Abstract
We investigated the functional roles of ceramide, an intracellular lipid mediator, in cell signaling pathways by monitoring the intracellular movement of protein kinase C (PKC) subtypes fused to green fluorescent protein (GFP) in HeLa living cells. C2-ceramide but not C2-dihydroceramide induced translocation of δPKC-GFP to the Golgi complex, while αPKC- and ζPKC-GFP did not respond to ceramide. The Golgi-associated δPKC-GFP induced by ceramide was further translocated to the plasma membrane by phorbol ester treatment. Ceramide itself accumulated to the Golgi complex where δPKC was translocated by ceramide. Gamma interferon also induced the δPKC-specific translocation from the cytoplasm to the Golgi complex via the activation of Janus kinase and Mg2+-dependent neutral sphingomyelinase. Photobleaching studies showed that ceramide does not evoke tight binding of δPKC-GFP to the Golgi complex but induces the continuous association and dissociation of δPKC with the Golgi complex. Ceramide inhibited the kinase activity of δPKC-GFP in the presence of phosphatidylserine and diolein in vitro, while the kinase activity of δPKC-GFP immunoprecipitated from ceramide-treated cells was increased. The immunoprecipitated δPKC-GFP was tyrosine phosphorylated after ceramide treatment. Tyrosine kinase inhibitor abolished the ceramide-induced activation and tyrosine phosphorylation of δPKC-GFP. These results suggested that gamma interferon stimulation followed by ceramide generation through Mg2+-dependent sphingomyelinase induced δPKC-specific translocation to the Golgi complex and that translocation results in δPKC activation through tyrosine phosphorylation of the enzyme.
Protein kinase C (PKC) is a family of phospholipid-dependent serine/threonine protein kinases consisting of at least 10 subspecies that can be classified into three subgroups, classical, novel, and atypical PKC (43, 44, 52, 54). The classical PKC members (α, βI, βII, and γ), each of which has a Ca2+ binding region (C2 region) and two cysteine-rich regions, are activated by Ca2+, phosphatidylserine (PS), and diacylglycerol (DG) or phorbol esters. The novel PKC members (δ, ɛ, η, and θ), lacking the C2 region, are activated by PS and DG or phorbol esters without Ca2+. The atypical PKC members (ζ and ι/λ), which lack the C2 region and have only one cysteine-rich region, are dependent on PS but are not affected by DG, phorbol esters, or Ca2+ (52). Although a considerable number of studies have demonstrated the involvement of PKC in various cellular functions (2, 6, 42, 45, 62), the individual roles of each PKC subtype in cellular functions remain unclear. Recent studies in living cells using green fluorescent protein (GFP)-tagged PKC have shown that each PKC subtype has a spatially and temporally different targeting mechanism that is dependent on the extracellular signals contributing to the subspecies-specific functions of PKC (46, 49, 56, 59). Based on these findings, it was proposed that the PKC targeting mechanism is a determinant for the specific function of each PKC subtype in response to various stimuli in various cell types.
Ceramide has recently emerged as an intracellular lipid mediator implicated in various cellular responses, such as programmed cell death, cell differentiation, growth inhibition, and long-term depression of synaptic transmission (5, 18, 19, 24, 47, 51, 64, 65). Ceramide is generated by transient hydrolysis of sphingomyelin, and many reports have indicated that ceramide is produced via receptor-mediated stimulation by various extracellular ligands, including vitamin D3 (50), gamma interferon (IFN-γ) (25), tumor necrosis factor alpha (TNF-α) (11, 25), interleukin-1 (36), and nerve growth factor (5, 10). Recently, the regulation of PKC activity by ceramide has been reported, but the results are still controversial; ceramide has been shown to activate αPKC or inhibit δPKC autophosphorylation in renal mesangial cells in vitro (22). Furthermore, it is also reported that ceramide induces the translocation of δPKC and ɛPKC from the membrane to the cytosol in human myelogenous leukemia HL-60 cells (57) or of αPKC from the cytosol to the membrane in renal mesangial cells and in smooth muscle cells (22, 23). These apparently contradictory results may have been due to differences not only in methods but also in time points and cell types examined, suggesting the necessity to observe the localization of each PKC subtype after ceramide treatment continuously in living cells.
In the present study, we investigated the intracellular movement of GFP-tagged PKC subtypes in living cells after treatment with various stimuli, such as ceramide and IFN-γ. We also examined the effect of ceramide on the kinase activity of PKC subtypes. We demonstrated here the δPKC-specific translocation to the Golgi complex by ceramide and the activation of δPKC through tyrosine phosphorylation of the enzyme.
MATERIALS AND METHODS
Materials.
d-Erythro-C2-ceramide and d-erythrodihydro-C2-ceramide were purchased from Biomol Research Laboratories (Plymouth Meeting, Pa.). d-Erythro-C6-ceramide and 6-{[N-(7-nitrobenz-2-oxa-1, 3-diazol-4-yl) amino] hexanoyl} sphingosine (C6-NBD-ceramide) were purchased from Molecular Probes (Eugene, Oreg.). 12-O-Tetradecanoylphorbol-13-acetate (TPA) was purchased from Sigma Chemical Co. (St. Louis, Mo.). D609 was purchased from Cayman Chemical (Ann Arbor, Mich.). IFN-γ was a kind gift from Shionogi Research Laboratory and Co., Ltd. (Osaka, Japan). TNF-α was purchased from Gibco BRL (Grand Island, N.Y.). Scyphostatin was a kind gift from Takeshi Ogita (Biomedical Research Laboratories, Sankyo Co., Ltd., Tokyo, Japan). Glutathione (GSH) was purchased from Nacalai Tesque Inc. (Kyoto, Japan). Tyrphostin AG490 and genistein were purchased from Calbiochem-Novabiochem Co. (La Jolla, Calif.). Anti-αPKC, δPKC, and ɛPKC monoclonal antibodies were purchased from Transduction Laboratories (Lexington, Ky.). Anti-δPKC and ηPKC polyclonal antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). Anti-ζPKC polyclonal antibody was purchased from Upstate Biotechnology (Lake Placid, N.Y.) and that used for immunoblotting was produced as described previously (53). Anti-phosphotyrosine antibody, clone 4G10, was purchased from Upstate Biotechnology. Peroxidase-conjugated goat anti-mouse or anti-rabbit immunoglobulin G (IgG) was purchased from Amersham Corp. (Arlington Heights, Ill.). Cy3-labeled goat anti-mouse or anti-rabbit IgG was purchased from Amersham Corp. Calf thymus H1 histone was purchased from Boehringer GmbH (Mannheim, Germany). Myelin basic protein (MBP) was purchased from Sigma Chemical Co. Sodium fluoride (NaF) was purchased from Nacalai Tesque Inc. Sodium orthovanadate was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All the other chemicals used were of analytical grade.
Cell culture.
HeLa cells and HEK293 cells were purchased from Riken Cell Bank (Tsukuba, Japan). The CHO-K1 cell strain was a gift from Masahiro Nishijima (National Institute of Health, Tokyo, Japan). HeLa cells were cultured in minimum essential medium (Gibco BRL) which was buffered with 44 mM NaHCO3 and supplemented with 10% fetal bovine serum (FBS) in 5% CO2 at 37°C in a humidified incubator. HEK293 cells were maintained in minimum essential medium supplemented with 44 mM NaHCO3 and 10% horse serum in a humidified atmosphere containing 5% CO2 at 37°C. CHO-K1 cells were cultured as described previously (49, 59). All media were supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml), and the FBS used was not heat inactivated.
Preparation of δPKC-GFP adenovirus.
The adenoviral plasmids (pAdEasy-1 and pAdEasy-2) and the shuttle vectors (pShuttle, pShuttle-CMV, pAdTrack, and pAdTrack-CMV) were gifts from Bert Vogelstein (Johns Hopkins University Oncology Center, Baltimore, Md.). Generation of an adenoviral vector including δPKC-GFP cDNA was performed according to the methods described on the web site of Johns Hopkins University Oncology Center (http://www.coloncancer.org/adeasy.html). Briefly, the plasmid encoding GFP (BS 348) was digested by BgIII and HindIII. The plasmid encoding δPKC (BS390) was digested with XhoI and BamHI, the digested products were subcloned into the XhoI and HindIII sites of the expression vector pShuttle-CMV, and the new plasmid was designated pBsAd010.
For the production of recombinant virus (δPKC-GFP in pAdEasy-1), pBsAd010 linearized with PmeI was cotransformed with supercoiled circular pAdEasy-1 into competent Escherichia coli BJ5183 cells by electroporation. Transformation yielded approximately 10 kanamycin-resistant clones, of which about two-thirds contained recombinants, based on the sizes of undigested miniprep plasmid DNA. Candidate clones were digested with several restriction endonucleases to verify proper recombination. HEK293 cells at 50 to 70% confluency were prepared in 25-cm2 flasks for transfection of adenoviral vector. Recombinant adenoviral vector DNA (∼4 μg) including δPKC-GFP was digested with PacI and transfected into HEK293 cells by lipofection using TransIT-LT2 (Mirus, Madison, Wis.) according to the manufacturer's standard protocol. Transfected cells were monitored for GFP expression and were collected 7 to 10 days after transfection and then resuspended in 2 ml of phosphate-buffered saline without Ca2+ or Mg2+ [PBS(−)]. After three cycles of freezing in a methanol-dry ice bath and rapid thawing at 37°C, 500 μl of viral lysate was used to infect 106 cells in 25-cm2 flasks. Three or four days later, viruses were harvested as described above. To generate higher titer viral stocks, this process was repeated three times. In the final round, a total of 108 cells in 24 75-cm2 flasks were used to obtain a multiplicity of infection of 5. Three to five days after the final infection, the resultant viruses were purified by CsCl banding, and final yields were measured as described previously (21). Final yields were generally 1011 to 1012 PFU.
Expression of αPKC-, δPKC-, and ζPKC-GFP or δPKC protein in HeLa cells.
GFP was fused to the C terminus of each PKC subtype. For lipofection, plasmids (∼5.5 μg) including αPKC-, δPKC-, and ζPKC-GFP or δPKC cDNA were transfected into 5 × 106 HeLa cells using TransIT-LT2 (Mirus) according to the manufacturer's standard protocol. For adenoviral infection, HeLa cells (5 × 106) were incubated in serum-free medium for 2 h with recombinant δPKC-GFP adenovirus with a multiplicity of infection of 10 in a humidified atmosphere containing 5% CO2 at 37°C. After infection, the viral supernatant was removed, and the cells were cultured in normal serum-containing medium. Adenoviral infection was performed in the immunoblotting experiments and kinase assays. The fluorescence of αPKC-, δPKC-, and ζPKC-GFP became detectable 16 h after transfection. All experiments were performed 2 days after transfection.
Observation of αPKC-, δPKC-, and ζ-PKC-GFP translocation.
HeLa cells expressing αPKC-, δPKC-, and ζPKC-GFP were spread onto glass-bottomed culture dishes (MatTek, Ashland, Mass.) and cultured for at least 16 h before observation. The culture medium was replaced with normal HEPES buffer composed of 135 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, and 10 mM glucose, pH 7.3. The fluorescence of GFP was monitored under a confocal laser scanning fluorescence microscope (LSM 410 or 510; Carl Zeiss, Jena, Germany) at a 488-nm excitation wavelength with a 515-nm-long pass or 510- to 525-nm band pass barrier filter. Translocation of the fusion protein was triggered by addition of various stimulators at high concentrations into the HEPES buffer to obtain the appropriate final concentrations. All experiments were performed at 37°C.
Immunostaining of endogenous αPKC, δPKC, and ζPKC or expressed δPKC.
Before and after treatment with 10 μM C2-ceramide or C6-NBD-ceramide, HeLa cells were fixed with a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.01 M PBS (pH 7.4) for 30 min. After washing twice with PBS, the cells were treated with PBS containing 0.3% Triton X-100 and 5% normal goat serum (NGS) for 10 min. The cells were then incubated with the anti-αPKC and -δPKC monoclonal antibodies (diluted 1:200) or the anti-ζPKC polyclonal antibody (Upstate Biotechnology) (diluted 1:200) in PBS with 0.03% Triton X-100 (PBS-T) and 5% NGS for 1 h at 25°C. After washing in PBS-T, the cells were incubated with Cy3-labeled goat anti-mouse or anti-rabbit IgG (diluted 1:1,000) for 30 min. After three washes with PBS-T for 10 min each time, the fluorescence of Cy3 was observed under a confocal laser scanning fluorescent microscope with excitation at 588 nm using a 590-nm-long pass barrier filter.
Codetection of the Golgi complex and δPKC-GFP translocated by ceramide.
Texas red-conjugated wheat germ agglutinin was used to monitor the Golgi complex. After translocation of δPKC-GFP by 10 μM C2-ceramide, the cells were fixed with a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.01 M PBS (pH 7.4) for 30 min. After washing twice with PBS, the cells were treated with PBS containing 0.3% Triton X-100 and 5% NGS for 10 min. The cells were then incubated with 1 μg of Texas red-conjugated wheat germ agglutinin (Molecular Probes, Leiden, The Netherlands) per ml for 40 min in PBS-T and 5% NGS. After three washes with PBS-T for 10 min each time, the fluorescence of Texas red and GFP was observed under a confocal laser scanning fluorescent microscope, with excitation at 488 nm using a 510- to 525-nm band pass barrier filter for the former and with excitation at 588 nm using a 590-nm-long pass barrier filter for the latter.
Cell fractionation and immunoprecipitation.
For detection of endogenous PKC isozymes in HeLa cells, cultured HeLa cells were harvested with 1 ml of homogenate buffer (250 mM sucrose, 10 mM EGTA, 2 mM EDTA, 20 mM Tris-HCl, 200 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, pH 7.4) and centrifuged at 2,000 × g. For immunoblot analysis of tyrosine phosphorylation, we also used the homogenate buffer containing 10 mM NaF and 1 mM Na3VO4. The cells were resuspended with 300 μl of homogenate buffer containing 1% Triton X-100 and sonicated (UD-210; Tomy Seiko Co. Ltd., Tokyo, Japan) (output, 5; duty, 50%) 10 times at 4°C, and the supernatant was used after centrifugation at 19,000 × g for 15 min.
For subcellular fractionation, the HeLa cells transfected with the adenoviral vector containing δPKC-GFP were treated with 10 μM C2-ceramide for 20 min at 37°C and then harvested with 1 ml of homogenate buffer and centrifuged at 2,000 × g. The cells were resuspended with 300 μl of homogenate buffer and sonicated as described above. The samples were centrifuged at 55,000 × g for 30 min at 4°C, and the supernatant was collected as the cytosol fraction. The pellet was sonicated with 300 μl of homogenate buffer containing 1% Triton X-100 and centrifuged at 19,000 × g for 15 min, and then the supernatant was collected as the particulate fraction.
For immunoprecipitation of endogenous αPKC, δPKC, or ζPKC or expressed δPKC-GFP, the cytosol, particulate, or total fraction was rotated with the subtype-specific antibodies (anti-αPKC, δPKC, or ζPKC) or anti-GFP polyclonal antibody (Molecular Probes) (diluted 1:50) for 2 h at 4°C and then with protein G-Sepharose for an additional 2 h. Samples were centrifuged at 2,000 × g for 5 min at 4°C, and pellets were washed three times with PBS(−).
Finally, the pellet was suspended in 50 μl of PBS(−) and used for phosphorylation or immunoblotting studies as described below.
Immunoblotting analysis.
The same amounts of samples from each fraction were subjected to sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis according to the method of Laemmli (28), and the separated proteins were electorophoretically transferred onto polyvinylidene difluoride (PVDF) filters (Millipore Co., Bedford, Mass.). Nonspecific binding sites on the PVDF filters were blocked by incubation with 5% nonfat milk or 2% bovine serum albumin in PBS-T for 18 h. The PVDF filters were then incubated with the anti-PKC monoclonal antibodies (αPKC, δPKC, or ɛPKC) (diluted 1:1,000), the anti-PKC polyclonal antibodies (δPKC, ηPKC, or ζPKC) (diluted 1:1,000), the anti-GFP polyclonal antibody (Clontech, Palo Alto, Calif.) (diluted 1:1,000), or anti-phosphotyrosine antibody (diluted 1:1,000) for 1 h at 25°C. After washing in PBS-T, the filters were incubated with peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG (diluted 1:10,000) for 30 min. After three rinses, the immunoreactive bands were visualized using an enhanced chemiluminescence detection kit (Amersham). After immunoblot using anti-phosphotyrosine antibody, the PVDF filter was washed according to the manufacturer's protocol for reprobing with anti-GFP polyclonal antibody.
Fluorescence photobleaching.
Photobleaching experiments were performed as follows. After translocation of δPKC-GFP by 10 μM C2-ceramide, a circular region in the Golgi complex or a square region of perikarya within a plane of the cell was photobleached by scanning for 15 s with an argon laser of the highest power. Recovery and fading of fluorescence in the selected regions were then analyzed by imaging the entire cell by confocal fluorescent microscopy with low laser power at the indicated times after photobleaching. For all of the images, the noise levels were reduced by line scan averaging.
In vitro and in vivo kinase assay of δPKC.
The immunoprecipitated samples (10 μl of suspended pellet) were used for both in vitro and in vivo kinase assays. In vitro kinase assays of δPKC-GFP expressed in HeLa cells were performed as described previously (49). Briefly, the kinase activity in 10 μl of each sample was assayed by measuring the incorporation of 32Pi into H1 histone from [γ-32P]ATP in the presence of PS (8 μg/ml), diolein (DO) (0.8 μg/ml), or C2-ceramide at various concentrations. For the in vivo kinase assay, endogenous αPKC, δPKC, or ζPKC or exogenous δPKC-GFP was immunoprecipitated from HeLa cells at various time points after stimulation with 10 μM C2-ceramide, and then the kinase activities were measured with H1 histone (αPKC and δPKC) or MBP (ζPKC) as the substrate without any PKC activators such as PS, DO, or C2-ceramide.
RESULTS
Expression of PKC isozymes in HeLa cells.
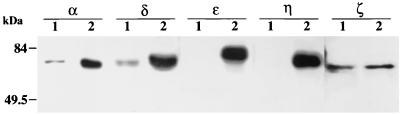
The expression of endogenous PKC subtypes in HeLa cells was examined by immunoblotting using subtype-specific antibodies. In HeLa cell lysates, each antibody against αPKC, δPKC, or ζPKC detected an immunoreactive band of reasonable molecular weight, while ɛ and η subtypes of PKC were not detected, indicating that the α, δ, and ζ subtypes of PKC are expressed in HeLa cells (Fig. 1). In this study, therefore, we focused on the localization of αPKC, δPKC, and ζPKC but not of ɛPKC or ηPKC.
FIG. 1.
Immunoblotting analysis of endogenous PKC subtypes in HeLa cells. Total cell lysates (25 μg) extracted from HeLa cells (lanes 1) were separated by sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and stained with antibodies against each PKC subtype. Rat brain homogenate was used as a positive control for αPKC, δPKC, ɛPKC, and ζPKC, and recombinant ηPKC expressed in CHO-K1 cells was used as a positive control for ηPKC (lanes 2). The α, δ, and ζ subtypes of PKC were detected with reasonable molecular masses in HeLa cells. The results shown are representative of two independent experiments.
Translocation of αPKC, δPKC, and ζPKC induced by C2-ceramide and phorbol ester.
When αPKC- and ζPKC-GFP were expressed in HeLa cells, intense and homogeneous fluorescence of αPKC- and ζPKC-GFP was observed throughout the cytoplasm, with no signals detected in the nucleus (Fig. 2A and C). In contrast, faint but significant fluorescence of δPKC-GFP was seen in the nucleus in addition to intense fluorescence in the cytoplasm, and occasionally, δPKC-GFP was found more densely in the perinuclear region than in the surrounding cytoplasm (Fig. 2A and C). Localization of the fluorescence did not change for at least 60 min when observed under a confocal laser scanning fluorescence microscope without stimulation.
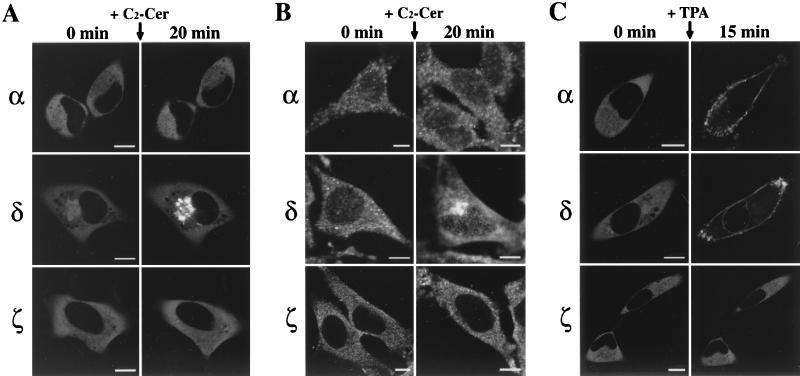
FIG. 2.
Ceramide- or TPA-induced translocation of PKC subtypes in HeLa cells. (A) C2-ceramide (C2-Cer)-induced translocation of αPKC-, δPKC-, and ζPKC-GFP overexpressed in HeLa cells. αPKC-, δPKC-, and ζPKC-GFP were seen throughout the cytoplasm of HeLa cells, and faint signals for δPKC-GFP were also seen in the nucleus. The addition of 10 μM C2-ceramide induced translocation of δPKC-GFP but not of αPKC- or ζPKC-GFP from the cytoplasm to the perinuclear region. (B) Immunocytochemical localization of endogenous αPKC, δPKC, and ζPKC before and after C2-ceramide treatment in HeLa cells. Endogenous αPKC, δPKC, and ζPKC were visualized by immunostaining with anti-αPKC, δPKC, or ζPKC antibodies and Cy3-labeled secondary antibodies. The addition of 10 μM C2-ceramide induced the accumulation of endogenous δPKC but not of αPKC or ζPKC to the perinuclear region. (C) TPA-induced translocation of αPKC-, δPKC-, and ζPKC-GFP overexpressed in HeLa cells. TPA at 1 μM induced translocation of αPKC-GFP from the cytoplasm to the plasma membrane. TPA at 1 μM induced translocation of δPKC-GFP from the cytoplasm and nucleoplasm to the plasma membrane and nuclear membrane, respectively. Application of 1 μM TPA failed to induce translocation of ζPKC-GFP. The results shown are representative of three independent experiments. Bars, 10 μm.
The effects of C2-ceramide, a membrane-permeable analogue of ceramide, on the cellular localization of αPKC-, δPKC, and ζPKC-GFP were investigated in HeLa cells. The δPKC-GFP accumulated significantly in the perinuclear region after treatment with C2-ceramide at 10 μM. The intensity of fluorescence in the perinuclear region reached the maximum level at 20 min after treatment. The δPKC-GFP in the nucleoplasm was not altered by C2-ceramide. The fluorescence remained in the perinuclear region for at least 60 min after C2-ceramide treatment and did not return to the cytoplasm. On the other hand, application of 10 μM C2-ceramide failed to induce any translocation of αPKC- or ζPKC-GFP (Fig. 2A). To verify the δPKC-specific translocation by ceramide, we further examined the effect of ceramide on endogenous αPKC, δPKC, and ζPKC in HeLa cells by immunocytochemistry. As shown in Fig. 2B, endogenous δPKC but not αPKC or ζPKC was significantly accumulated in the perinuclear region after the treatment, as seen in the case of δPKC-GFP. TPA at 1 μM induced translocation of both αPKC- and δPKC-GFP but not of ζPKC-GFP from the cytoplasm to the plasma membrane within 15 min. Translocation from the nucleoplasm to the nuclear membrane was also observed only in the case of δPKC-GFP (Fig. 2C). The fluorescence of αPKC- and δPKC-GFP remained on the plasma membrane or on the nuclear membrane for at least 60 min after TPA treatment.
C2-dihydroceramide, a derivative of C2-ceramide lacking the C4-C5 double bond of the sphingoid backbone (4), did not induce significant translocation of δPKC-GFP (Fig. 3A). C2-ceramide is known to be converted to C2-sphingomyelin by sphingomyelin synthase (SMS) (35, 55). To determine whether C2-ceramide but not C2-sphingomyelin translocates δPKC-GFP, we studied the effects of D609, an inhibitor of SMS (35, 41, 58), on the C2-ceramide-induced translocation of δPKC-GFP. Pretreatment with 200 μg of D609/ml for 30 min failed to inhibit the C2-ceramide-induced translocation of δPKC-GFP (Fig. 3B). After C2-ceramide-induced translocation of δPKC-GFP to the perinuclear region, TPA treatment (1 μM, 3 min) induced translocation of both the perinuclear and cytosolic δPKC-GFP to the plasma membrane (Fig. 3C). The δPKC-GFP in the nucleoplasm was translocated to the nuclear membrane 10 min after TPA treatment (data not shown).
FIG. 3.
Characterization of the ceramide-induced translocation of δPKC-GFP. (A) C2-dihydroceramide (DH-C2-Cer) (10 μM), an inactive ceramide, failed to induce translocation of δPKC-GFP. (B) Preincubation with D609 (200 μg/ml) for 30 min did not affect the C2-ceramide (C2-Cer)-induced translocation of δPKC-GFP. (C) After translocation of δPKC-GFP to the perinuclear region by treatment with 10 μM C2-ceramide for 20 min, 1 μM TPA irreversibly induced translocation of all the δPKC-GFP to the plasma membrane within 3 min. The results shown are representative of three independent experiments. Bars, 10 μm.
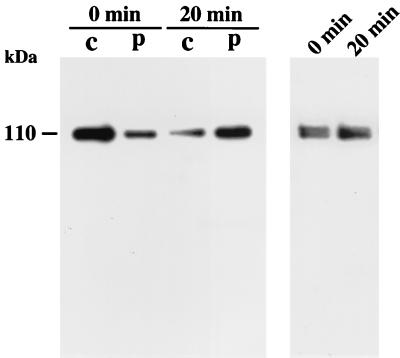
Translocation of δPKC-GFP by ceramide was further examined by immunoblotting analysis. To determine whether δPKC-GFP was translocated from the cytosol to the particulate fraction by C2-ceramide, immunoblotting analysis was performed. δPKC-GFP was immunoprecipitated from the transfected HeLa cells using anti-N terminus of δPKC monoclonal antibody and stained with anti-C terminus of δPKC polyclonal antibody as described in Materials and Methods. As shown in Fig. 4 (left panel), δPKC-GFP was predominantly present in the cytosolic fraction before C2-ceramide treatment, and C2-ceramide (10 μM, 20 min) caused translocation of δPKC-GFP from the cytosol to the particulate fraction. In addition, we obtained the same results using anti-GFP antibody instead of anti-N terminus of δPKC antibody for immunoprecipitation (data not shown). These results suggested that no degradation of δPKC-GFP occurred in the nontreated cells or even in the cells treated with C2-ceramide. We further examined whether the total amounts of δPKC-GFP were altered during C2-ceramide treatment by immunoblot analysis using anti-δPKC monoclonal antibody. The amount of δPKC-GFP in the total homogenate of transfected HeLa cells was not altered by C2-ceramide treatment (Fig. 4, right panel).
FIG. 4.
Ceramide-induced translocation of δPKC-GFP assessed by immunoblotting analysis. With immunoblotting analysis, δPKC-GFP was detected as a 110-kDa band that was more abundant in the cytosolic fraction (c). C2-ceramide treatment (10 μM, 20 min) induced translocation of δPKC-GFP from the cytosolic fraction to the particulate fraction (p). No degradation products were detected before or after treatment with C2-ceramide (left panel). The level of δPKC-GFP in the total homogenate was not changed after ceramide treatment for 20 min (right panel). The results shown are representative of three independent experiments.
Intracellular movement of ceramide.
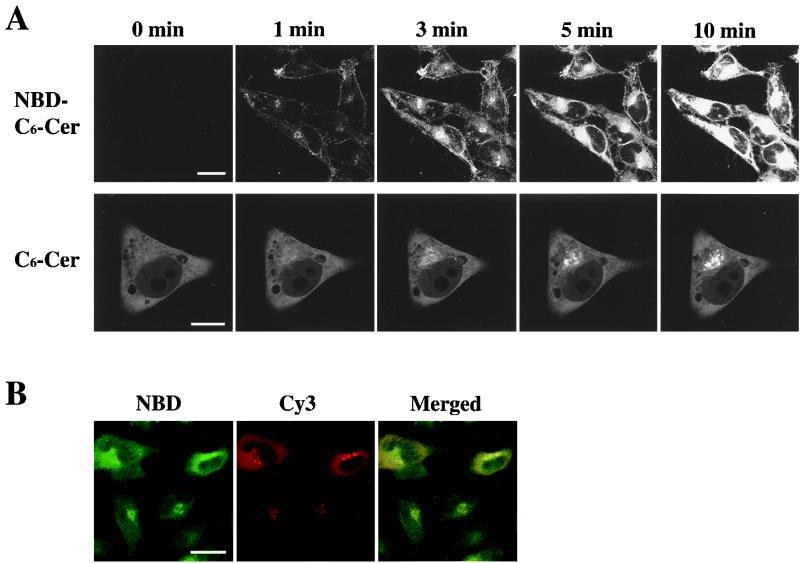
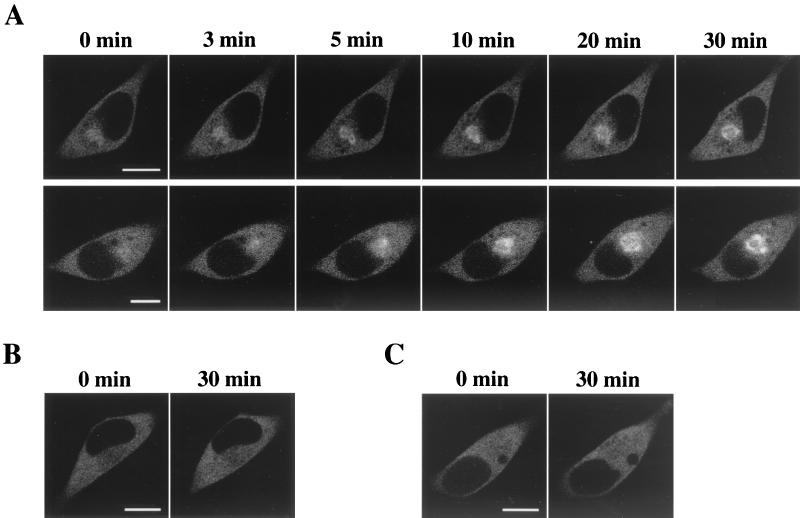
To compare the time course of δPKC-GFP translocation with permeation of ceramide into cells, we monitored the movement of ceramide in HeLa cells using a fluorescent analogue of ceramide, C6-NBD-ceramide. After application of 10 μM C6-NBD-ceramide to HeLa cells, the fluorescence of C6-NBD-ceramide was first detected on the plasma membrane at 1 min, and weak signals were in the perinuclear region, and then the intensity of the fluorescence on the plasma membrane gradually increased until 10 min. Obvious accumulation of the fluorescence was seen in the perinuclear region at 3 min, and the intensity of fluorescence was markedly increased at 10 min (Fig. 5A, top row). The C6-NBD-ceramide accumulated at the perinuclear region was not altered by TPA treatment (1 μM, 15 min) (data not shown). C6-ceramide also induced the translocation of δPKC-GFP similarly to the effect of C2-ceramide. The accumulation of δPKC-GFP was first seen at 1 min and was apparent 3 min after treatment with C6-ceramide, and the intensity of GFP fluorescence in the perinuclear region increased until 10 min (Fig. 5A, bottom row) and reached a maximum at 20 min (data not shown).
FIG. 5.
Localization of fluorescent ceramide (C6-NBD-ceramide) in HeLa cells. (A) C6-NBD-ceramide (NBD-C6-Cer) at 10 μM rapidly accumulated on the plasma membrane and perinuclear region of HeLa cells 1 min after the treatment, and the fluorescence in the perinuclear region increased significantly until 10 min (top row). C6-ceramide (C6-Cer)-induced translocation of δPKC-GFP showed a similar time course to that of C6-NBD-ceramide (bottom row). (B) HeLa cells transfected with δPKC were fixed after treatment with 10 μM C6-NBD-ceramide for 20 min. Cells were immunostained with anti-δPKC monoclonal antibody and with Cy3-labeled IgG as secondary antibody to make the expressed δPKC visible. The localization of C6-NBD-ceramide (NBD) is shown in green (left) and of δPKC is shown in red (center). On the merged image, the overlapped signals of C6-NBD-ceramide and Cy3 appear in yellow (right). The results shown are representative of three independent experiments. Bars, 10 μm (A, top row) and 20 μm (A, bottom row, and B).
To identify whether C6-ceramide translocates δPKC-GFP to the same intracellular compartment that C6-NBD-ceramide accumulates in, the δPKC was visualized with anti-δPKC monoclonal antibody in HeLa cells overexpressing δPKC after C6-NBD-ceramide treatment. As shown in Fig. 5B, intense NBD fluorescence was present in the perinuclear region. δPKC immunoreactivity also accumulated in the perinuclear region. Merged images showed that the fluorescence of NBD and δPKC immunoreactivity were colocalized in the perinuclear region, indicating that C6-NBD-ceramide and δPKC are targeted to the same perinuclear compartment.
Translocation of δPKC-GFP induced by IFN-γ.
The effect of IFN-γ, which hydrolyzes sphingomyelin to generate ceramide, on the translocation of δPKC-GFP was investigated in HeLa cells, since these cells are known to express IFN-γ receptors (33). IFN-γ at 100 U/ml induced significant δPKC-GFP translocation from the cytoplasm to the perinuclear region within 5 min, and the intensity of fluorescence increased in the perinuclear region until 30 min (Fig. 6A, top row). We examined the influence of serum deprivation on the translocation of δPKC-GFP induced by IFN-γ. When the culture medium was replaced with serum-free medium, IFN-γ induced the same translocation of δPKC-GFP as seen in the presence of FBS (Fig. 6A, bottom row). Serum deprivation did not alter the localization of δPKC-GFP until at least 60 min after treatment (data not shown). The application of 100 U of IFN-γ per ml failed to induce αPKC- or ζPKC-GFP translocation for at least 60 min (Fig. 6B and C).
FIG. 6.
Effects of IFN-γ on PKC subtype translocation in HeLa cells. (A) Treatment with IFN-γ (100 U/ml) induced translocation of δPKC-GFP from the cytoplasm to the perinuclear region within 5 min after treatment of HeLa cells in culture medium containing FBS (top row). The translocation of δPKC-GFP was not altered by eliminating FBS from the culture medium (bottom row). (B) IFN-γ (100 U/ml) did not affect the localization of αPKC-GFP expressed in HeLa cells. (C) IFN-γ (100 U/ml) did not affect the localization of ζPKC-GFP expressed in HeLa cells. The results shown are representative of three independent experiments. Bars, 10 μm.
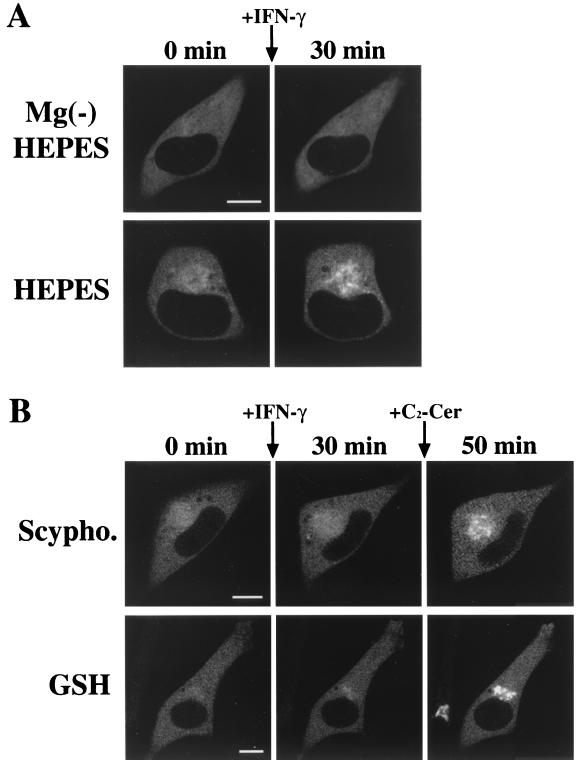
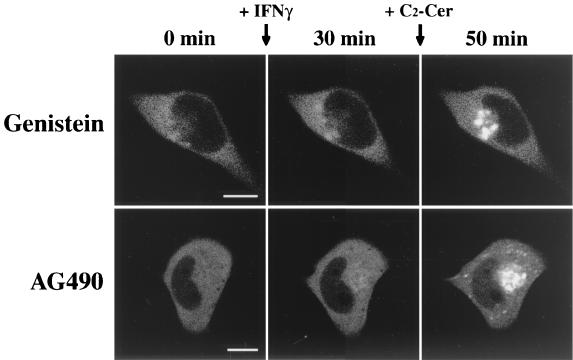
We further examined the downstream signaling pathway after activation of the IFN-γ receptor, which contributes to the translocation of δPKC. IFN-γ has been known to cause the activation of sphingomyelinase and then produce ceramide from sphingomyelin (25). To determine whether the IFN-γ-induced translocation of δPKC-GFP is mediated by the activation of sphingomyelinase, we first examined the effect of the Mg2+ chelator EDTA, which inhibits Mg2+-dependent neutral sphingomyelinase, a subtype of sphingomyelinase (5, 14). As shown in Fig. 7A, pretreatment with Mg2+-free HEPES buffer containing 0.5 mM EDTA for 30 min entirely blocked the IFN-γ-induced translocation of δPKC-GFP (Fig. 7A, top row), while in normal HEPES buffer IFN-γ induced translocation of δPKC-GFP from the cytoplasm to the perinuclear region (Fig. 7A, bottom row). The effects of other inhibitors of Mg2+-dependent neutral sphingomyelinase (scyphostatin and GSH) (3, 34, 63) were further examined. Pretreatment with 50 μM scyphostatin for 15 min effectively blocked the translocation of δPKC-GFP induced by IFN-γ, and the further application of 10 μM C2-ceramide also rescued the perinuclear translocation of δPKC-GFP (Fig. 7B, top row). Similarly, pretreatment with 5 mM GSH for 30 min effectively inhibited the translocation of δPKC-GFP induced by IFN-γ, and the further application of 10 μM C2-ceramide rescued the perinuclear translocation of δPKC-GFP fluorescence (Fig. 7B, bottom row). Tyrosine kinases such as JAK1 and JAK2 are involved in the downstream stages of IFN-γ signaling pathways (40). To clarify whether the perinuclear translocation of δPKC-GFP induced by IFN-γ is mediated by the activation of JAK1 and JAK2 in HeLa cells, we investigated the effects of genistein or tyrphostin AG490 on the IFN-γ-induced translocation of δPKC-GFP. Pretreatment with 100 μM genistein, a nonspecific tyrosine kinase inhibitor, for 30 min effectively blocked the translocation of δPKC-GFP induced by IFN-γ, and further application of 10 μM C2-ceramide rescued the perinuclear translocation of δPKC-GFP (Fig. 8, top row). Pretreatment with 100 μM tyrphostin AG490, a specific inhibitor of JAK2 tyrosine kinase (1, 37, 66), for 30 min also blocked the translocation of δPKC-GFP induced by IFN-γ, and the further application of 10 μM C2-ceramide rescued the perinuclear translocation of δPKC-GFP fluorescence as seen in the case of treatment with sphingomyelinase inhibitors (Fig. 8, bottom row).
FIG. 7.
Effects of sphingomyelinase inhibitors on IFN-γ-induced translocation of δPKC-GFP. (A) Treatment with Mg2+-free HEPES [Mg(−) HEPES] buffer containing 0.5 mM EDTA for 30 min blocked the IFN-γ (100 U/ml)-induced translocation of δPKC-GFP (top row). IFN-γ induced translocation of δPKC-GFP in normal HEPES buffer containing 1 mM Mg2+ (bottom row). (B) IFN-γ-induced translocation of δPKC-GFP was inhibited by pretreatment with 50 μM scyphostatin (Scypho.) for 15 min. However, scyphostatin did not inhibit the 10 μM C2-ceramide (C2-Cer)-induced translocation of δPKC-GFP (top row). GSH treatment (5 mM, 30 min) also abolished IFN-γ- but not C2-ceramide-induced translocation of δPKC-GFP (bottom row). The results shown are representative of three independent experiments. Bars, 10 μm.
FIG. 8.
Effects of tyrosine kinase inhibitors on IFN-γ-induced translocation of δPKC-GFP. IFN-γ-induced translocation of δPKC-GFP was inhibited by pretreatment with 100 μM genistein for 30 min. However, genistein did not alter 10-μM ceramide (C2-Cer)-induced translocation of δPKC-GFP (top row). Similarly, preincubation with tyrphostin AG490 (100 μM) for 30 min abolished IFN-γ-but not C2-ceramide-induced translocation of δPKC-GFP (bottom row). The results shown are representative of three independent experiments. Bars, 10 μm.
Ceramide is also generated by the activation of TNF-α receptors, which are expressed in HeLa cells (11, 13, 25, 38). We studied the effects of TNF-α on the translocation of δPKC-GFP. TNF-α at 100 U/ml induced apparent δPKC-GFP translocation from the cytoplasm to the perinuclear region within 20 min, and the intensity of the fluorescence increased slowly in the perinuclear region until 60 min (data not shown).
Target site of δPKC-GFP in response to ceramide.
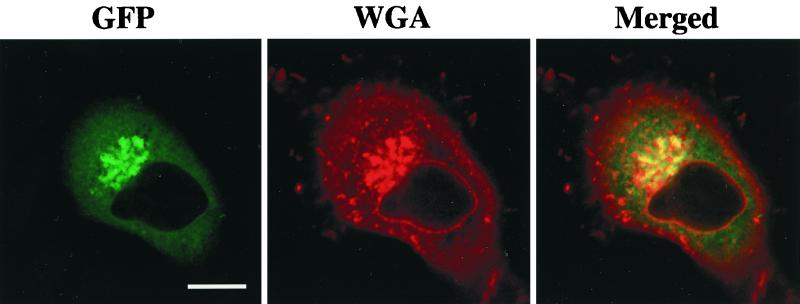
To identify the intracellular compartment where δPKC-GFP accumulated in response to C2-ceramide, the Golgi complex was visualized with Texas red-conjugated wheat germ agglutinin in HeLa cells expressing δPKC-GFP after ceramide treatment. As shown in Fig. 9, intense GFP fluorescence was present in the perinuclear region in addition to moderate fluorescence throughout the cytoplasm. Texas red fluorescence accumulated in the perinuclear region and was also seen on the nuclear membrane. Merged images showed that the fluorescence of GFP and that of Texas red were colocalized in the perinuclear region, indicating that δPKC-GFP is targeted to the Golgi complex in response to ceramide. Colocalization of δPKC-GFP and Texas red-conjugated wheat germ agglutinin was also seen after stimulation with C6-ceramide or IFN-γ (data not shown).
FIG. 9.
Colocalization of δPKC-GFP and wheat germ agglutinin binding sites in δPKC-GFP-expressing HeLa cells treated with ceramide. HeLa cells transfected with δPKC-GFP were fixed after treatment with 10 μM C2-ceramide for 20 min. Cells were treated with Texas red-conjugated wheat germ agglutinin (WGA) to make the Golgi complex visible. The localization of δPKC-GFP is shown in green (left). The Golgi complex is shown in red (center). On the merged image, overlapping GFP and Texas red signals appear yellow (right). The results are representative of four independent experiments. Bar, 10 μm.
FRAP of δPKC-GFP translocated by ceramide.
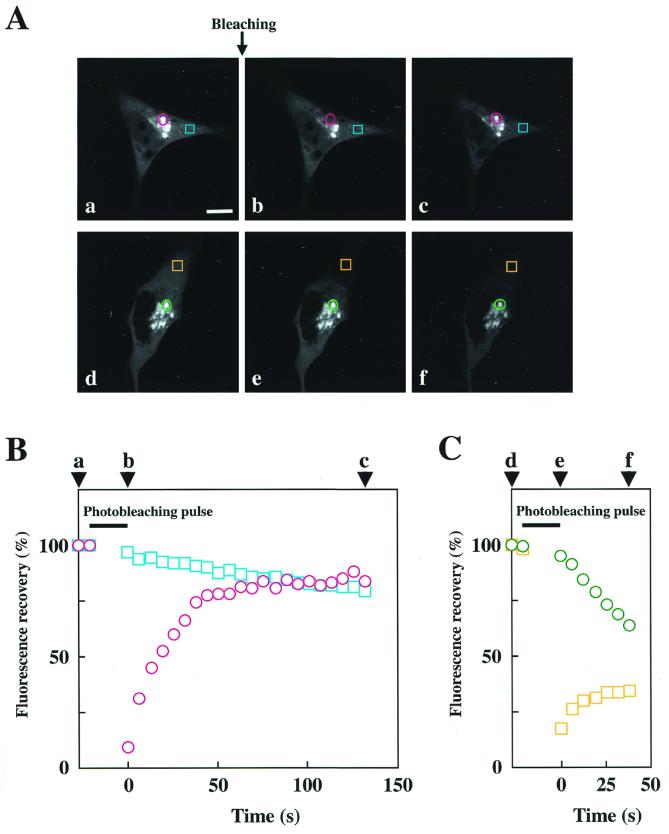
We investigated the interaction of δPKC-GFP with the Golgi complex by fluorescence recovery after photobleaching (FRAP). We measured the fluorescence recovery of the δPKC-GFP in the bleached area and also the fluorescence fading in the unbleached area after photobleaching with an argon laser at 488 nm. As shown in Fig. 10, after treatment with 10 μM C2-ceramide for 30 min, photobleaching of a circular area in the Golgi complex abolished the fluorescence of δPKC-GFP in the circle. The GFP fluorescence in the circle recovered within 40 s to a level similar to that in the unbleached Golgi complex. The recovery of fluorescence was significantly faster than the translocation of δPKC-GFP induced by ceramide (Fig. 5A). In contrast, the fluorescence in the unbleached perikarya faded gradually. Photobleaching was also applied to a square area in perikarya (Fig. 10). GFP fluorescence of the bleached area (perikarya) rapidly recovered (within 30 s), and the fluorescence in the Golgi complex rapidly faded.
FIG. 10.
FRAP of δPKC-GFP after translocation induced by ceramide. (A) Fluorescence recovery of δPKC-GFP after photobleaching of the Golgi complex (a to c) or of the cytoplasm (d to f). The images were obtained before (a and d) and 0 s (b and e), 132 s (c), or 38 s (f) after photobleaching. The bleached areas are shown in red circles (a to c) and orange squares (d to f). The blue squares (a to c) and green circles (d to f) show the areas where fluorescence fading was measured. (B and C) Measurement of fluorescence recovery of δPKC-GFP after photobleaching of the Golgi complex (B) or of the cytoplasm (C). Time-dependent recovery (red circle and orange square) of fluorescence in the bleached areas and fading (blue square and green circle) of the fluorescence in the unbleached areas are shown as percentages of the fluorescence before bleaching. Arrowheads (a to f) indicate the time points of the pictures in panel A. The results shown are representative of three independent experiments.
Changes in kinase activity of δPKC by C2-ceramide, in vitro and in vivo.
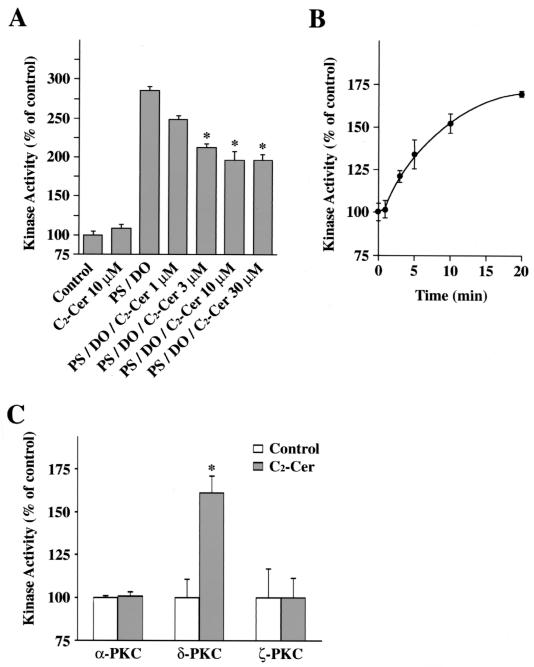
The effects of C2-ceramide on the kinase activity of δPKC-GFP were examined by an in vitro kinase assay. As shown in Fig. 11A, C2-ceramide at 10 μM failed to activate δPKC-GFP in vitro. In the presence of PS and DO, the kinase activity of δPKC-GFP was increased 2.9-fold, and C2-ceramide inhibited the activation of δPKC-GFP by PS and DO. The activity of δPKC-GFP in the presence of the cofactors was dose-dependently inhibited by C2-ceramide, and the maximal level was seen at 10 μM (31% inhibition).
FIG. 11.
Effects of ceramide on kinase activity of PKC subspecies, in vitro and in vivo. (A) Effects of C2-ceramide on kinase activity of δPKC-GFP assessed by in vitro kinase assay. Kinase activities of the immunoprecipitated δPKC-GFP were measured in the presence of various concentrations of C2-ceramide (C2-Cer) or activators of δPKC such as PS and DO. Data are expressed as percentages of the control level. Statistical significance: ∗, P < 0.05 versus kinase activity of PS and DO. (B) Changes in kinase activity of δPKC-GFP in HeLa cells after C2-ceramide treatment assessed by in vivo kinase assay. δPKC-GFP was immunoprecipitated from HeLa cells overexpressing δPKC-GFP at various time points after ceramide treatment. The kinase activity of δPKC-GFP was assayed with H1 histone as the substrate without any activators such as PS or DO. Data are expressed as percentages of the control level (the kinase activity before stimulation). (C) Effects of C2-ceramide on kinase activity of endogenous PKC subtypes assessed by in vivo kinase assay. Endogenous αPKC, δPKC, and ζPKC were immunoprecipitated from HeLa cells before and after C2-ceramide (C2-Cer) treatment. The kinase activity of αPKC, δPKC, and ζPKC was assayed with H1 histone (αPKC and δPKC) or MBP (ζPKC) as the substrate without any activators such as PS or DO. Data are expressed as percentages of the control level (the kinase activity of each PKC subtype immunoprecipitated from untreated cells). Statistical significance: ∗, P < 0.01 versus kinase activity of control. All results represent the means and standard errors of more than three determinations.
In contrast, the in vivo kinase assay indicated that the kinase activity of the immunoprecipitated δPKC-GFP was increased in HeLa cells treated with C2-ceramide. Treatment with 10 μM C2-ceramide increased the kinase activity of the immunoprecipitated δPKC-GFP in a time-dependent manner, and at 20 min after treatment with C2-ceramide, the kinase activity was increased 1.7-fold (Fig. 11B). To examine whether endogenous δPKC is also activated by ceramide, we performed the in vivo kinase assay of endogenous αPKC, δPKC, and ζPKC in untransfected HeLa cells. After the treatment with 10 μM C2-ceramide for 20 min, the kinase activity of δPKC but not of αPKC or ζPKC was significantly increased 1.6-fold (Fig. 11C).
Tyrosine phosphorylation of δPKC-GFP after treatment with C2-ceramide in HeLa cells.
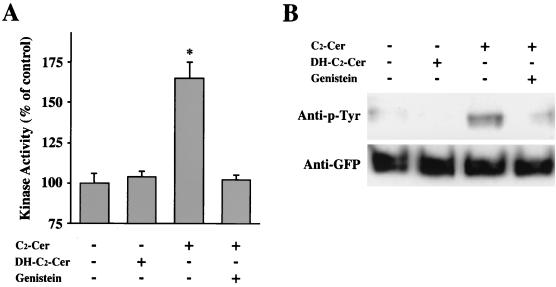
It has been reported that δPKC is activated by tyrosine phosphorylation after various stimulations (8, 9, 17, 27, 30, 31, 60). To elucidate whether or not the ceramide-induced activation of δPKC-GFP is through tyrosine phosphorylation, we investigated the effect of tyrosine kinase inhibitor on the C2-ceramide-induced activation of δPKC-GFP. Treatment with 10 μM C2-ceramide induced significant activation of δPKC-GFP, while treatment with 10 μM C2-dihydroceramide for 20 min did not induce significant activation of δPKC-GFP (Fig. 12A). The activation of δPKC-GFP by C2-ceramide was abolished by the pretreatment with 200 μM genistein, a nonspecific tyrosine kinase inhibitor (Fig. 12A). To determine whether or not δPKC-GFP was tyrosine phosphorylated after the treatment with C2-ceramide, tyrosine phosphorylation of δPKC-GFP was analyzed by immunoblotting using an anti-phosphotyrosine antibody. Although C2-dihydroceramide did not cause any tyrosine phosphorylation of δPKC-GFP, δPKC-GFP was significantly tyrosine phosphorylated by treatment with C2-ceramide (Fig. 12B). In addition, pretreatment with 200 μM genistein effectively blocked the tyrosine phosphorylation of δPKC-GFP induced by C2-ceramide. Immunoblotting with the anti-GFP antibody revealed that similar amounts of δPKC-GFP were immunoprecipitated in all samples.
FIG. 12.
Effects of a tyrosine kinase inhibitor on kinase activity and tyrosine phosphorylation of δPKC-GFP after C2-ceramide treatment. (A) Effect of genistein on the ceramide-induced activation of δPKC-GFP assessed by in vivo kinase assay. After pretreatment with genistein (200 μM), the δPKC-GFP was immunoprecipitated from ceramide (C2-Cer)- or C2-dihydroceramide (DH-C2-Cer)-treated cells. The kinase activities of the immunoprecipitated δPKC-GFP were assayed with H1 histone as the substrate without any activators such as PS or DO. Data are expressed as percentages of the control level (the kinase activity of δPKC-GFP from untreated cells). Statistical significance: ∗, P < 0.01 versus kinase activity of control. (B) Effect of genistein on tyrosine phosphorylation of δPKC-GFP in transfected HeLa cells treated with C2-ceramide. The δPKC-GFP was prepared as described for panel A, and tyrosine phosphorylation of δPKC-GFP was analyzed by immunoblotting using anti-phosphotyrosine (anti-p-Tyr) antibody (top row). Immunoblotting of the same membrane was performed using anti-GFP polyclonal antibody as described in Materials and Methods (bottom row). All results represent the means and standard errors of more than three determinations.
DISCUSSION
We have studied the targeting mechanism of PKC subtypes in living cells using GFP fusion proteins to elucidate the individual function of each PKC subtype. In CHO-K1 cells overexpressing γPKC-, δPKC-, and ɛPKC-GFP, spatial and temporal targeting varied depending on PKC subtype and extracellular stimulus (49, 56, 59). We further demonstrated that PKC translocation to the specific intracellular compartment (PKC targeting) is necessary for the recognition and phosphorylation of the substrates in the compartment (48). This subtype-and stimulus-specific targeting strongly suggested that the targeting mechanisms of PKC subtypes determine their individual roles in cell signaling pathways. In the present study, we examined the effects of ceramide on PKC translocation to clarify how ceramide is involved in various cell responses, especially in PKC-mediated signaling pathways.
Since HeLa cells express IFN-γ receptors coupled to the sphingomyelin-ceramide pathway, we used HeLa cells that endogenously express αPKC, δPKC, and ζPKC for the present study. Among these three endogenously expressed PKC subtypes, only δPKC was translocated to the Golgi complex by a permeable ceramide analogue. Immunoblotting analysis indicated that ceramide induced translocation of δPKC from the cytosol to the particulate fraction, suggesting that δPKC was associated with the Golgi membrane after ceramide treatment. Immunocytochemical studies also revealed that ceramide translocated endogenous δPKC but not αPKC or ζPKC to the Golgi complex. The results of the present study in living cells suggested that only δPKC, at least in HeLa cells, is responsible for the ceramide-induced cellular responses, although it is possible that other PKC subtypes expressed at undetectable levels in HeLa cells are also involved in the responses or that ceramide acts on PKC without translocation of PKC to specific subcellular compartments. Shirai et al. demonstrated that among γPKC, δPKC, and ɛPKC, only δPKC was insensitive to various fatty acids, including arachidonic acid, which induced translocation of ɛPKC to the Golgi complex (59). Ceramide also translocated ɛPKC but not γPKC to the Golgi complex in the present study (data not shown). Since ceramide translocates both ɛPKC and δPKC to the Golgi complex and arachidonic acid translocates only ɛPKC but not δPKC, it is suggested that arachidonic acid-induced translocation of ɛPKC to the Golgi complex may occur by a mechanism different from that involved in ceramide-induced translocation of δPKC and ɛPKC to the Golgi complex. Previous biochemical studies, however, showed that αPKC and δPKC have ceramide-binding abilities and that treatment with ceramide translocated ɛPKC as well as δPKC from the membrane to the cytosol fraction (57). It was also reported that ceramide induced translocation of αPKC from the cytosol to the membrane fraction (22) and that ζPKC was translocated to the perinuclear region by ceramide (16). In the present study using living HeLa cells, neither αPKC nor ζPKC responded to ceramide 60 min after treatment. The precise reason for this discrepancy is not clear, but it may have been due to differences in the cell types or experimental conditions used.
Many studies have shown that ceramide is produced through sphingomyelin hydrolysis after exposure to various extracellular stimuli, including IFN-γ (25) and TNF-α (11, 25). As shown in Fig. 6, physiological receptor stimulation by IFN-γ evoked translocation of only δPKC, but not αPKC or ζPKC, from the cytoplasm to the Golgi complex as seen when treated with ceramide. Since Mg2+-dependent neutral sphingomyelinase inhibitors such as scyphostatin and GSH inhibited IFN-γ- but not ceramide-induced translocation of δPKC, it is likely that the translocation of δPKC occurred downstream of the Mg2+-dependent neutral sphingomyelinase pathway. Furthermore, the chelation of extracellular Mg2+ completely blocked the translocation of δPKC, demonstrating that the Mg2+-dependent neutral sphingomyelinase is activated outside the plasma membrane. Since D609, an inhibitor of SMS, did not alter the ceramide-induced translocation of δPKC or that induced by IFN-γ, ceramide generated by hydrolysis of sphingomyelin, but not sphingomyelin generated from ceramide, induced PKC translocation. Although serum deprivation has been reported to induce sphingomyelin hydrolysis and generation of ceramide within 10 h after treatment (24), the serum deprivation did not alter localization of δPKC, at least within the 60-min observation period in the present study. Furthermore, because IFN-γ induced translocation both in the presence and absence of serum, translocation of δPKC did not occur through an unknown effect of serum. The TNF-α receptor is also known to be expressed in HeLa cells (13, 38), and TNF-α also induced similar but slower translocation of δPKC (data not shown). From the present findings that both AG490, a JAK2 inhibitor, and genistein, a tyrosine kinase inhibitor, completely blocked IFN-γ-induced translocation of δPKC, it is likely that Mg2+-dependent neutral sphingomyelinase is activated downstream of the IFN-γ receptor-JAK pathway and that ceramide is subsequently produced, leading to translocation of δPKC to the Golgi complex, although the detailed pathway between JAK2 and Mg2+-dependent sphingomyelinase is currently unclear.
Ceramide is widely used as a marker for the Golgi complex, as ceramide accumulates in this organelle (32). As shown in Fig. 5, C6-NBD-ceramide accumulated to the perinuclear region with a time course similar to that of C6-ceramide-induced translocation of δPKC, and finally, ceramide and δPKC accumulated to the same compartment, the Golgi complex (Fig. 5B). This simultaneous translocation of δPKC with ceramide to the Golgi complex suggested that the translocation of δPKC was due to its association with ceramide accumulating in the Golgi complex. However, NBD-ceramide was transiently accumulated on the plasma membrane just after application, but ceramide treatment did not cause translocation of δPKC to the plasma membrane. These observations suggested that ceramide may act on δPKC only at the Golgi complex but not at the plasma membrane. Although it is unclear whether δPKC binds ceramide directly or indirectly, it is possible that other components, such as anchoring protein, are necessary for the association of δPKC with ceramide in the Golgi complex. While the Golgi-associated δPKC following ceramide treatment was further translocated to the plasma membrane by TPA, the Golgi-associated NBD-ceramide was not altered by TPA treatment. This strongly suggested that the binding of δPKC to the Golgi complex is reversible and that the association and dissociation of δPKC with the Golgi complex occurred continuously. Rapid recovery of fluorescence into the bleached areas and fading of the fluorescence in the unbleached areas (Fig. 10) suggested that δPKC does not bind tightly to the Golgi complex but continuously moves in both directions between the Golgi complex and the cytoplasm.
The effects of ceramide on PKC activity are controversial; ceramide has been reported to inhibit αPKC activity by an indirect mechanism (29) and to have no direct effect on PKC activity (20), while Huwiler et al. reported an inhibitory effect of ceramide on δPKC activity in the presence of PKC activators such as DG and PS (22) in vitro. We found that ceramide did not affect the basal activity of δPKC but dose-dependently inhibited the kinase activity in the presence of PS and DO in vitro. It is noteworthy, however, that the activity of the immunoprecipitated δPKC was increased after ceramide treatment in vivo (Fig. 11B). An increase in kinase activity of immunoprecipitated δPKC was also seen after treatment with IFN-γ (1.6-fold increase) but not with dihydroceramide (data not shown). These results suggested that δPKC is not activated by a direct interaction with ceramide but is activated by unknown factors that are modulated by ceramide in the Golgi complex. Tyrosine phosphorylation is a candidate to explain the unknown factor activating δPKC in the Golgi complex. There is increasing evidence that δPKC is activated by its tyrosine phosphorylation after various stimulations of the cells (8, 9, 17, 27, 30, 31, 60). To elucidate the involvement of tyrosine phosphorylation in ceramide-induced activation of δPKC, we examined the effect of a tyrosine kinase inhibitor on ceramide-induced activation of δPKC and also on the ceramide-induced tyrosine phosphorylation of δPKC. As shown in Fig. 12, ceramide induced tyrosine phosphorylation of δPKC as well as the activation of δPKC, and genistein, a tyrosine kinase inibitor, abolished both tyrosine phosphorylation and activation of δPKC. Considering that genistein did not block the ceramide-induced translocation of the δPKC (Fig. 8), tyrosine phosphorylation of δPKC is not necessary for the translocation of δPKC to the Golgi complex. Currently, the tyrosine kinase which phosphorylates δPKC in response to ceramide remains unclear. These results strongly suggest that ceramide induces the δPKC-specific translocation to the Golgi complex and also induces the δPKC-specific activation by tyrosine phosphorylation in the Golgi complex.
Ceramide, one of the most important second messengers, has been shown to regulate various biological processes (19, 24, 47, 51, 64, 65). Among these multiple functions, ceramide has attracted attention as an intracellular mediator of apoptosis. C2-ceramide as well as ceramide generation after treatment with TNF-α caused DNA fragmentation (15, 47). Furthermore, Chin et al. reported that IFN-γ also induced apoptosis through the STAT signaling pathway (7). The involvement of δPKC in apoptosis in various types of cells was demonstrated after exposure to various extracellular stimuli (12, 26, 39, 61). These reports strongly suggested that ceramide-induced translocation of δPKC to the Golgi complex is an important step for apoptosis. However, Obeid et al. showed that PKC activation by TPA inhibited ceramide-induced DNA fragmentation (47) and suggested the involvement of two signaling pathways, ceramide- and PKC-associated pathways, in the regulation of apoptosis. The present study showed that TPA evokes translocation of δPKC to the plasma membrane even after ceramide translocates δPKC to the Golgi complex, suggesting that the inhibitory effect of TPA on DNA fragmentation is due to the different targeting of PKC but not to the activation of PKC in the same intracellular compartment to which PKC was translocated by ceramide.
In conclusion, IFN-γ stimulation followed by ceramide generation through Mg2+-dependent neutral sphingomyelinase induced δPKC-specific translocation to the Golgi complex, and this translocation resulted in δPKC activation through tyrosine phosphorylation of the enzyme.
ACKNOWLEDGMENT
This work was supported by grants from the Ministry of Education, Science, Sports, and Culture in Japan (09NP0601, 11780448, 12680754, 12210107), the Sankyo Foundation of Life Science, the Uehara Memorial Foundation, and the Hyogo Science and Technology Association.
REFERENCES
- 1.Abe J, Berk B C. Fyn and JAK2 mediate Ras activation by reactive oxygen species. J Biol Chem. 1999;274:21003–21010. doi: 10.1074/jbc.274.30.21003. [DOI] [PubMed] [Google Scholar]
- 2.Abeliovich A, Chen C, Goda Y, Silva A J, Stevens C F, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo K, Krut O, Wiegmann K, Kreder D, Micheli M, Schafer R, Sickman A, Schmidt W E, Schmidt J M, Schroder J M, Meyer H E, Sandhoff K, Kronke M. Purification and characterization of a magnesium-dependent neutral sphingomyelinase from bovine brain. J Biol Chem. 2000;275:7641–7647. doi: 10.1074/jbc.275.11.7641. [DOI] [PubMed] [Google Scholar]
- 4.Bielawska A, Crane H M, Liotta D, Obeid L M, Hannun Y A. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J Biol Chem. 1993;268:26226–26232. [PubMed] [Google Scholar]
- 5.Brann A B, Scott R, Neuberger Y, Abulafia D, Boldin S, Fainzilber M, Futerman A H. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim J J, Hashimoto K, Thompson R F, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC gamma mutant mice. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 7.Chin Y E, Kitagawa M, Kuida K, Flavell R A, Fu X Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning M F, Dlugosz A A, Howett M K, Yuspa S H. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C delta. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 9.Denning M F, Dlugosz A A, Threadgill D W, Magnuson T, Yuspa S H. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C delta. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 10.Dobrowsky R T, Werner M H, Castellino A M, Chao M V, Hannun Y A. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 11.Dressler K A, Mathias S, Kolesnick R N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- 12.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong W W, Kamen R, Weichselbaum R, et al. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelmann H, Holtmann H, Brakebusch C, Avni Y S, Sarov I, Nophar Y, Hadas E, Leitner O, Wallach D. Antibodies to a soluble form of a tumor necrosis factor (TNF) receptor have TNF-like activity. J Biol Chem. 1990;265:14497–14504. [PubMed] [Google Scholar]
- 14.Futerman A H, Stieger B, Hubbard A L, Pagano R E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medical cisternae of the Golgi apparatus. J Biol Chem. 1990;265:8650–8657. [PubMed] [Google Scholar]
- 15.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galve Roperh I, Haro A, Diaz Laviada I. Ceramide-induced translocation of protein kinase C zeta in primary cultures of astrocytes. FEBS Lett. 1997;415:271–274. doi: 10.1016/s0014-5793(97)00985-x. [DOI] [PubMed] [Google Scholar]
- 17.Gschwendt M, Kielbassa K, Kittstein W, Marks F. Tyrosine phosphorylation and stimulation of protein kinase C delta from porcine spleen by src in vitro. Dependence on the activated state of protein kinase C delta. FEBS Lett. 1994;347:85–89. doi: 10.1016/0014-5793(94)00514-1. [DOI] [PubMed] [Google Scholar]
- 18.Hannun Y A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 19.Hannun Y A. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 20.Hannun Y A, Loomis C R, Merrill A H, Jr, Bell R M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261:12604–12609. [PubMed] [Google Scholar]
- 21.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huwiler A, Fabbro D, Pfeilschifter J. Selective ceramide binding to protein kinase C-alpha and -delta isoenzymes in renal mesangial cells. Biochemistry. 1998;37:14556–14562. doi: 10.1021/bi981401i. [DOI] [PubMed] [Google Scholar]
- 23.Ibitayo A I, Sladick J, Tuteja S, Louis Jacques O, Yamada H, Groblewski G, Welsh M, Bitar K N. HSP27 in signal transduction and association with contractile proteins in smooth muscle cells. Am J Physiol. 1999;277:G445–G554. doi: 10.1152/ajpgi.1999.277.2.G445. [DOI] [PubMed] [Google Scholar]
- 24.Jayadev S, Liu B, Bielawska A E, Lee J Y, Nazaire F, Pushkareva M, Obeid L M, Hannun Y A. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 25.Kim M Y, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- 26.Konishi H, Matsuzaki H, Takaishi H, Yamamoto T, Fukunaga M, Ono Y, Kikkawa U. Opposing effects of protein kinase C delta and protein kinase B alpha on H(2)O(2)-induced apoptosis in CHO cells. Biochem Biophys Res Commun. 1999;264:840–846. doi: 10.1006/bbrc.1999.1579. [DOI] [PubMed] [Google Scholar]
- 27.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lee J Y, Hannun Y A, Obeid L M. Ceramide inactivates cellular protein kinase C alpha. J Biol Chem. 1996;271:13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Mischak H, Yu J C, Wang L M, Mushinski J F, Heidaran M A, Pierce J H. Tyrosine phosphorylation of protein kinase C-delta in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- 31.Li W, Yu J-C, Michieli P, Beeler J F, Ellmore N, Heidaran M A, Pierce J H. Stimulation of the platelet-derived growth factor β receptor signaling pathway activates protein kinase C-δ. Mol Cell Biol. 1994;14:6727–6735. doi: 10.1128/mcb.14.10.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipsky N G, Pagano R E. Intracellular translocation of fluorescent sphingolipids in cultured fibroblasts: endogenously synthesized sphingomyelin and glucocerebroside analogues pass through the Golgi apparatus en route to the plasma membrane. J Cell Biol. 1985;100:27–34. doi: 10.1083/jcb.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littman S J, Faltynek C R, Baglioni C. Binding of human recombinant 125I-interferon gamma to receptors on human cells. J Biol Chem. 1985;260:1191–1195. [PubMed] [Google Scholar]
- 34.Liu B, Hannun Y A. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- 35.Luberto C, Hannun Y A. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J Biol Chem. 1998;273:14550–14559. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- 36.Mathias S, Younes A, Kan C C, Orlow I, Joseph C, Kolesnick R N. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science. 1993;259:519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- 37.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder J S, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman C M. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature (London) 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 38.Miura M, Friedlander R M, Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuno K, Kubo K, Saido T C, Akita Y, Osada S, Kuroki T, Ohno S, Suzuki K. Structure and properties of a ubiquitously expressed protein kinase C, nPKC delta. Eur J Biochem. 1991;202:931–940. doi: 10.1111/j.1432-1033.1991.tb16453.x. [DOI] [PubMed] [Google Scholar]
- 40.Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur A G, Barbieri G, Witthuhn B A, Schindler C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature (London) 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 41.Muller Decker K. Interruption of TPA-induced signals by an antiviral and antitumoral xanthate compound: inhibition of a phospholipase C-type reaction. Biochem Biophys Res Commun. 1989;162:198–205. doi: 10.1016/0006-291x(89)91981-5. [DOI] [PubMed] [Google Scholar]
- 42.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature (London) 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 43.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature (London) 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 44.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 45.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 46.Oancea E, Teruel M, Quest A, Meyer T. Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. J Cell Biol. 1998;140:485–498. doi: 10.1083/jcb.140.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obeid L M, Linardic C M, Karolak L A, Hannun Y A. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 48.Ohmori S, Sakai N, Shirai Y, Yamamoto H, Miyamoto E, Shimizu N, Saito N. Importance of PKC targeting for the phosphorylation of its substrate, myristoylated alanine-rich C-kinase substrate (MARCKS) J Biol Chem. 2000;275:26449–26457. doi: 10.1074/jbc.M003588200. [DOI] [PubMed] [Google Scholar]
- 49.Ohmori S, Shirai Y, Sakai N, Fujii M, Konishi H, Kikkawa U, Saito N. Three distinct mechanisms for translocation and activation of the δ subspecies of protein kinase C. Mol Cell Biol. 1998;18:5263–5271. doi: 10.1128/mcb.18.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okazaki T, Bell R M, Hannun Y A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- 51.Okazaki T, Bielawska A, Bell R M, Hannun Y A. Role of ceramide as a lipid mediator of 1 alpha, 25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 52.Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, Nishizuka Y. Phorbol ester binding to protein kinase C requires a cystein-rich zinc-finger-like sequence. Proc Natl Acad Sci USA. 1989;86:4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci USA. 1989;86:3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osada S-I, Mizuno K, Saido T C, Suzuki K, Kuroki T, Ohno S. A new member of the protein kinase C family, nPKCθ, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridgway N D, Merriam D L. Metabolism of short-chain ceramide and dihydroceramide analogues in Chinese hamster ovary (CHO) cells. Biochim Biophys Acta. 1995;1256:57–70. doi: 10.1016/0005-2760(95)00010-a. [DOI] [PubMed] [Google Scholar]
- 56.Sakai N, Sasaki K, Ikegaki N, Shirai Y, Ono Y, Saito N. Direct visualization of the translocation of the gamma-subspecies of protein kinase C in living cells using fusion proteins with green fluorescent protein. J Cell Biol. 1997;139:1465–1476. doi: 10.1083/jcb.139.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawai H, Okazaki T, Takeda Y, Tashima M, Sawada H, Okuma M, Kishi S, Umehara H, Domae N. Ceramide-induced translocation of protein kinase C-delta and -epsilon to the cytosol. Implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- 58.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 59.Shirai Y, Kashiwagi K, Yagi K, Sakai N, Saito N. Distinct effects of fatty acids on translocation of gamma- and epsilon-subspecies of protein kinase C. J Cell Biol. 1998;143:511–521. doi: 10.1083/jcb.143.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soltoff S P, Toker A. Carbachol, substance P, and phorbol ester promote the tyrosine phosphorylation of protein kinase C delta in salivary gland epithelial cells. J Biol Chem. 1995;270:13490–13495. doi: 10.1074/jbc.270.22.13490. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Mukai H, Toshimori M, Miyamoto M, Ono Y. Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc Natl Acad Sci USA. 1998;95:11566–11571. doi: 10.1073/pnas.95.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka M, Nara F, Suzuki Konagai K, Hosoya T, Ogita T. Structural elucidation of sciphostatin, an inhibitor of membrane-bound neutral sphingomyelinase. J Am Chem Soc. 1997;119:7871–7872. [Google Scholar]
- 64.Venable M E, Lee J Y, Smyth M J, Bielawska A, Obeid L M. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 65.Yang S N. Ceramide-induced sustained depression of synaptic currents mediated by ionotropic glutamate receptors in the hippocampus: an essential role of postsynaptic protein phosphatases. Neuroscience. 2000;96:253–258. doi: 10.1016/s0306-4522(99)00582-5. [DOI] [PubMed] [Google Scholar]
- 66.Zhu T, Lobie P E. Janus kinase 2-dependent activation of p38 mitogen-activated protein kinase by growth hormone. Resultant transcriptional activation of ATF-2 and CHOP, cytoskeletal re-organization and mitogenesis. J Biol Chem. 2000;275:2103–2114. doi: 10.1074/jbc.275.3.2103. [DOI] [PubMed] [Google Scholar]