Abstract
The authors assessed the early antihypertensive efficacy of olmesartan medoxomil (OM) in a 12‐week prospective observational study. Of 2221 patients with untreated hypertension who received OM (mainly 10 or 20 mg), 331 patients whose blood pressure (BP) was measured at 1 week after initiation of treatment were defined as the ``early BP determination group,'' whereas the remaining 1890 patients were defined as the ``standard BP determination group.'' Baseline characteristics, doses of OM, concomitant drugs used, and BP during treatment did not differ between the 2 groups. The achievement rate of BP target (<140/90 mm Hg) was 28.4% at 1 week in the early BP determination group and 28.3% at 2 weeks in the standard BP determination group (P=NS). Rates of adverse drug reactions in the 2 groups were similar. The present study suggests that OM is safe and effective in reducing BP at an early time point of treatment.
Large‐scale clinical studies have demonstrated that lowering blood pressure (BP) in hypertensive patients reduces the incidence of cardiovascular events; results of some studies have suggested that prompt and more effective BP control may be important for the prevention of cardiovascular events. 1 , 2 , 3
There is a perception that calcium channel blockers (CCBs) have a relatively rapid‐onset antihypertensive effect, whereas angiotensin II type 1 (AT1) receptor blockers (ARBs) may have a slower‐onset effect. 4 Olmesartan medoxomil (OM) is an ARB with high affinity 5 , 6 , 7 and selectivity toward AT1 receptors. 8 OM has been shown to elicit more potent antihypertensive effects at 2 weeks after starting treatment compared with other ARBs tested. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16
While previous studies have suggested that OM lowers BP levels as early as 1 week after commencing treatment, 17 , 18 the early treatment effects of OM have not been studied in a large number of patients.
The present study is a secondary analysis of a previously reported 12‐week prospective observational study in hypertensive patients who received OM in the setting of daily clinical practice in Japan between July 2004 and September 2005. 19 , 20 In this report, we assessed BP levels at early time points (1 or 2 weeks) of treatment with OM monotherapy in 2221 patients.
Methods
Study Design and Participants
This study followed an open, prospective cohort design. The study protocol was approved by the In‐House Ethical Committee of Sankyo (presently Daiichi Sankyo following merger) and was based on the pharmaceutical affairs law in Japan. The protocol was submitted to and approved by the Ministry of Health, Labour and Welfare of Japan before study commencement. This study was carried out in medical institutions registered according to good postmarketing surveillance practice in Japan.
Participants were OM‐naive and had essential hypertension. Physicians in several medical institutions were asked to select and register patients at the registration center within 14 days of starting OM therapy. The registration period was 1 year, from July 2004 to June 2005. OM (mainly 10 or 20 mg) was administered at each participating physician’s discretion. Neither prior treatment nor combination drugs were restricted. The standard observation period was 12 weeks. BP, clinical laboratory data, and adverse events were recorded.
In this secondary analysis, we selected patients with untreated hypertension who received OM monotherapy and whose BP was determined before and within 3 weeks (4–21 days) of starting OM treatment. Patients were divided into 2 groups, those whose BP was and those whose BP was not measured at 1 week (7±3 days) after starting OM (“early BP determination group” and “standard BP determination group,” respectively). The main efficacy variable was the change from baseline in BP and achievement rate of BP target (<140/90 mm Hg) at the earliest time point measured (1 or 2 weeks).
Statistical Analysis
Continuous variables and categorical variables were expressed as mean±SD and rate (%), respectively, and were compared between the 2 groups by t‐test and Fisher’s exact test as appropriate. The time course of changes in BP was analyzed by Dunnett’s multiple comparison test. Multiple logistic regression analysis adjusted for baseline characteristics of the patients was used to test whether BP determination at 1 or 2 weeks affected the achievement rate of the BP target at the end of the study. The covariate factors used were sex, age (younger than 65, 65–74, and 75 years or older), presence or absence of comorbid conditions, and systolic BP (SBP)/diastolic BP (DBP) before treatment with OM.
P values <.05 were defined as significant. Statistical analyses were performed using SAS System Release 8.2 (SAS Institute Inc., Cary, NC).
Results
Patient Disposition
Of 6261 patients included in the main study, those who had been receiving other antihypertensive agents when treatment with OM was started (n=3306) and those whose BP was not determined before or within 4 to 21 days after initiation of OM treatment (n=734) were excluded. Hence, 2221 patients were analyzed in the present study. The early BP determination and standard BP determination groups comprised 331 and 1890 of the 2221 patients, respectively. The patient disposition is shown in Figure 1.
Figure 1.
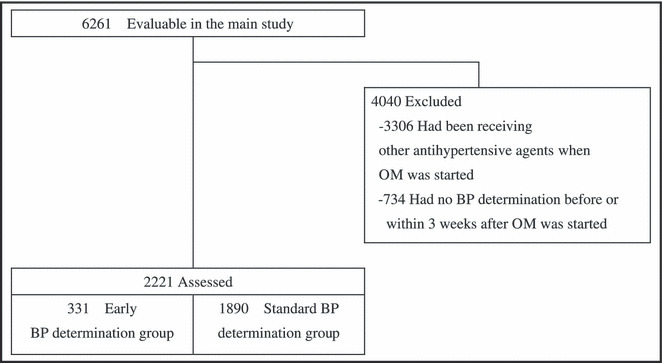
Study population and patient disposition. OM indicates olmesartan medoxomil; BP, blood pressure.
Baseline Characteristics and Drug Administration
Baseline characteristics of the 2 groups are shown in Table I. No significant difference was noted between the 2 groups at baseline, except mean age.
Table I.
Baseline Characteristics of Patients
| Early BP Determination Group (n=331) | Standard BP Determination Group (n=1890) | |
|---|---|---|
| Women | 176 (53.2) | 1027 (54.3) |
| Age, y | 62.2±11.9 | 63.8±11.7a |
| Age range, y | ||
| <65 | 182 (55.0) | 961 (50.8) |
| 65–74 | 101 (30.5) | 571 (30.2) |
| ≥75 | 48 (14.5) | 358 (18.9) |
| BMI, kg/m2 | 24.5±3.5 | 24.2±3.5 |
| SBP, mm Hg | 169.0±16.7 | 168.2±16.8 |
| DBP, mm Hg | 95.6±12.5 | 95.2±12.0 |
| Pulse rate, beats/min | 73.7±10.2 | 75.0±10.5 |
| Comorbid conditions | 204 (61.6) | 1200 (63.5) |
| Hyperlipidemia | 129 (39.0) | 629 (33.3) |
| Diabetes mellitus | 37 (11.2) | 268 (14.2) |
| Heart disease | 16 (4.8) | 133 (7.0) |
| Cerebrovascular disorder | 10 (3.0) | 92 (4.9) |
| Liver disease | 39 (11.8) | 171 (9.0) |
| Renal disease | 7 (2.1) | 57 (3.0) |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.Values are mean ± SD or No. (%). a P<.05.
Initial mean dosage of OM in the early and standard BP determination groups was 16.8±4.8 and 17.1±4.8 mg/d, respectively; approximately 70% and 30% of patients in both groups started OM at 20 and 10 mg/d, respectively.
In the early BP determination group, 330/331 (99.7%) and 161/186 patients (86.6%) remained on OM monotherapy at 1 and 12 weeks of treatment. Similarly, 1856/1890 (98.2%) and 1187/1372 patients (86.5%) in the standard BP determination group remained on OM monotherapy at 2 and 12 weeks, respectively. Thus, roughly the same proportion of patients remained on OM monotherapy at the end of the study in both groups (Table II). The most frequently added antihypertensive drug class was CCBs, which were given to 10.8% (20/186 patients) and 10.5% (144/1372 patients) in the 2 groups, respectively (Table II).
Table II.
Patients’ Distribution According to Number and Class of Additional Antihypertensive Drugs Being Taken at Each Time Point of BP Determination (1 or 2 and 12 Weeks of Treatment)
| Early BP Determination Group | Standard BP Determination Group | |||
|---|---|---|---|---|
| 1 Week (n=331) | 12 Weeks (n=186) | 2 Weeks (n=1890) | 12 Weeks (n=1372) | |
| No. | ||||
| 0 | 330 (99.7) | 161 (86.6) | 1856 (98.2) | 1187 (86.5) |
| 1 | 1 (0.3) | 20 (10.8) | 33 (1.7) | 163 (11.9) |
| 2 | 0 (0.0) | 5 (2.7) | 1 (0.1) | 21 (1.5) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| ≥4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Class | ||||
| Diuretic | 0 (0.0) | 5 (2.7) | 9 (0.5) | 35 (2.6) |
| α‐Blocker | 0 (0.0) | 2 (1.1) | 1 (0.1) | 10 (0.7) |
| β‐Blocker | 0 (0.0) | 1 (0.5) | 1 (0.1) | 14 (1.0) |
| CCB | 1 (0.3) | 20 (10.8) | 23 (1.2) | 144 (10.5) |
| ACE inhibitor | 0 (0.0) | 0 (0.0) | 1 (0.1) | 4 (0.3) |
| ARB | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 2 (1.1) | 0 (0.0) | 0 (0.0) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin II type 1 receptor blocker; BP, blood pressure; CCB, calcium channel blocker. Values are No. (%). The patients’ distribution represents that in 1 day prior to BP determination at 1, 2, and 12 weeks.
Efficacy
Changes of BP from baseline at the earliest time point measured (1 or 2 weeks) and at 12 weeks are shown in Figure 2. Mean±SD change in SBP/DBP from baseline was 21.9±17.1/10.0±10.9 mm Hg at 1 week in the early BP determination group and 21.7±18.1/10.4±10.8 mm Hg in the standard BP determination group (P=NS). No significant difference was detected between the 2 groups. Achievement rate of the BP target (<140/90 mm Hg) in patients in the early BP determination group before OM administration was 0.3%, while the rates at 1 and 12 weeks after initiation of OM were 28.4% and 52.2%, respectively. In the standard BP determination group, the rates before and at 2 and 12 weeks were 1.1%, 28.3%, and 50.5%, respectively. No significant difference was detected between the achievement rate at 1 week in the early BP determination group and that at 2 weeks in the standard BP determination group (P=.947).
Figure 2.
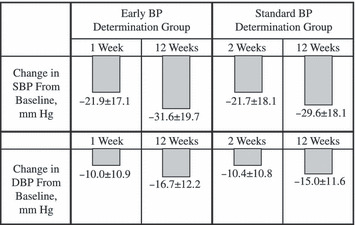
Changes from baseline in blood pressure (BP) at early time point measured (1 or 2 weeks) and at 12 weeks. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
The time course of changes in BP is shown in Figure 3. SBP and DBP in the early BP determination group were significantly (P<.0001) reduced to 147.0±15.7/85.6±10.5 mm Hg at 1 week. Likewise, SBP and DBP in the standard BP determination group were significantly (P<.0001) and comparably reduced to 146.5±17.3/84.7±11.0 mm Hg at 2 weeks. SBP and DBP in the 2 groups were not significantly different at 4, 8, and 12 weeks of OM administration (t‐test).
Figure 3.
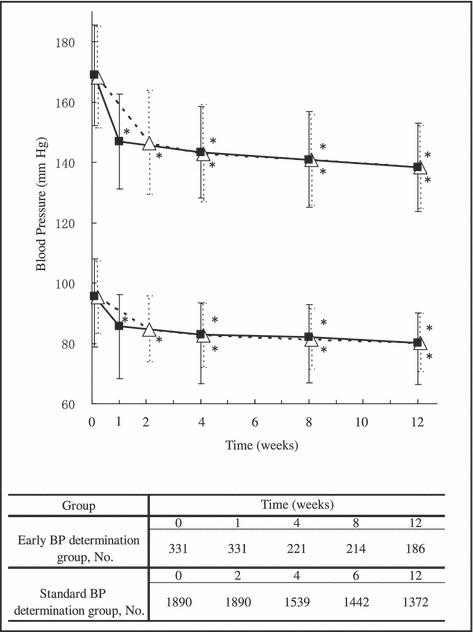
Time course of changes in blood pressure (BP). Upper and lower symbols represent changes in mean (SD) systolic BP and diastolic BP, respectively. Filled small squares and open large triangles were values obtained from the early determination group and standard determination group, respectively. *Significant (P<.0001) difference vs before treatment by Dunnett’s multiple comparison test. The table at the bottom of the figure shows the number of patients whose BP was determined at each time point.
The odds ratio for achieving the BP target at the end of the study in the early BP determination vs standard BP determination group was 1.071 (95% confidence interval, 0.837–1.370), indicating no significant difference between the 2 groups.
Safety
The incidence rates of adverse drug reactions in the early BP determination group and the standard BP determination group were 2.7% (9/331 patients) and 3.7% (70/1890 patients), respectively, indicating that there was no significant difference between groups. Furthermore, incidence rates of adverse drug reactions associated with excessive BP lowering such as dizziness, postural dizziness, and hypotension were 0.6% (2/331 patients) and 1.0% (18/1890 patients) in the early BP determination group and in the standard BP determination group, respectively, again indicating no significant difference between groups. All patients recovered from their adverse drug reactions.
Discussion
This report describes a secondary analysis of efficacy data from a previously published 12‐week prospective observational study of the antihypertensive effects of OM. 19 , 20 The present study indicates that OM monotherapy provided significant and safe BP reduction as early as the first week of treatment. The achievement rate of the BP target was 28.4% at 1 week and 28.3% at 2 weeks after initiation of OM treatment.
To assess whether the results achieved at 1 week of treatment in 331 patients could be extrapolated to 1890 previously untreated patients whose BP was not assessed at 1 week, a comparison was made between the 2 groups regarding baseline characteristics, efficacy, safety, and BP target achievement rates. No significant difference in these parameters was detected between the 2 groups, with the exception of the mean age of the patients. It is considered unlikely that the small difference of age would affect the pharmacokinetics of OM in the 2 groups; thus, it is concluded that BP findings at 1 week in the early BP determination group might possibly be extrapolated to the standard BP determination group.
Furthermore, we tested whether determination of BP level at 1 week affected the achievement rate of the BP target at the end of the study using multiple logistic regression analysis. The odds ratio of achieving the BP target in the early vs standard BP determination group was 1.071, suggesting that measuring BP at 1 week vs at 2 weeks does not predict a different outcome of achieving BP target.
Several factors contribute to the prompt BP‐lowering effects of OM, including high affinity and persistent binding to AT1 receptors and favorable pharmacokinetics. Angiotensin II levels are increased shortly after administration of an ARB due to the blockade of normal feedback inhibition of renin release. Thus, administration of ARBs with weak AT1 receptor affinity may not sufficiently antagonize the stimulated AT1 receptors, resulting in gradual BP lowering. However, ARBs with a high affinity for AT1 receptors, such as OM, may achieve more complete blockade of AT1 receptors, thereby causing more rapid BP lowering. It may be possible that BP reduction at 1 week is achievable with other antihypertensive agents.
The incidence rate of adverse drug reactions in the early BP determination group was 2.7%, whereas that in the standard BP determination group was 3.7% (P=NS). Furthermore, adverse drug reactions associated with excessive BP lowering occurred in 0.6% in the early BP determination group and 1.0% in the standard BP determination group (P=NS). These similar results further underscore the contention that patients in the 2 groups responded identically to OM treatment.
The BP‐lowering efficacies of various ARBs at early time points have been reported previously. In the study by Kassler‐Taub and associates, 21 achievement rates of the DBP target (<90 mm Hg) at 1 week were 48% with irbesartan 300 mg, 31% with losartan potassium 100 mg, and 26% with placebo. In an analysis of the BP‐lowering efficacy of incremental doses of valsartan given either alone or together with hydrochlorothiazide, the achievement rates of the BP target (<140/90 mm Hg) at 1 week were 5% to 28%. 22
Successful early reduction of BP may confer additional benefit over slower BP reduction. 1 , 2 , 3 Moreover, it is believed that rapid reduction of BP as early as the first week of treatment may improve patient adherence, thereby enhancing reduction of cardiovascular risk. 23
Some limitations of this study should be considered. The design of the study was to represent the “real world” of clinical practice, and consequently patients were not blinded to treatment and no placebo comparison was used. Moreover, this is a secondary analysis of existing clinical trial data that was not designed to assess early antihypertensive efficacy. It was not possible to compare BP at 1 week and at 2 weeks in the same patient because the number of patients with BP determinations at both time points was limited.
In conclusion, the present study suggests that OM monotherapy is safe and effective in producing rapid BP reduction in the first week of treatment.
Acknowledgments and disclosures:
The authors express their deep gratitude to the many physicians who cooperated in this study. We are thankful to Suzanne Oparil, MD, Vascular Biology and Hypertension Program, University of Alabama at Birmingham, for her comments and suggestions in the preparation of the manuscript. This study was supported for funding and data collection by Sankyo Co., Ltd. (presently Daiichi Sankyo following merger). Statistical analysis was conducted by Daiichi Sankyo Co., Ltd. and ACRONET Corp. Ikuo Saito, MD, and Toshio Kushiro, MD, provided intellectual advice for the study concept, design, and scientific interpretation of the study results. Mayumi Ishikawa, Yasuyuki Matsushita, Kei Sagawa, Katsutoshi Hiramatsu, and Masahiro Komiya are employees of Daiichi Sankyo.
References
- 1. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2231. [DOI] [PubMed] [Google Scholar]
- 2. Staessen JA, Thijis L, Fagard R, et al. Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens. 2004;22:847–857. [DOI] [PubMed] [Google Scholar]
- 3. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 4. Guideline Subcommittee . Japanese Society of Hypertension Guidelines for the Management of Hypertension 2004. Hypertens Res. 2006;29(suppl):S1–S105. [DOI] [PubMed] [Google Scholar]
- 5. Mizuno M, Sada T, Ikeda M, et al. Pharmacology of CS‐866, a novel nonpeptide angiotensin II receptor antagonist. Eur J Pharmacol. 1995;285:181–188. [DOI] [PubMed] [Google Scholar]
- 6. Yanagisawa H, Amemiya Y, Kanazaki T, et al. Nonpeptide angiotensin II receptor antagonists: synthesis, biological activities, and structure‐activity relationships of imidazole‐5‐carboxylic acids bearing alkyl, alkenyl, and hydroxyalkyl substituents at the 4‐position and their related compounds. J Med Chem. 1996;39:323–338. [DOI] [PubMed] [Google Scholar]
- 7. Miura S, Fujino M, Saku K, et al. Angiotensin II receptor blocker as an inverse agonist: a current perspective. Curr Hypertens Rev. 2005;1:115–121. [Google Scholar]
- 8. Koike H, Konse T, Sada T, et al. Olmesartan medoxomil, a novel potent angiotensin II blocker. Annu Rep Sankyo Lab. 2003;55:1–99. [Google Scholar]
- 9. Brunner HR, Laeis P. Clinical efficacy of olmesartan medoxomil. J Hypertens. 2003;21(suppl):S43–S46. [DOI] [PubMed] [Google Scholar]
- 10. Stumpe KO. Olmesartan compared with other angiotensin II receptor antagonists: head‐to‐head trials. Clin Ther. 2004;26(suppl A):A33–A37. [DOI] [PubMed] [Google Scholar]
- 11. Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens. 2001;3:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neutel JM. Clinical studies of CS‐866, the newest angiotensin II receptor antagonist. Am J Cardiol. 2001;87: 37–43C. [DOI] [PubMed] [Google Scholar]
- 13. Stumpe KO, Ludwig M. Antihypertensive efficacy of olmesartan compared with other antihypertensive drugs. J Hum Hypertens. 2002;16(suppl 2):S24–S28. [DOI] [PubMed] [Google Scholar]
- 14. Fabia MJ, Abdilla N, Oltra R, et al. Antihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24 h ambulatory blood pressure monitoring. J Hypertens. 2007;25:1327–1336. [DOI] [PubMed] [Google Scholar]
- 15. Zannad F, Fay R. Blood pressure‐lowering efficacy of olmesartan relative to other angiotensin II receptor antagonists: an overview of randomized controlled studies. Fundam Clin Pharmacol. 2007;21:181–190. [DOI] [PubMed] [Google Scholar]
- 16. Chrysant SG, Melino M, Karki S, et al. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double‐blind, placebo‐controlled, 8‐week factorial efficacy and safety study. Clin Ther. 2008;30:587–604. [DOI] [PubMed] [Google Scholar]
- 17. Brunner HR, Stumpe KO, Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24‐hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Invest. 2003;23:419–430. [DOI] [PubMed] [Google Scholar]
- 18. Brunner HR, Arakawa K. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil in achieving 24‐hour blood pressure reductions and ambulatory blood pressure goals. Clin Drug Invest. 2006;26:185–193. [DOI] [PubMed] [Google Scholar]
- 19. Saito I, Kushiro T, Yamakawa M, et al. Drug use result survey of Olmetec® Tablets (olmesartan medoxomil). J Clin Ther Med. 2006;22:873–893. (In Japanese) [Google Scholar]
- 20. Saito I, Kushiro T, Ishikawa M, et al. Drug use result survey of Olmetec® Tablets (olmesartan medoxomil) 2nd report. J Clin Ther Med. 2007;23:695–705. (In Japanese) [Google Scholar]
- 21. Kassler‐Taub K, Littlejohn T, Elliott W, et al. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan, in mild‐to‐moderate hypertension. Am J Hypertens. 1998;11:445–453. [DOI] [PubMed] [Google Scholar]
- 22. Weir MR, Levy D, Crikelair N, et al. Time to achieve blood‐pressure goal: Influence of dose of valsartan monotherapy and valsartan and hydrochlorothiazide combination therapy. Am J Hypertens. 2007;20:807–815. [DOI] [PubMed] [Google Scholar]
- 23. Neutel JM, Smith DHG, Weber MA. Low‐dose combination therapy: An important first‐line treatment in the management of hypertension. Am J Hypertens. 2001;14: 286–292. [DOI] [PubMed] [Google Scholar]


