Abstract
Endothelial dysfunction is a major determinant of atherosclerosis and a negative prognostic factor in patients with coronary artery disease and hypertension. Recovery of endothelial dysfunction has been associated with improved prognosis in these patients. The aim of the present study was to verify whether antagonism of angiotensin II AT1 receptors with an angiotensin receptor blocker, candesartan, improved endothelial function in patients with hypertension, stable coronary artery disease, and endothelial dysfunction. We studied 26 patients who were receiving β‐blockers with optimal blood pressure control, in a randomized, double blind study. Patients were randomized to placebo (n=13) or to candesartan 16 mg/d (n=13) for 2 months. Endothelial function was assessed by ultrasound using hyperemic flow‐mediated dilation of the brachial artery. Mean arterial blood pressure was unchanged in both groups (from 93.3±9.2 to 93.2±17.3 mm Hg in the candesartan group and from 101.3±14.2 to 102.3±13.9 mm Hg in the placebo group; both P=ns). Maximal blood flow was similar between placebo and candesartan groups at baseline and at the end of the study, whereas flow‐mediated dilation significantly increased in the candesartan group (from 5.27%±1.69% to 7.15%±2.67%; P=0.01) but remained unchanged in the placebo group (from 4.49%±1.97% to 5.88%±2.30%; P=ns). AT1 receptor antagonism with candesartan, in addition to β‐blocker therapy, improves endothelial function in high‐risk hypertensive patients.
Endothelial dysfunction (ED) is a major determinant of progression and destabilization of atherosclerosis. 1 , 2 It is common in subjects with or at risk for ischemic heart disease, including hypertensives, people with hypercholesterolemia, smokers and diabetics. 3 , 4 , 5 , 6 , 7 It represents a common pathophysiological pathway through which major cardiovascular risk factors promote atherosclerosis. In these categories of patients, ED, measured at the coronary or peripheral level, represents an independent prognostic indicator for the occurrence of adverse ischemic cerebrovascular and cardiovascular events. 3 , 4 , 5 , 6 , 7 Improvement of ED has been reported to be associated with a more favorable prognosis in hypertensive women 8 and in patients with acute coronary syndromes. 9 Thus, ED is emerging as a possible relevant therapeutic target for pharmacologic treatment that may play a role in influencing the choice of drugs in high‐risk patients. 8 , 9
Angiotensin‐converting enzyme inhibitors improve endothelial function. 10 More recently, this action has also been reported for AT1 receptor antagonists in different patient populations. 11 , 12 , 13 , 14 No one study, however, has thus far assessed the effects of AT1 receptor antagonism in high‐risk patients with hypertension and coronary artery disease (CAD) with sustained ED despite conventional antiischemic treatment and blood pressure control.
Methods
Study Population and Design
This was a randomized, double‐blinded, placebo‐controlled, phase III add‐on study. To enter the study, eligible patients had to fulfill the following criteria: (1) angiographically documented CAD defined as the presence of a coronary stenosis ≥50% of the vessel diameter in at least one major coronary artery; (2) no acute coronary syndromes in the previous 3 months; (3) history of hypertension; (4) controlled blood pressure values (ie, systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg, measured according to recommendations of the European Society of Cardiology guidelines 15 ) while on β‐blocker therapy; (5) no previous use of angiotensin‐converting enzyme inhibitors or AT1 receptor antagonists; and (6) sustained endothelial dysfunction by brachial hyperemic flow‐mediated dilation (FMD), defined as FMD <9% in 2 consecutive measurements 15 days apart.
The protocol was approved by the Institutional Ethics Committee and each patient gave written informed consent to participate.
FMD Assessment
FMD evaluation was carried out in the morning, after an overnight fast, in a quiet room at a constant temperature of 21±1°C, as previously reported. 16 All subjects abstained from smoking and intake of caffeine‐containing food or beverages for at least 12 hours before the study. Endothelial function in the form of FMD was measured according to recent guidelines, 16 by ultrasound, using a 7.5 MHz linear‐array transducer. Briefly, FMD was assessed by measuring with ultrasound unit electronic calipers, the change in brachial artery diameter after 60 seconds of reactive hyperemia compared with baseline measurements after deflation of a cuff placed around the forearm that had been inflated to 50 mm Hg above systolic blood pressure for 5 minutes. The response of the vessel diameter to reactive hyperemia was expressed as a percent change relative to the diameter immediately before cuff inflation, at baseline and at end of study protocol. In our laboratory, the intraobserver variability for repeated measurements of resting arterial diameter is 0.01±0.02 mm. 6 When reactive hyperemia studies are performed on 2 different days, the between‐occasion, within‐patients difference for measurement of FMD is 1.5%± 0.7%. 6 FMD data obtained in the patient population were compared to those of a historical reference population represented by 28 healthy subjects without evidence of atherosclerotic disease previously reported from our laboratory. 17
Statistical Analysis
Data are expressed as mean ± standard deviation. Comparisons among groups were performed by univariate analysis of variance (ANOVA) test for multiple comparisons. Comparisons between baseline and 2‐months values were performed by paired Student t‐test. A value of P<0.05 (two‐sided) was considered significant.
Results
Of 32 consecutive patients screened, 28 demonstrated persistent impairment of FMD at visit 2 and were randomized to placebo (n=14) or candesartan (n=14) in addition to β‐blocker therapy which consisted of atenolol in 15 (54%), carvedilol in 3 (11%), and metoprolol in 10 (35%) patients. As reported in the Table, characteristics of the 2 groups were similar with regards to age, gender, hemodynamic, and lipid parameters. One patient in each group refused to continue the study; final analysis therefore includes 26 patients.
Table.
Demographic and Clinical Characteristics of Study Population
| Total | Candesartan | Placebo | P | |
|---|---|---|---|---|
| Age | 58±10 | 55±8 | 60±12 | NS |
| Male | 27 (96%) | 13 (93%) | 14 (100%) | NS |
| Smoke | 25 (89%) | 13 (93%) | 12 (86%) | NS |
| Alcohol | 7 (25%) | 3 (21%) | 4 (29%) | NS |
| Months from diagnosis of hypertension | 69±50 | 62±55 | 75±44 | NS |
| β‐Blockers | ||||
| Atenolol | 15 (54%) | 5 (36%) | 10 (71%) | NS |
| Carvedilol | 3 (11%) | 3 (21%) | 0 (0%) | |
| Metoprolol | 10 (35%) | 6 (43%) | 4 (29%) | |
| Blood pressure | ||||
| Systolic (mm Hg) | 123±13 | 119±9 | 126±15 | NS |
| Diastolic (mm Hg) | 78±8 | 79±9 | 78±7 | NS |
| FMD | 4.8±0.8 | 4.6±0.9 | 5.0±0.7 | NS |
Abbreviations: NS, not significant; FMD, flow‐mediated dilation.
Hemodynamic Effects of Therapy
Heart rate did not change in patients assigned to placebo from randomization to end of study (from 64±7 to 65±7; P=ns) whereas it significantly decreased in patients assigned to candesartan (from 64±9 to 58±4; P<0.01). Mean arterial blood pressure did not change significantly in the placebo group (from 101.3±14.2 mm Hg to 102.3±13.9 mm Hg; P=ns) and in the candesartan group (from 93.3±9.2 mm Hg to 93.2±17.3 mm Hg; P=ns). There was no correlation between change in blood pressure between baseline and end of treatment and change of FMD (R=0.154; P=0.452).
FMD Evaluation
Basal and hyperemic diameter of the brachial artery at baseline and at end of study did not change in both groups (basal: 4.59±0.9 vs. 4.48±0.71, P=ns, and hyperemia: 4.82±0.89 vs. 4.80±0.74, P=ns, for candesartan; basal: 5.04±0.71 vs. 5.02±0.25, P=ns, and hyperemia: 5.26±0.7 vs. 5.31±0.66, P=ns, for placebo). Similarly, no differences were observed in basal and hyperemic flow from baseline to end of study in both groups of patients (basal: 101.1±28 vs. 107.7±31.6, P=ns, and hyperemia: 360.2±87.9 vs. 373.8±62.5, P=ns, in the candesartan group; basal: 105.2±23.7 vs. 101.8±20.6, P=ns and hyperemia: 326.6±88.5 vs. 342.4±65.9, P=ns, in the placebo group) (Figure 1). FMD at baseline was significantly reduced (ANOVA P value <0.0001) in both groups of patients compared to controls (11.4%±3.4%; P<0.01 for both), but it did not differ between candesartan and placebo groups (Figure 2). After 2 months of therapy, FMD significantly (ANOVA P value=0.025) increased in the candesartan group (from 5.27%±1.69% to 7.15%±2.67%; P=0.01) but did not significantly change in the placebo group (from 4.49±1.97 to 5.88±2.30; P=ns; Figure 2).
Figure 1.
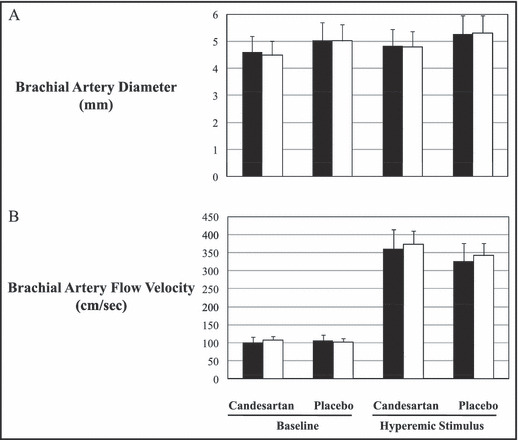
(A) Basal and hyperemic diameter of the brachial artery at randomization (black bars) and at end of study (white bars). (B) Basal and hyperemic flow of the brachial artery at randomization (black bars) and at end of study (white bars).
Figure 2.
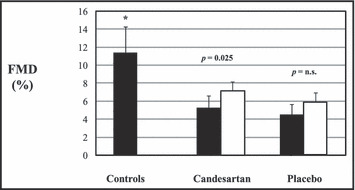
Flow‐mediated dilation (FMD) of brachial artery at baseline (black bars) and after 2 months of therapy (white bars). *P<0.01 vs both candesartan and placebo groups. n.s. indicates not significant.
Discussion
The findings of the present study demonstrated that AT1 receptor antagonism with candesartan, at the commonly employed dose of 16 mg/d for 8 weeks, improves endothelial function in hypertensive patients with stable CAD and with sustained ED. A relevant finding of the study was that candesartan was administered in addition to standard antiischemic therapy and in patients with well controlled blood pressure values.
Previous Studies
To our knowledge this is the first study in which the effects of an AT1 receptor antagonist on endothelial function were assessed in a randomized, double blind, placebo‐controlled group of patients affected by CAD and hypertension, with sustained ED despite optimal blood pressure control. In addition, no previous randomized studies have utilized hyperemic brachial FMD evaluation to assess endothelial function. This currently represents a well validated, non‐invasive tool for endothelial function testing in humans. 18
Previous studies have reported a beneficial effect of candesartan on endothelial function in different patient populations, using different methodological approaches. This drug was shown to improve endothelial function, evaluated by venous occlusion plethysmography, in normotensive hypercholesterolemic subjects. 13 Ghiadoni et al. 11 reported improved endothelial function, evaluated by strain gauge venous plethysmography, after chronic administration of candesartan in patients with essential hypertension and basal elevated blood pressure. In that study angiotensin receptor blocker treatment resulted in a significant reduction of blood pressure compared to baseline levels. Using a brachial hyperemic FMD, Isobe et al. 12 also reported in a nonrandomized trial, that candesartan administration improved endothelial function in hypertensive patients with elevated blood pressure. Again, in that study treatment was associated with a substantial blood pressure decrease. In addition, in a population of hypertensive patients with uncontrolled blood pressure receiving no other drugs, Koh et al., 19 in a randomized placebo‐controlled trial, reported that the administration of 16 mg/d of candesartan for 2 months significantly decreased blood pressure and improved endothelial function evaluated by brachial FMD. Compared to the current study, the patients enrolled by Koh et al. did not have concomitant CAD, had elevated blood pressure, and were not selected on the basis of ED. In patients with CAD, acute intrabrachial administration of candesartan has been shown to enhance endothelial function evaluated by brachial FMD. 14 In the chronic setting, the same group also reported that 4 weeks’ administration of losartan significantly improved endothelial function evaluated by radial artery ultrasound in patients with CAD. 20 Other studies with different angiotensin receptor blockers and other renin‐angiotensin inhibitors have also demonstrated improvement in ED.
Mechanisms of Endothelial Function Improvement Using AT1 Receptor Antagonists
Pathophysiological mechanisms which account for the beneficial effects of AT1 receptor antagonists on endothelial function have not been clarified. Experimental evidence indicates that AT1 receptor antagonism exerts an antioxidative effect mediated through inhibition of angiotensin II stimulation of NADPH activity which leads to reduced superoxide production and nitric oxide (NO) degradation. 21 In addition, AT1 antagonism as well as angiotensin‐converting enzyme inhibition enhances the antioxidant activity of the superoxide dismutase, contributing to increased NO availability. 20 More recently, it has been also reported that AT1 receptor antagonism, likely through stimulation of the AT2 receptors, activates bradykinin/B2 receptor‐mediated NO production, which may also represent a relevant mechanism for endothelial function amelioration. 14 Finally, it has been demonstrated that AT1 receptor antagonists reduce inflammation in patients with CAD. This may represent the final favorable effect of the antioxidant properties of these drugs, contributing to endothelial function improvement. 22
Clinical Value of Endothelial Function Evaluation
The independent prognostic role of endothelial dysfunction has been repeatedly reported in patients at risk for cardiovascular disease as well as in those with known CAD or peripheral arterial disease. 3 , 4 , 5 , 6 , 7 Recent studies also indicate that endothelial dysfunction is not only a marker of prognosis, but may be a relevant therapeutic target. 8 , 9 It has been reported 8 that in hypertensive women treated with different pharmacologic therapies, cardiovascular risk over a follow‐up of 67 months was reduced only in those patients in whom ED was ameliorated. Very recently, in patients with acute myocardial infarction, Fichtlscherer et al. 9 reported that prognosis over a follow‐up of 47 months was significantly more favorable in those patients who demonstrated improved endothelial function 8 weeks after the acute coronary syndrome. These observations strongly suggest that the presence of ED is associated with an adverse prognosis in patients with known CAD, and suggest that reversal of ED may be associated with reduced cardiovascular risk in these patients. However, these observations do not prove a cause‐effect relationship between enhancement of endothelial function and reduction of cardiovascular adverse events; further prospective studies are warranted to verify the value of endothelial function as an intermediate end point to which therapy might be targeted.
Conclusions
In hypertensive coronary patients, with sustained ED despite blood pressure control, antagonism of AT1 receptors with candesartan improves ED. Follow‐up studies are warranted to evaluate whether improvement of ED is associated with a cardioprotective effect of their agent, candesartan, against adverse cardiac events in these patients.
References
- 1. Ross E. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 2. Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:S32–S39. [DOI] [PubMed] [Google Scholar]
- 3. Suwaidi JA, Hamasaki S, Higano ST, et al. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 4. Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. [DOI] [PubMed] [Google Scholar]
- 5. Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. [DOI] [PubMed] [Google Scholar]
- 6. Brevetti G, Silvetsro A, Schiano V, et al. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease. Additive value of flow‐mediated dilation to ankle‐brachial pressure index. Circulation. 2003;108:2093–2098. [DOI] [PubMed] [Google Scholar]
- 7. Bugiardini R, Manfrini O, Pizzi C, et al. Endothelial function predicts future development of coronary artery disease. A study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–2523. [DOI] [PubMed] [Google Scholar]
- 8. Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. [DOI] [PubMed] [Google Scholar]
- 9. Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes. Further evidence foe the existence of the “vulnerable” patient. Circulation. 2004;110:1926–1932. [DOI] [PubMed] [Google Scholar]
- 10. Mancini GB, Henry GC, Macaya C, et al. Angiotensin‐converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing Endothelial Dysfunction) Study. Circulation. 1996;94:258–265. [DOI] [PubMed] [Google Scholar]
- 11. Ghiadoni L, Virdis A, Magagna A, et al. Effect of the angiotensin type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;35:501–506. [DOI] [PubMed] [Google Scholar]
- 12. Isobe N, Taniguchi K, Oshima S, et al. Candesartan cilexetil improves left ventricular function, left ventricular hypertrophy, and endothelial function in patients with hypertensive heart disease. Circ J. 2002;66:993–999. [DOI] [PubMed] [Google Scholar]
- 13. Wassmann S, Hilgers S, Laufs U, et al. Angiotensin II type 1 receptor antagonism improves hypercholesterolemia‐associated endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2002;22:1208–1212. [DOI] [PubMed] [Google Scholar]
- 14. Hornig B, Kohler C, Schlink D, et al. AT1‐receptor antagonism improves endothelial function in coronary artery disease by a bradykinin/B2‐receptor‐dependent mechanism. Hypertension. 2003;41:1092–1095. [DOI] [PubMed] [Google Scholar]
- 15. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. [DOI] [PubMed] [Google Scholar]
- 16. Corretti MC, Anderson TJ, Benjamin EJ, et al. International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 17. Brevetti G, Silvestro A, Di Giacomo S, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–379. [DOI] [PubMed] [Google Scholar]
- 18. Widlansky ME, Gokce N, Keaney JF, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. [DOI] [PubMed] [Google Scholar]
- 19. Koh KK, Ahn JY, Han SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–910. [DOI] [PubMed] [Google Scholar]
- 20. Hornig B, Landmesser U, Kobler C, et al. Comparative effect of ACE inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease. Role of superoxide dismutase. Circulation. 2001;103:799–805. [DOI] [PubMed] [Google Scholar]
- 21. Warnholtz A, Nickenig G, Schulz E, et al. Increased NADH‐oxidase–mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin‐angiotensin system. Circulation. 1999;99:2027–2033. [DOI] [PubMed] [Google Scholar]
- 22. Schieffer B, Bunte C, Witte J, et al. Comparative effects of AT1‐antagonism and angiotensin‐converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44:362–368. [DOI] [PubMed] [Google Scholar]


