hg or an HR of 110 beats per minute in any patient; values reported are 30‐minute averages. Beginning with the start of the fenoldopam infusion, urine samples were collected every 30 minutes. Blood was obtained at the start of the infusion and at the end of each hour for measurements of serum Na, K, Cr, and Osm; the same measurements were obtained for all urine samples. Urinary clearances (ClX) were calculated by the standard formula: 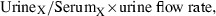 where X can be Na, K, Cr, or Osm. Fractional excretion (FE X) was calculated as
where X can be Na, K, Cr, or Osm. Fractional excretion (FE X) was calculated as 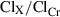 . Urinary‐free water clearance (ClH2O) was calculated as urine flow rate−ClOsm. Distal Na to K exchange was calculated as
. Urinary‐free water clearance (ClH2O) was calculated as urine flow rate−ClOsm. Distal Na to K exchange was calculated as 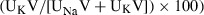 where V is urine volume.
6
where V is urine volume.
6
Figure 1.
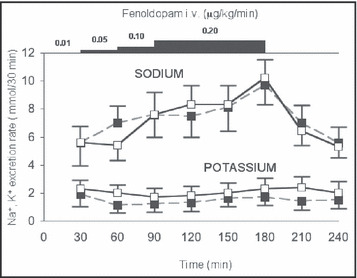
Sodium (Na) and serum potassium (K) excretion (mean ± standard error) in blacks (n=14, filled squares) and whites (n=17, open squares) during graded infusion of fenoldopam in study 2. Responses in Na excretion between blacks and whites were not significantly different. Excretion of K did not change significantly during the infusion and was significantly greater in whites throughout the study.
Statistical comparisons between races were by unpaired t test, and trends over time were assessed by analysis of variance (ANOVA). For urine studies, the main racial comparison was between the average values for the 90 minutes of the highest‐dose (0.2 mg/kg/min) fenoldopam infusion. Statistical significance was accepted at the P<.05 level. All data are expressed as mean ± standard deviation, except in the Figures, where standard error is used for graphic clarity.
Results
Study 1
Characteristics of patients are shown in Table I. There was no racial difference in the rate of Na or DA excretion during the daytime or nighttime periods (Table II). The total amount of DA excreted during the entire urine collection period was higher in blacks, but the difference did not reach statistical significance (515.3±349.1 in blacks vs 321.3±133.5 μg/d in whites; P=.10). There were no differences between men and women within each race or for the entire study population (data not shown). The change in the average rate of DA excretion between day and night was directionally opposite, rising in blacks and falling in whites (Table II), and the day to night differences in the excretion rates were of borderline significance (6.5±12.2 μg/h in blacks [night to day] and −2.0±9.4 μg/h in whites; P=.07). There were no significant correlations between the urinary DA and Na during the daytime, at night, or during the entire period. Average BP declined in both groups at night (from 121.0±10.0/77.0±9.6 to 107.7±12.7/62.5± 11.6 mm Hg in blacks, day vs night; 119.9±9.3/ 73.5±5.5 to 108.8±8.3/61.7±7.2 mm Hg in whites, day vs night), but changes in BP were not significantly different between the races and did not correlate with the changes in DA excretion rates.
Table I.
Baseline Characteristics of Patients in Study 1
| Parameter | Blacks (n=11) | Whites (n=17) | P Valuea |
|---|---|---|---|
| Female sex, No. (%) | 8 (72.7) | 8 (47.1) | .23 |
| Age, y | 32.5±9.5 | 27.2±5.6 | .13 |
| Ambulatory blood pressure, mm Hgb | |||
| Systolic | 115.2±10.6 | 116.3±8.2 | .75 |
| Diastolic | 72.0±8.9 | 69.8±5.7 | .43 |
| BMI, kg/m2 | 28.5±8.7 | 25.0±5.8 | .21 |
| ClCr, mL/min | 94.7±14.5 | 94.0±14.8 | .90 |
| Serum Cr, mg/dL | 0.94±0.14 | 0.93±0.18 | .91 |
| Serum Na, mmol/L | 139.0±1.9 | 140.5±2.9 | .14 |
| Serum K, mmol/L | 4.2±0.17 | 4.3±0.32 | .25 |
Values are mean ± standard deviation unless otherwise indicated. aBlacks vs whites (t test). bAverage for 24 hours by oscillometric automated device.
Table II.
Day and Night Urine Volume, Flow Rate, and Composition for Study 1
| Parameter | Blacks (n=11) | Whites (n=17) | P Valuea |
|---|---|---|---|
| Day | |||
| Volume, mL | 934±751 | 1070±403 | .59 |
| Flow rate, mL/min | 1.18±0.95 | 1.38±0.48 | .55 |
| Na excretion, mmol | 83.4±59.8 | 101.0±57.3 | .45 |
| K excretion, mmol | 26.1±16.7 | 45.6±20.9 | .01 |
| DA excretion rate, μg/h | 9.5±4.0 | 12.2±8.1 | .26 |
| Night | |||
| Volume, mL | 455±212 | 478±207 | .78 |
| Flow rate, mL/min | 0.94±0.48 | 0.93±0.36 | .94 |
| Na excretion, mmol | 43.3±32.4 | 55.2±31.7 | .35 |
| K excretion, mmol | 13.5±12.0 | 13.6±9.6 | .99 |
| DA excretion rate, μg/h | 16.1±14.2 | 10.1±3.7 | .20 |
Abbreviations: DA, urinary dopamine; K, potassium; Na, sodium. Values are mean ± standard deviation unless otherwise indicated. aBlacks vs whites (t test).
Study 2
Characteristics of patients are shown in Table III. The only significant difference was for diastolic BP. Graded infusion of fenoldopam resulted in a prompt and progressive increase in Na excretion in both blacks and whites, which fell promptly with discontinuation of the drug infusion (Figure 1). ClCr did not change significantly and, thus, FE Na also increased significantly (P=.005 in whites and P=.02 in blacks). Mean Na excretion and FE Na were comparable in the black and white groups. Values for Na excretion and FE Na during the highest dose of fenoldopam are shown in Table IV. K excretion and FE K did not change significantly in either racial group during the infusion (Figure 1). Both measures of K excretion (as well as the Na/K ratio) were significantly lower in blacks than whites throughout the infusion (all P≤.05). Values for K excretion and FE K during the highest dose of fenoldopam are shown in Table IV. Distal Na‐to‐K exchange did not change significantly during the infusion and was lower in blacks throughout the infusion (all P<.03). Values during the highest dose of fenoldopam were 16.2±4.9% vs 20.7±6.6% in blacks vs whites, respectively (P=.04).
Table III.
Baseline Characteristics of the Patients for Study 2
| Parameter | Blacks (n=14) | Whites (n=17) | P Valuea |
|---|---|---|---|
| Female sex, No. (%) | 9 (64.3) | 7 (41.2) | .21 |
| Age, y | 32.1±10.3 | 29.3±9.3 | .43 |
| Blood pressure, mm Hgb | |||
| Systolic | 118.1±11.3 | 116.9±11.4 | .77 |
| Diastolic | 74.4±7.0 | 65.2±5.9 | .01 |
| BMI, kg/m2 | 26.3±6.0 | 29.4±6.5 | .19 |
| ClCr, mL/min | 93.6±11.5 | 90.0±11.8 | .67 |
| Serum Cr, mg/dL | 0.98±0.16 | 0.94±0.14 | .45 |
| Serum Na, mmol/L | 138.6±3.4 | 137.8±1.3 | .38 |
| Serum K, mmol/L | 4.0±0.3 | 4.1±0.2 | .35 |
| Serum osmolality, mOsm/L | 283.5±3.5 | 285.3±4.7 | .23 |
Abbreviations: BMI, body mass index; ClCr, creatinine clearance; Cr, creatinine; K, potassium; Na, sodium. Values are expressed as mean ± standard deviation unless otherwise indicated. aBlacks vs whites (t test). bAverage of 2 seated auscultatory readings.
Table IV.
Renal Responses to Fenoldopam Infusion at the Highest Dose (0.2 μg/kg/min) in Blacks and Whites in Study 2
| Parameter | Blacks (n=14) | Whites (n=17) | P Valuea |
|---|---|---|---|
| Urine excretion | |||
| Na, mmol 90/min | 8.9±4.9 | 8.5±3.5 | .76 |
| K, mmol 90/min | 1.5±0.7 | 2.0±0.7 | .05 |
| Osmolality, mOsm 90/min | 28.5±13.8 | 32.9±9.0 | .32 |
| ClCr, mL/min | 124.3±26.9 | 135.2±18.7 | .21 |
| FE Na, % | 1.70±0.74 | 1.55±0.67 | .56 |
| FE K, % | 10.60±2.77 | 13.24±4.30 | .05 |
| Distal Na to K exchange, % | 16.2±4.9 | 20.7±6.6 | .04 |
Abbreviations: ClCr, creatinine clearance; Cr, creatinine; FE, fractional excretion; K, potassium; Na, sodium. Values are expressed as mean ± standard deviation unless otherwise indicated. aBlacks vs whites (t test).
Urine flow rate and ClH2O increased progressively during the study and reached a plateau during the period in which the maximal dose of fenoldopam was infused. During the 90 minutes of high‐dose fenoldopam, both were greater in whites (Figure 2). Minimum Uosm reached during the maximal dose of fenoldopam infused was similar between the groups (blacks, 66.6±16.3 mOsm/L; whites, 63.8±18.1 mOsm/L [P=.65]).
Figure 2.
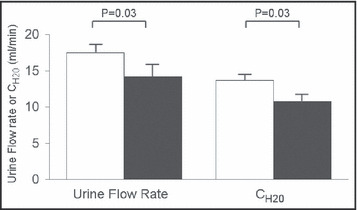
Maximal rates of urine flow and CH2O in blacks (n=14, filled bars) and whites (n=17, open bars) in study 2 during infusion of fenoldopam at 0.20 μg/kg/min. Values shown are mean ± standard error and statistical comparisons are by t test.
There were no significant changes in systolic BP for the entire infusion period (whites: P=.94 by ANOVA, range in whites 116.4±10.6 mm Hg at baseline, minimum of 115.4±9.9 mm Hg; blacks: P=.97 by ANOVA, range in blacks 117.8±9.6 mm Hg at baseline, minimum of 114.1±13.1 mm Hg) or HR (whites: P=.27 by ANOVA, range in whites 68.2±12.7 beats per minute at baseline to a maximum of 77.8±10.0 beats per minute; blacks: P=.13 by ANOVA, range in blacks 67.0±12.8 beats per minute to a maximum of 79.9±15.5 beats per minute). Diastolic BP declined significantly in whites (P=.002 by ANOVA, range 69.2± 7.8 mm Hg at baseline to a minimum of 62.2±6.5 mm Hg) and to a similar magnitude in blacks, although the change was of only borderline significance (P=.09 by ANOVA, 74.0±9.6 mm Hg to a minimum of 65.5±7.0 mm Hg).
Discussion
The natriuretic effect of endogenous DA contributes to the maintenance of Na balance, and blunted DA activity may play a role in the pathogenesis of hypertension, particularly in the SS subtype. The possibility that there are racial differences in DA activity has been suggested by observations in blacks of a lack of a relationship between urinary DA and Na excretion observed in whites 9 , 10 by the lesser DA excretion provoked by dietary Na loading in normotensive SS blacks compared with whites 11 and by the failure of a low‐dose DA infusion to promote a significant increase in Na excretion in blacks. 17 In the current report we demonstrate that there is no race‐related difference in urinary DA excretion, no relationship between DA and Na excretion in either race, and no difference in the increase of Na excretion resulting from infusion of the DA‐1 agonist fenoldopam. Our studies were performed in normotensive patients, so these results do not exclude a pathophysiologic role for DA in hypertension or in mediating BP responses to Na, but they suggest that any differences in DA excretion or DA‐1 responses between SS and NSS individuals are not likely to be primarily related to race.
We have previously used data from these studies to demonstrate that blacks excrete a free water load more slowly than do whites. 20 Delivery of tubular fluid from the proximal tubule to the distal diluting segment is an important determinant of free water generation, and in our original report of some of the findings derived from study 1, we showed that FE of lithium (a measure of proximal tubular Na reabsorption) did not differ between blacks and whites, suggesting that differences in proximal tubular Na reabsorption did not underlie the racial difference in CH2O. The results of study 2 reported here further support the contention that delivery of fluid to the diluting segment does not explain the racial difference in water handling since fenoldopam suppresses proximal tubular Na reabsorption and markedly increases distal delivery but did not abolish the racial difference in CH2O. Minimum urine osmolality achieved during the fenoldopam infusion was the same in blacks and whites, reinforcing our earlier conclusion that it is the capacity for free water generation that is impaired, and that the renal mechanisms responsible for generating the maximal transtubular osmolal gradient function similarly in both races. 20
The failure of fenoldopam to provoke the kaliuresis that might be expected when distal tubular Na load is increased has been observed consistently and may reflect the effect of DA‐1 activation at distal tubular sites. 6 , 18 , 19 We saw no significant changes in urinary K excretion or FE K during fenoldopam administration, and we further note that fenoldopam‐induced natriuresis does not abrogate the difference in urinary K excretion that is almost invariably observed in blacks compared with whites (including our patients in study 1). 22 Although differences in urinary K excretion could simply result from differences in dietary K intake, differences in renal tubular K handling may also contribute. 22 It appears that DA‐1 receptor‐mediated tubular processes do not contribute to the racial difference in K excretion, which here appear to be related to differences in distal Na to K exchange.
Conclusions
We report here that urinary DA excretion and natriuretic responses to fenoldopam infusion are similar in whites and blacks. Fenoldopam does not affect previously described racial differences in renal CH2O or urinary K excretion.
Disclosures: This study was supported in part by the Michigan Institute for Clinical and Health Research National Institutes of Health grant UL1RR024986 and by the Faculty Group Practice of the University of Michigan.
References
- 1. Oates NS, Ball SG, Perkins CM, et al. Plasma and urine dopamine in man given sodium chloride in the diet. Clin Sci. 1979;56:261–264. [DOI] [PubMed] [Google Scholar]
- 2. Harvey JN, Casson IF, Clayden AD, et al. A paradoxical fall in urine dopamine output when patients with essential hypertension are given added dietary salt. Clin Sci. 1984;67:83–88. [DOI] [PubMed] [Google Scholar]
- 3. Goldstein DS, Stull R, Eisenhofer G, et al. Urinary excretion of dihydroxyphenyalanine and dopamine during alterations of dietary salt intake in humans. Clin Sci. 1989;76:517–522. [DOI] [PubMed] [Google Scholar]
- 4. Alexander RW, Gill JR, Yamabe H, et al. Effects of sodium and of acute saline infusion on the interrelationship between dopamine excretion and adrenergic activity in man. J Clin Invest. 1974;54:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krishna GG, Danovitch GM, Beck FWJ, et al. Dopaminergic modulation of the natriuretic response to volume expansion. J Lab Clin Med. 1985;105:214–218. [PubMed] [Google Scholar]
- 6. O’Connell DP, Ragsdale NV, Boyd DG, et al. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension. 1997;29:115–122. [DOI] [PubMed] [Google Scholar]
- 7. Gill JR, Gullner HG, Lake CR, et al. Plasma and urinary catecholamines in salt‐sensitive idiopathic hypertension. Hypertension. 1988;11:312–319. [DOI] [PubMed] [Google Scholar]
- 8. Shikuma R, Yoshimura M, Kambara S, et al. Dopaminergic modulation of salt sensitivity in patients with essential hypertension. Life Sci. 1985;38:915–921. [DOI] [PubMed] [Google Scholar]
- 9. Critchley JA, Lee MR. No correlation between UNa and UDA in West Africans and no increase in UDA with Na loading. Lancet. 1986;2:460. [DOI] [PubMed] [Google Scholar]
- 10. Lee MR, Critchley JA, Gordon CJ, et al. Ethnic differences in the renal sodium dopamine relationship. A possible explanation for regional variations in the prevalence of hypertension?. Am J Hypertens. 1990;3:100S–103S. [DOI] [PubMed] [Google Scholar]
- 11. Sowers JR, Zemel MB, Zemel P, et al. Salt sensitivity in blacks. Salt intake and natriuretic substances. Hypertension. 1988;12:485–490. [DOI] [PubMed] [Google Scholar]
- 12. Damasceno A, Santos A, Serrao P, et al. Deficiency of renal dopaminergic‐dependent natriuretic response to acute sodium load in black salt‐sensitive subjects in contrast to salt‐resistant subjects. J Hypertens. 1999;17: 1995–2001. [DOI] [PubMed] [Google Scholar]
- 13. McDonald RH, Goldberg LI, McNay JL, et al. Effects of dopamine in man: augmentation of sodium excretion, glomerular filtration rate and renal plasma flow. J Clin Invest. 1964;43:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olsen NV, Hansen JM, Ladefoged SD, et al. Renal tubular reabsorption of sodium and water during infusion of low‐dose dopamine in normal man. Clin Sci. 1990; 78:503–507. [DOI] [PubMed] [Google Scholar]
- 15. Olsen NV, Olsen MH, Fogh‐Andersen N, et al. Lithium clearance method and the renal response to low‐dose dopamine in man: a randomized, controlled study. Clin Sci. 1993;84:237–242. [DOI] [PubMed] [Google Scholar]
- 16. Olsen NV, Lund J, Jensen PF, et al. Dopamine, dobutamine, and dopexamine. A comparison of renal effects in unanesthetized human volunteers. Anesthesiology. 1993; 79:685–694. [DOI] [PubMed] [Google Scholar]
- 17. Marinac JS, Willsie SK, Dew M, et al. Pharmacodynamic effects of dopamine stratified by race. Am J Ther. 2001;8: 27–34. [DOI] [PubMed] [Google Scholar]
- 18. Ragsdale NV, Lynd M, Chevalier RL, Felder RA, et al. Selective peripheral dopamine‐1 receptor stimulation. Differential responses in sodium loading and depletion in humans. Hypertension. 1990;15:914–921. [DOI] [PubMed] [Google Scholar]
- 19. Hughes JM, Ragsdale V, Felder RA, et al. Diuresis and natriuresis during continuous dopamine‐1 receptor stimulation. Hypertension. 1988;11(suppl I):I‐69–I‐74. [DOI] [PubMed] [Google Scholar]
- 20. Weder AB, Gleiberman L, Sachdeva A. Whites excrete a water load more rapidly than blacks. Hypertension. 2009;53:715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moyer TP, Jiang NS, Tyce GM, et al. Analysis for urinary catecholamines by liquid chromatography with amperometric detection: methodology and clinical interpretation of results. Clin Chem. 1979;25:256–263. [PubMed] [Google Scholar]
- 22. Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–713. [DOI] [PubMed] [Google Scholar]


