Abstract
Regular physical activity and training are associated with reductions in blood pressure (BP), yet elevated BP is one of the most common abnormalities found during the pre‐participation physical evaluation of athletes. Hypertension (HTN) remains the most common cardiovascular condition encountered in athletic populations, therefore all athletes require screening for HTN. Because athletes often have white coat HTN, BP recordings outside the office are also necessary. The 36th Bethesda Conference classified sports according to their varying physiologic demands and provided specific recommendations for the evaluation, treatment, and sport participation of athletes with HTN. In general, angiotensin‐converting enzyme inhibitors and other vasodilators are the medications of choice for active and athletic patients because of their limited interference with cardiovascular conditioning. Other agents can be used but some sports governing bodies proscribe the use of certain antihypertensive medications such as β‐blockers for elite athletes.
Aerobic exercise, defined as rhythmic contraction of large muscle groups (eg, running, walking, cycling, swimming, rowing) increases respiratory and heart rates and oxygen consumption. 1 , 2 Aerobic exercise tends to increase heart rate and venous return (cardiac preload), while resistance exercises such as power lifting increase peripheral vascular resistance (PVR) and left ventricular afterload. Exercise training affects blood pressure (BP) via chronic effects on autonomic control mechanisms and vascular remodeling. 2 Cardiorespiratory fitness, a physiologic attribute related to the efficiency of the oxygenation during sustained physical activity, is also increased by progressive aerobic exercise training. 1
Epidemiologic studies have shown that physical activity 3 and cardiorespiratory fitness 4 are inversely related to BP and the prevalence of hypertension (HTN). These trends have been corroborated by randomized trials showing that physical activity can reduce BP in normotensive, prehypertensive, and hypertensive persons. 5 In general, aerobic exercise training reduces mean systolic BP (SBP) by about 2 mm Hg to 7 mm Hg, with the greatest reduction in hypertensive patients. 5 Moderate intensity resistance training with lower weights and higher repetition regimens can also lower mean SBP by about 3 mm Hg to 6 mm Hg. 6
BP Patterns in Athletes and Physically Active Persons
Sustained HTN
While the overall prevalence of HTN in the physically active is approximately 50% lower than in the general population, 7 athletes who are black, elderly, obese, diabetic, or who have chronic kidney disease develop essential HTN more frequently than those who don’t have these conditions. Almost 80% of adolescent athletes with an elevated BP of >142/92 mm Hg during a sports preseason screening examination were found in one study to eventually develop sustained HTN, 8 so BP should be closely monitored irrespective of the level of physical fitness. The prevalence of secondary HTN is the same as in the general population, 9 with the exception that wheelchair athletes with spinal cord injuries may have severe episodic HTN related to autonomic or bladder dysfunction. 10
White Coat HTN
Out‐of‐office BP recordings (home BP in the morning and evening or 24‐hour ambulatory BP monitoring [ABPM]) should be obtained in anyone with elevated office readings because of the high prevalence of the white coat effect (marked home‐office BP difference). In a study of 410 athletes (aged 16.4±2.6 years), 11 18 had elevated office BP readings but 16 of these “office hypertensives” (88%) had normal ABPM (24‐hour average, daytime and nocturnal BP). White coat HTN (WCH) was defined, however, as 24‐hour SBP <140 mm Hg and 24‐hour diastolic BP (DBP) <90 mm Hg. These threshold values are higher than are currently recommended for adults. This study also did not compare patient ABPM data against normative ABPM data. The only study to use normative ABPM data to investigate WCH in children (not specifically athletes) 12 defined WCH as mean 24‐hour BP <95th percentile in the Second Task Force on Blood Pressure Control in Children and Adolescents. 13 WCH was also defined as 24‐hour mean BP <95th percentile using normative ambulatory BP (ABP) data. 14 The prevalence of WCH in all evaluated children was 62% by Task Force criteria and 56% by normative ABP criteria. The prevalence of WCH in the patients with documented clinic HTN was 53% by Task Force criteria and 45% by normative ABP criteria. These results confirm that WCH is as prevalent in children as in adults but there is insufficient evidence to suggest that WCH is more prevalent in young athletes than in nonathletes. The prognostic significance of WCH is not certain. Evidence of an exaggerated exercise BP response and/or increased left ventricular mass index in 62% of (nonathlete) patients with WCH suggest that this diagnosis in children may represent a prehypertensive state. 15 Prospective data suggest that there are no differences in cardiovascular (CV) disease risk between normotensives and those with WCH defined by an average daytime ABP <130/80 mm Hg, whereas the risk increases and is not different from those with ambulatory HTN in the WCH group with mean daily BP between 130/80 mm Hg and 135/85 mm Hg. 16 Thus, athletes with WCH whose mean daily BP is <130/80 mm Hg should not require drug therapy but should be monitored on a yearly basis. Athletes with mean daily BP >130/80 mm Hg but ≤135/85 mm Hg may or may not require drug therapy but should be counseled on lifestyle modifications to reduce BP (see below) and should be monitored more frequently, with appropriate steps taken if greater home BP elevation occurs.
Isolated Systolic HTN
Many conditioned athletes (particularly young men) have “athlete’s heart”: very high resting stroke volume and cardiac output with low PVR and heart rate. 17 Pulse pressure and SBP are high in these individuals, very often in the range of pre‐HTN and occasionally in the range of stage 1 HTN, because cardiac stroke volume is increased. 18 , 19 DBP is usually normal.
Another anomaly of SBP in athletes is “spurious systolic HTN (SSH),” which occurs as a result of exaggerated pulse pressure amplification (PPA) in the arm. PPA occurs as a result of the progressive decrease in arterial diameter from the heart to the arm and the corresponding increase in arterial impedance. In affected individuals, central (aortic) SBP is much less (up to 30–40 mm Hg less) than brachial SBP and central pressure contours are normal. SSH can be detected clinically by radial arterial tonometry, but the long‐term significance of this condition is unknown. Using a definition for SSH as a brachial SBP >140 mm Hg with a central SBP <125 mm Hg, Hulsen and colleagues 20 found 57 cases in young men and 3 cases in young women among the 750 participants in the Atherosclerosis Risk in Young Adults study. The central aortic pressure in athletes was still approximately 20 mm Hg greater than in young normotensive persons (who typically have central aortic pressures <100 mm Hg). 21 This may reflect the greater stroke volume of athletes. 17 The reported 20‐year Framingham risk scores in the SSH group were elevated but not statistically different from those in the normotensive group. SSH may represent an intermediate form between normotension and sustained HTN. Since, however, the brachial DBP correlates better than SBP with central pressures in youth, 22 further study is required. As central aortic BP is not routinely measured, the concept of SSH is not yet ready to be applied in the clinical realm.
Evaluation of HTN in Athletes
History
Family history of HTN and premature CV disease as well as behavioral factors including high intake of sodium and saturated fats (especially in processed and “fast” foods), alcohol, drugs (specifically, stimulants and cocaine), tobacco, human growth hormone, or anabolic steroids (Table I) should be elicited. Alcohol consumption is particularly frequent in scholastic athletes 23 and may lead to increased early morning BP readings. Other substances that increase BP include nonsteroidal anti‐inflammatory drugs (NSAIDs), caffeine, diet pills, decongestants, and herbal and dietary supplements, which often contain “natural” stimulants such as guarana, ma huang, or ephedra. Women should be questioned about oral contraceptives since about 5% develop elevated BP during a 5‐year period. 24 Symptoms related to BP elevation should also be identified, particularly in older athletes, including unusual exertional chest pain, dyspnea, or declining performance.
Table I.
Behaviors That Can Increase Blood Pressure
| Sodium and saturated fat intake (fast foods) |
| Alcohol |
| Tobacco (any form) |
| Over‐the‐counter medications Cold remedies, decongestants “Diet pills” |
| Ergogenic aides Caffeine Sudafed Cocaine Human growth hormone Anabolic steroids |
| Prescription medications Non‐steroidal anti‐inflammatory drugs Oral contraceptives |
| Dietary supplements Guarana Ephedra (banned) Ma huang |
Physical Examination
At least 2 pressures should be recorded for each visit according to standardized guidelines: undisturbed in a quiet room after at least 5 minutes, back supported in a chair, feet on the floor, arm supported at the level of the heart, and without talking. Proper cuff size (bladder encircles at least 80% of arm circumference 24 ; large cuff when mid‐arm circumference >33 cm, small cuff for mid‐arm circumference <23 cm 25 , 26 ). The cuff must be inflated to at least 20 mm greater than disappearance of the radial pulse to avoid the “auscultatory gap” and so potentially underestimate BP. 25 , 26 BP in both arms should be recorded prominently in the chart at the initial visit with follow‐up based on values from the higher arm. If the initial values are elevated, at least one other set of values should be recorded at least 1 week later. 27
In adolescents, BP varies by age, sex, and height 28 according to easily accessible tables (http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm). Normal BP is defined as <90th percentile; BP between the 90th and 95th percentile is pre‐HTN. In adolescents, BP ≥120/80 mm Hg is pre‐HTN, as in adults, even if this figure is <90th percentile; HTN is ≥95th percentile. Stage 1 HTN is 95th to 99th percentile +5 mm Hg and stage 2 HTN is >99th percentile +5 mm Hg for age, sex, and height, respectively (Table II). These categories correspond to the adult categories of normal (BP <120/80 mm Hg), pre‐HTN (SBP 120–139 mm Hg or DBP 80–89 mm Hg), stage I (SBP 140–159 mm Hg or DBP 90–99 mm Hg), and stage II (SBP ≥160 mm Hg or DBP ≥100 mm Hg) HTN.
Table II.
Classification of Hypertension in Children and Adolescents, With Measurement Frequency and Therapy Recommendations
| SBP or DBP Percentilea | Frequency of Blood Pressure Measurement | Therapeutic Lifestyle Changes | Pharmacologic Therapy | |
|---|---|---|---|---|
| Normal | <90th | Recheck at next scheduled physical examination | Encourage healthy diet, sleep, and physical activity | – |
| Prehypertension | 90th to <95th or if BP exceeds 120/80 mm Hg even if <90th percentile up to <95th percentileb | Recheck in 6 mo | Weight‐management counseling if overweight; introduce physical activity and diet managementc | None unless compelling indications such as chronic kidney disease, diabetes mellitus, heart failure, or left ventricular hypertrophy exist |
| Stage 1 hypertension | 95th–99th percentile +5 mm Hg | Recheck in 1–2 wk or sooner if the patient is symptomatic; if persistently elevated on 2 additional occasions, evaluate or refer to source of care within 1 mo | Weight‐management counseling if overweight; introduce physical activity and diet managementc | Initiate therapy based on indications such as symptomatic hypertension, secondary hypertension, hypertensive target‐organ damage, diabetes (types 1 and 2), and persistent hypertension despite nonpharmacologic measures, or if compelling indications existe |
| Stage 2 hypertension | >99th percentile +5 mm Hg | Evaluate or refer to source of care within 1 wk or immediately if the patient is symptomatic | Weight‐management counseling if overweight; introduce physical activity and diet managementb | Initiate therapyd |
aFor sex, age, and height measured on at least 3 separate occasions; if systolic and diastolic categories are different, categorize by the higher value. bThis occurs typically at 12 years old for systolic blood pressure (SBP) and at 16 years old for diastolic BP (DBP). cParents and children trying to modify the eating plan to the Dietary Approaches to Stop Hypertension Study eating plan could benefit from consultation with a registered or licensed nutritionist to get them started. dMore than 1 drug may be required.eSymptomatic or secondary hypertension, target‐organ damage, or persistent hypertension despite non‐pharmacologic measures. Reproduced with permission from Pediatrics. 114:555–576. Copyright ©2004 by the American Academy of Pediatrics.
If the arm pressure is elevated, palpitation of the femoral pulses and a measurement in one leg (particularly in patients older than 30 years) is indicated using a thigh cuff with popliteal auscultation. 29 Leg SBP is often 10% to 20% greater than the brachial SBP. 30 If the leg SBP is less than the brachial SBP, peripheral arterial disease (older patients) or coarctation of the aorta (younger patients) may be present. If an elevated BP is found, a funduscopic examination, thyroid gland palpation, cardiac auscultation, and abdominal auscultation (for a renal bruit) are indicated. Because of the prevalence of the white coat effect in athletes, elevated office readings should be confirmed by ABPM.
Additional Assessment
Once a diagnosis of HTN has been established in athletes or nonathletes, either in adults or children, further assessment of target organs should be considered. Athletes with pre‐HTN (90–95th percentile in adolescents) or stage I HTN (95–99th percentile +5 mm Hg in adolescents) should have blood chemistries and a lipid profile, hematocrit, urinalysis, and electrocardiography performed. Athletes with stage 2 HTN (>99th percentile +5 mm Hg in adolescents), abnormal laboratory results, or a possible secondary cause of HTN should be referred for additional study, often including echocardiography. 27 , 31 Since secondary HTN is present in only a few percent of cases, 9 workup is not always required and most physicians will employ an observation period before initiating additional studies. Secondary HTN tends to occur somewhat more frequently in younger patients; for example, fibromuscular hyperplasia of one or both renal arteries should be suspected in young sedentary as well as athletic women with HTN who are not taking oral contraceptives. 32
It is reasonable to recommend that any athlete with sustained HTN have an echocardiogram performed, 27 which must be interpreted by an expert. Hypertensive athletes with ambiguous findings on echocardiography require cardiology consultation. In general, athletes develop a pattern of physiologic “eccentric hypertrophy” in which increased left ventricular (LV) mass is associated with normal or increased fractional mid‐wall shortening, high LV volume, and normal or minimally increased LV wall thickness. At the extremes, this pattern is easily differentiated from the pathologic “concentric hypertrophy” of HTN, where the LV chamber size is normal or reduced and the LV wall thickness is increased. 33 Concentric hypertrophy is also found in bodybuilders. LV hypertrophy (LVH) beyond the “athlete’s heart” should limit participation until the BP is normalized 27 and the possibility of hypertrophic cardiomyopathy has been fully assessed. 17 , 31
Participation Recommendations for Athletes With HTN
The 36th Bethesda Conference provided recommendations for athletes with CV or structural abnormalities to prevent sudden cardiac death or disease progression (Table III). 27 Sports were categorized into 2 general types: dynamic (producing a volume load on the left ventricle) or static (producing a pressure load on the left ventricle), 34 and further classified according to level of intensity (low, medium, and high) and presence or absence of a contact/collision component (Figure). This system allows recommendations for participation in competitive sports for athletes with HTN and suggests activities suitable for cross‐training, but such decisions must be individualized.
Table III.
Recommendations of the 36th Bethesda Conference for Hypertension in Athletes
| Before individuals commence training for competitive athletics, they should undergo careful assessment of blood pressure (BP), and those with initially high levels (>140/90 mm Hg) should have out‐of‐office measurements to exclude isolated office white coat hypertension. Those with prehypertension (120/80 mm Hg–139/89 mm Hg) should be encouraged to modify lifestyle but should not be restricted from physical activity. Those with sustained hypertension should have echocardiography. Left ventricular hypertrophy (LVH) beyond that seen with “athletes’ heart” should limit participation until BP is normalized by appropriate drug therapy. |
| The presence of stage 1 hypertension in the absence of target organ damage including LVH or concomitant heart disease should not limit the eligibility for any competitive sport. Once having begun a training program, the hypertensive athlete should have BP remeasured every 2–4 months (or more frequently, if indicated) to monitor the impact of exercise. |
| Athletes with more severe hypertension (stage 2), even without evidence of target organ damage such as LVH, should be restricted, particularly from high static sports (classes IIIA–IIIC), until their hypertension is controlled by either lifestyle modification or drug therapy. |
| All drugs being taken must be registered with appropriate governing bodies to obtain a therapeutic exemption. |
| When hypertension coexists with another cardiovascular disease, eligibility for participation in competitive athletics is usually based on the type and severity of the associated condition. |
Reprinted with permission from Kaplan et al. 27
Figure.
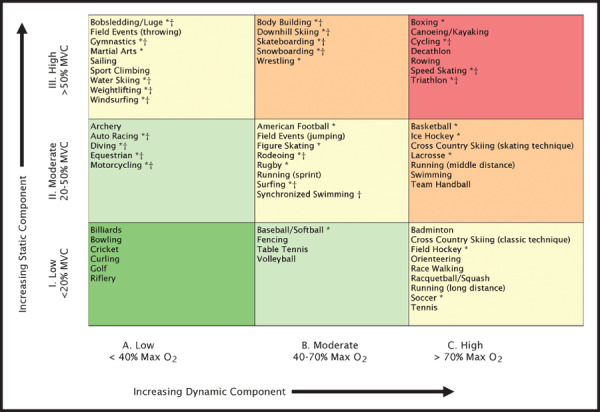
Classification of sports. This classification is based on peak static and dynamic components achieved during competition. It should be noted, however, that higher values may be reached during training. The increasing dynamic component is defined in terms of the estimated percent of maximal oxygen uptake (Max O2) achieved and results in an increasing cardiac output. The increasing static component is related to the estimated percent of maximal voluntary contraction (MVC) reached and results in an increasing blood pressure load. The lowest total cardiovascular demands (cardiac output and blood pressure) are shown in green and the highest in red. Blue, yellow, and orange depict low moderate, moderate, and high moderate total cardiovascular demands, respectively. *Danger of bodily collision. †Increased risk if syncope occurs. Reprinted with permission from Mitchell et al. 34
There are intrinsic limitations to the Bethesda classification system because most physical activities involve a spectrum of static and dynamic components. For example, distance running has low static and high dynamic demands, water skiing has high static and low dynamic demands, and rowing has both high static and dynamic demands. 34 This necessitates a somewhat more complex nomenclature such as IIIC (high static, high dynamic), IIB (moderate static, moderate dynamic), and IA (low static, low dynamic). For example, an athlete with stage II HTN would be advised to avoid sports classified as IIIA, IIIB, and IIIC but may be able to participate in IA sports until evaluation is complete and the BP is under control. Hypertensive athletes in sports that do not impose heavy static demands (eg, tennis, basketball) 34 who use heavy resistance weight training (high static and low dynamic demand) may be advised to modify such training regimens. During the evaluation and management phase, it is reasonable for the majority of patients to begin moderate intensity exercise (40%–60% maximum oxygen consumption) such as walking or other “talk exercise.” 2
Asymptomatic individuals with controlled HTN and no CV disease or renal complications may participate in exercise or competitive athletics but should be closely monitored. 2 Preliminary peak or symptom‐limited exercise testing may be warranted, especially for men older than 45 and women older than 55 years who are planning a vigorous exercise program (ie, ≥60% maximum oxygen consumption or, to make it more clinically relevant, exercise that produces breathlessness so that patients cannot carry on a conversation: “can’t talk exercise”). Further stress imaging is appropriate in higher‐risk individuals who are symptomatic (ie, exertional chest pain or dyspnea), whose BP exceeds 180/90 mm Hg, or who are diabetic.
With respect to youth athletes, the American Academy of Pediatrics recommends that children and adolescents who have systemic HTN be allowed to participate in competitive athletics provided that there is no evidence of target organ damage or concomitant heart disease and that BP is measured every 2 months in the physician’s office to monitor the impact of exercise on BP. 35 Young persons with severe HTN should be restricted from competitive sport and highly static (isometric) activities until the BP is under control and there is no evidence of target organ damage. The guidelines allow, however, for modest CV conditioning during evaluation and treatment in these athletes.
Treatment of the Hypertensive Athlete
Health care providers should be aware that recreational, scholastic, and professional athletes have unique physiologic and psychological attributes and that one of their goals should be to keep athletes as active as possible. Nevertheless, athletes can still have HTN and must be periodically monitored. Untreated HTN in athletes may be accompanied by a varying degree of limitation in exercise performance. 36
Nonpharmacologic Therapy
Healthy lifestyle behaviors may not eliminate the need for antihypertensive agents but may reduce the amount of medication needed (Table IV). The most effective dietary and lifestyle changes in athletes include losing weight and decreasing sodium intake, 37 especially by reducing processed food intake. These lifestyle changes are particularly important for high‐risk individuals such as blacks, the elderly, and people with diabetes. Also of potential importance are increased potassium intake, decreased alcohol consumption, avoidance of tobacco (in any form) and drugs of abuse (especially sympathomimetics such as cocaine or ephedra). Other drugs to avoid include androgens, anabolic steroids, growth hormone, and NSAIDs. For athletes in static sports, performing regular aerobic exercise is desirable.
Table IV.
Lifestyle Modifications to Reduce Blood Pressure in Athletes
| Reduce sodium intake: blacks, elderly, diabetics |
| Increase potassium intake: endurance athletes |
| Weight loss |
| Reduce alcohol intake |
| No tobacco (any form) |
| Avoid non‐steroidal anti‐inflammatory drugs, herbals, sympathomimetics, human growth hormone, anabolic steroids |
| Relaxation techniques: meditation, yoga, Tai Chi |
| Light aerobic exercise |
Antihypertensive Drugs
The most common and best tolerated medications used for the treatment of HTN in athletes are vasodilators, 38 especially angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). These agents have no major adverse effects on energy metabolism and do not impair maximum oxygen uptake. 39 ARBs produce similar BP‐lowering and hemodynamic patterns as ACE inhibitors but have fewer side effects, especially cough and angioedema. In older athletes and black athletes, calcium channel blockers or thiazide diuretics are useful alternatives. Combination drug therapy may be needed, preferably with a combination of ACE inhibitor or ARB with thiazide diuretic or calcium channel blocker. Some athletes benefit from β‐blockade, but these agents are banned in certain precision sports. Older agents such as alpha‐methyldopa are rarely needed. 40 , 41
Drug side effects in athletes are generally similar to those seen in nonathletes, with the exception anecdotally that the common side effect of pedal edema appears to be far less frequent in endurance athletes than sedentary individuals. ACE inhibitors and ARBs carry the risk of fetal injury if used during pregnancy. 42 The standard of care among experts in pediatric HTN is not to avoid these agents but to prescribe them if indicated to women of child‐bearing potential and counsel them regarding pregnancy avoidance/contraception. If stopped immediately upon confirmation of pregnancy, there appears to be little risk to the fetus from these agents. 42 There have been anecdotal reports of postural hypotension after intense exercise in patients taking ACE inhibitors, so an adequate cool‐down period is recommended. 38 Both antihypertensive potency and potassium‐sparing effect of ACE inhibitors may be increased when they are taken concomitantly with NSAIDs. 43 Possible side effects of thiazides within the first month include increased urinary loss of potassium and magnesium that can lead to muscle cramps and cardiac arrhythmias, particularly in warm weather. Initial hypovolemia and orthostatic hypotension can occur with thiazides but beyond the first week of treatment, plasma and extracellular volume tend to return to pretreatment levels and the sustained BP‐lowering effects are attributable to systemic arteriolar dilation. 44
Banned Agents
The World Anti‐Doping Agency, the US Olympic Committee, and the National Collegiate Athletic Association have banned the use of some antihypertensive medications. 45 The only drug therapy recommendation that differs from recommendations for nonathletes is that youth and adult athletes with HTN should avoid diuretics and β‐blockers because these agents are proscribed by certain sports governing bodies. β‐Blockers are banned in precision sports such as archery, shooting, diving, and figure skating. 45 All diuretics are banned because they can mask the presence of anabolic steroids; thus, thiazides cannot be used by elite athletes who must undergo drug testing. 45
References
- 1. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 2. Pescatello LS, Franklin BA, Fagard R, et al. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 5. Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure‐regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. [DOI] [PubMed] [Google Scholar]
- 6. Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta‐analysis of randomized controlled trials. J Hypertens. 2005;23:251–259. [DOI] [PubMed] [Google Scholar]
- 7. Lehmann M, Durr H, Merkelbach H, et al. Hypertension and sports activities: institutional experience. Clin Cardiol. 1990;13:197–208. [DOI] [PubMed] [Google Scholar]
- 8. Tanji JL. Tracking of elevated blood pressure values in adolescent athletes at 1‐year follow‐up. Am J Dis Child. 1991;145:665–667. [DOI] [PubMed] [Google Scholar]
- 9. Hanson P, Andrea BE. Treatment of hypertension in athletes. In: Delee J, Drez D, Stanitski CL, eds. Orthopaedic Sports Medicine: Principles and Practice. Baltimore, MD: Saunders; 1994:307–319. [Google Scholar]
- 10. Schmid A, Schmidt‐Trucksass A, Huonker M, et al. Catecholamines response of high performance wheelchair athletes at rest and during exercise with autonomic dysreflexia. Int J Sports Med. 2001;22:2–7. [DOI] [PubMed] [Google Scholar]
- 11. Kouidi E, Fahadidou‐Tsiligiroglou A, Tassoulas E, et al. White coat hypertension detected during screening of male adolescent athletes. Am J Hypertens. 1999;12:223–226. [DOI] [PubMed] [Google Scholar]
- 12. Sorof JM, Portman RJ. White coat hypertension in children with elevated casual blood pressure. J Pediatr. 2000;137:493–497. [DOI] [PubMed] [Google Scholar]
- 13. Report of the Second Task Force on Blood Pressure Control in Children – 1987. Task Force on Blood Pressure Control in Children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 14. Soergel M, Kirschstein M, Busch C, et al. Oscillometric twenty‐four‐hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. [DOI] [PubMed] [Google Scholar]
- 15. Kavey RE, Kveselis DA, Atallah N, et al. White coat hypertension in childhood: evidence for end‐organ effect. J Pediatr. 2007;150:491–497. [DOI] [PubMed] [Google Scholar]
- 16. Verdecchia P, Angeli F, Gattobigio R, et al. The clinical significance of white‐coat and masked hypertension. Blood Press Monit. 2007;12:387–389. [DOI] [PubMed] [Google Scholar]
- 17. Pelliccia A. Athlete’s heart and hypertrophic cardiomyopathy. Curr Cardiol Rep. 2000;2:166–171. [DOI] [PubMed] [Google Scholar]
- 18. Dlin RA, Dotan R, Inbar O, et al. Exaggerated systolic blood pressure response to exercise in a water polo team. Med Sci Sports Exerc. 1984;16:294–298. [PubMed] [Google Scholar]
- 19. Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens. 2003;16:229–232. [DOI] [PubMed] [Google Scholar]
- 20. Hulsen HT, Nijdam ME, Bos WJ, et al. Spurious systolic hypertension in young adults; prevalence of high brachial systolic blood pressure and low central pressure and its determinants. J Hypertens. 2006;24:1027–1032. [DOI] [PubMed] [Google Scholar]
- 21. McEniery CM, Yasmin, Wallace S, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. [DOI] [PubMed] [Google Scholar]
- 22. Wilkinson IB, Franklin SS, Hall IR, et al. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38:1461–1466. [DOI] [PubMed] [Google Scholar]
- 23. Nelson TF, Wechsler H. Alcohol and college athletes. Med Sci Sports Exerc. 2001;33:43–47. [DOI] [PubMed] [Google Scholar]
- 24. Kaplan NM, Lieberman E. Clinical Hypertension, 6th ed. Baltimore, MD: Williams & Wilkins; 1994. [Google Scholar]
- 25. Baker RH, Ende J. Confounders of auscultatory blood pressure measurement. J Gen Intern Med. 1995;10:223–231. [DOI] [PubMed] [Google Scholar]
- 26. Hall WD. Pitfalls in the diagnosis and management of systolic hypertension. South Med J. 2000;93:256–259. [PubMed] [Google Scholar]
- 27. Kaplan NM, Gidding SS, Pickering TG, et al. Task Force 5: systemic hypertension. J Am Coll Cardiol. 2005;45:1346–1348. [DOI] [PubMed] [Google Scholar]
- 28. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 29. Bates B. A Guide to the Physical Examination and History Taking, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1991. [Google Scholar]
- 30. Rahiala E, Tikanoja T. Suspicion of aortic coarctation in an outpatient clinic: how should blood pressure measurements be performed? Clin Physiol. 2001;21:100–104. [DOI] [PubMed] [Google Scholar]
- 31. O’Connor FG, Meyering CD, Patel R, et al. Hypertension, athletes, and the sports physician: implications of JNC VII, the Fourth Report, and the 36th Bethesda Conference Guidelines. Curr Sports Med Rep. 2007;6:80–84. [PubMed] [Google Scholar]
- 32. Rangarajan U, Kochar MS. Hypertension in women. WMJ. 2000;99:65–70. [PubMed] [Google Scholar]
- 33. Maron BJ. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol. 1986;7:190–203. [DOI] [PubMed] [Google Scholar]
- 34. Mitchell JH, Haskell W, Snell P, et al. Task Force 8: classification of sports. J Am Coll Cardiol. 2005;45:1364–1367. [DOI] [PubMed] [Google Scholar]
- 35. American Academy of Pediatrics Committee on Sports Medicine and Fitness . Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 1997;99:637–638. [DOI] [PubMed] [Google Scholar]
- 36. Missault L, Duprez D, De Buyzere M, et al. Decreased exercise capacity in mild essential hypertension: non‐invasive indicators of limiting factors. J Hum Hypertens. 1992;6:151–155. [PubMed] [Google Scholar]
- 37. Kojuri J, Rahimi R. Effect of “no added salt diet” on blood pressure control and 24 hour urinary sodium excretion in mild to moderate hypertension. BMC Cardiovasc Disord. 2007;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niedfeldt MW. Managing hypertension in athletes and physically active patients. Am Fam Physician. 2002;66:445–452. [PubMed] [Google Scholar]
- 39. Chick TW, Halperin AK, Gacek EM. The effect of antihypertensive medications on exercise performance: a review. Med Sci Sports Exerc. 1988;20:447–454. [PubMed] [Google Scholar]
- 40. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 41. Handler J. Managing chronic severe hypertension in pregnancy. J Clin Hypertens (Greenwich). 2006;8:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung NA, Beevers DG, Lip GY. Management of hypertension in pregnancy. Am J Cardiovasc Drugs. 2001;1:253–262. [DOI] [PubMed] [Google Scholar]
- 43. Gifford RW Jr. Antihypertensive therapy. Angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, and calcium antagonists. Med Clin North Am. 1997;81:1319–1333. [DOI] [PubMed] [Google Scholar]
- 44. Hughes AD. How do thiazide and thiazide‐like diuretics lower blood pressure? J Renin Angiotensin Aldosterone Syst. 2004;5:155–160. [DOI] [PubMed] [Google Scholar]
- 45. WADA . The World Anti‐Doping Code. The 2007 Prohibited List. International Standard. Montreal, Canada: WADA; 2007. [Google Scholar]


