Pheochromocytoma is a well‐described cause of paroxysmal hypertension. 1 However, pheochromocytoma presenting with cyclical hypertension and hypotension is uncommon. 2 , 3 Cardiomyopathy in pheochromocytoma is a well‐known phenomenon. Only recently has the inverted takotsubo pattern, in which the left ventriculogram shows hypokinetic bases and preserved apical function, been recognized as being associated with such tumors. 4 , 5 , 6 This pattern is in contrast with standard takotsubo cardiomyopathy, which is characterized simply by apical ballooning. Here, we describe a case of pheochromocytoma presenting with cyclic blood pressure (BP) fluctuations as well as the inverted takotsubo cardiomyopathy. Three forms of takotsubo cardiomyopathy exist. One involves apical akinesis, another is the midventricular variant, and the other is the newly noted inverted takotsubo variant, as seen in our patient. 7 This presence of the inverted variant should arouse suspicion of a pheochromocytoma. We discuss how the excess catecholamines may contribute to this association.
A 55‐year‐old Caucasian man with no known medical problems awoke from sleep with palpitations and chest pain followed by a severe headache. The patient had intermittent palpitations and chest discomfort for several weeks prior to the emergency department visit, with a flu‐like illness about 2 months prior. He denied having any sweating or drenching night sweats. He had a remote history of alcohol abuse and smoked 1 pack a day of cigarettes for many years. He did not take any medications and had no family history of coronary artery disease.
On initial presentation he was pale and had a pulse rate of 180 beats per minute, with a BP of 80/55 mm Hg. He complained of ongoing severe substernal chest pain and shortness of breath. The physical examination revealed tachycardia without any murmurs, rubs or gallops, or diffuse rales in half the lung fields and no abdominal tenderness or new skin rashes or lesions. There was no clubbing, cyanosis, or edema of the extremities. Telemetry revealed unstable ventricular tachycardia, and the patient was cardioverted to a normal sinus rhythm. Follow‐up examination showed significant hypertension (175/90 mm Hg in the left arm and 190/99 mm Hg in the right arm), a heart rate of 80 beats per minute, a respiratory rate of 26 breaths per minute, and hypoxemia at 84% on room air.
Electrocardiographic (ECG) results after cardioversion showed a 3‐mm ST‐segment depression in the inferior leads II, III, and aVF, and leads V3 through V6. ECG findings prior to the arrhythmia had consistently revealed evidence of left ventricular (LV) hypertrophy by multiple ECG criteria (including those of Soklow‐Lyons), but no ST‐segment changes. Inverted T waves were consistently present in lead III. Ventricular fibrillation and atrial fibrillation were recorded, although sinus tachycardia was the most consistent rhythm. Laboratory results were notable for troponin I at 0.26 (Trop I, ultra range [0.00–0.78 ng/mL]), and a chest radiograph showed diffuse pulmonary edema. At this time, he received 80 mg of intravenous furosemide. Due to the acute decompensation, a broad differential diagnosis was considered, including myocardial infarction, aortic dissection, acute mitral valve insufficiency, takotsubo cardiomyopathy, and fulminant myocarditis.
Emergent coronary angiography was normal, with wide systolic BP variation indicating, in retrospect, that the cyclic nature of the BPs started on the day of presentation. BP did not change with the addition of intravenous contrast. The ejection fraction (EF) was severely depressed at 15% to 20%, with hypokinetic bases and preserved apical function. LV function by echocardiography was 30%. These LV dysfunction findings were similar to findings referred to as inverted takotsubo cardiomyopathy. His end‐diastolic pressure was elevated at 26 mm Hg, suggesting either decreased ventricular compliance or increased ventricular volume. The end‐diastolic diameter was 5.9 cm by echocardiogram.
Initial management focused on treatment of a likely nonischemic cardiomyopathy, with standard treatment of angiotensin‐converting enzyme inhibition, low‐dose β‐blockade, and acute diuresis. The patient received only a single dose of carvedilol 6.25 mg orally and then developed respiratory failure and paroxysms of hypertension and hypotension. The BP fluctuations (Figure 1) were managed with intermittent vasopressors including dopamine and norepinephrine. Dopamine was the initial pressor used at intermediate doses (3–7.5 μg/kg/min); this stimulates β‐receptors on the heart and peripheral circulations. Norepinephrine was added at low doses (2–4 μg/min) in an attempt to further increase the cardiac output. The cyclical nature of the BP led us to suspect a pheochromocytoma, and an endocrine consultation was performed.
Figure 1.
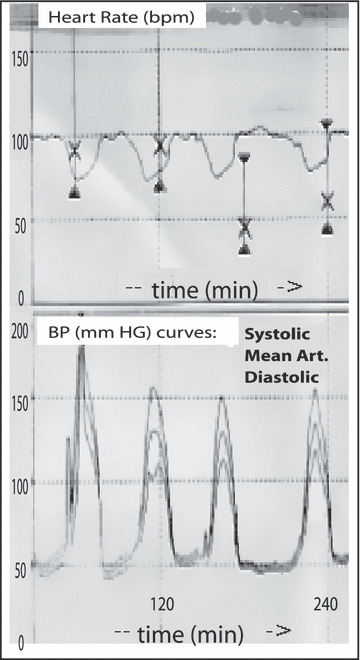
Findings showing the heart rate and blood pressure curves as inversely related. The cyclic variation in blood pressure has a similar cycle length except for the last cycle, which is of somewhat longer duration.
A pulmonary artery catheter was placed, which showed an elevated wedge pressure of 22 mm Hg and a severely decreased cardiac output and index of 3.9 L/min and 2.1 L/min/m2. In addition, wide swings in systemic vascular resistance (SVR), along with a proportional change in cardiac output were noted. Intravenous fluids helped maintain urine output but did not affect the cyclic BP. After the patient received 6 L of fluid, his wedge pressure and BP was unchanged. To maintain hemodynamic stability, the patient needed periodic intravenous vasoconstrictors; however, due to the extreme episodic hypotension, phentolamine—a pure α‐blocker—could not be safely initiated. Vasoconstrictors were continued, but for additional hemodynamic support, an intra‐aortic balloon pump was placed.
An abdominal computed tomography scan showed a right adrenal mass measuring approximately 5.2×4.3×5 cm, with approximately 20 to 35 Hounsfield units density. The preoperative values suggesting pheochromocytoma were as follows: serum epinephrine, 18,820 pg/mL (normal, 10–200 pg/mL); norepinephrine, 17,973 pg/mL (normal, 80–520 pg/mL); dopamine, 198,687 pg/mL (normal, 0–20 pg/mL); plasma metanephrine, 14.4 nmol/L (normal, 0.00–0.49 nmol/L); and normetanephrine, 8.19 nmol/L (0.00–0.89 nmol/L).
The patient underwent resection of the adrenal mass within the next 12 hours and had an unremarkable postoperative course. During the operation, even minimal touching of the mass caused large swings in BP. During surgery, his BP rose from 50 mm Hg systolic to 220 mm Hg systolic in a matter of 5 to 10 seconds. Surgery had to be discontinued on several occasions because of wide swings in BP. Hypertension and the BP variability rapidly resolved after removal of the tumor. The pathology of the adrenal mass measuring 3.5×3.2×2.8 cm was consistent with a pheochromocytoma with extensive hemorrhagic necrosis but without any vascular invasion. It stained strongly positive for chromogranin A and synaptophysin. The patient is doing well 3 months post‐discharge. ECG abnormalities of T‐wave inversions and LV hypertrophy had resolved prior to discharge. A post‐resection 24‐hour urine study showed normal values of dopamine, 175 μg/d (normal, 60–440); epinephrine, <3 μg/d (normal, 0–25); norepinephrine, 27 μg/d (normal, 0–100); normetanephrine, 306 μg/d (normal, 50–650); and metanephrine, 70 μg/d (normal, 30–350). LVEF was 55% by transthoracic echocardiography 3 days before discharge. This was a dramatic improvement from the LVEF of 30% initially seen by echocardiography on admission.
Case Discussion
Takotsubo cardiomyopathy is a recently recognized entity, first identified in the Japanese literature. The name takotsubo derives from the shape of a particular type of octopus trapping pot. Patients with takotsubo cardiomyopathy generally present with chest pain and ECG findings of an acute coronary syndrome. Results on coronary angiography are generally normal, and a typical cardiomyopathy exhibiting apical akinesis with hyperkinetic bases is present. The LV dysfunction does not correspond to a vascular territory. 8 The underlying mechanism for this cardiomyopathy is unknown, but high catecholamine levels exist in these patients, whose symptoms are triggered by an emotional stressor. The majority of takotsubo cardiomyopathy cases occur in postmenopausal women but have been reported in other patients as well. The course is generally self‐limited, with resolution of the cardiomyopathy in 6 to 8 weeks with supportive care.
(2)
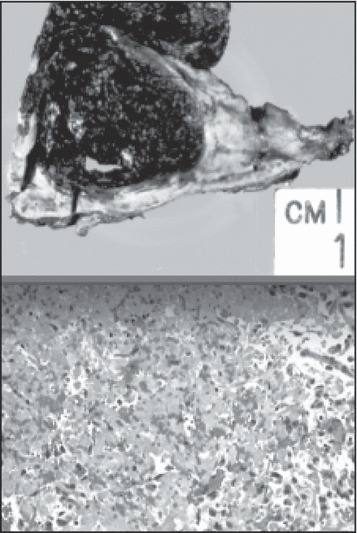
[ The surgically excised specimen shows extensive necrosis (top). Histologic slide of the pheochromocytoma (bottom). ]
Pheochromocytoma is an unusual cause of secondary hypertension and can present with symptoms suggestive of an acute coronary syndrome. 9 , 10 Initial symptoms such as headache, palpitations and sweating, nausea and vomiting, pallor, flushing, and abdominal pain are nonspecific 11 and, thus, the diagnosis can be challenging. ECG changes of T‐wave inversions, atrial and ventricular hypertrophy, axis abnormalities, QT‐interval prolongations, and various arrhythmias have been reported. 12 The T‐wave inversions and LV hypertrophy seen in this case resolved shortly after tumor removal. In this case, we did not suspect a pheochromocytoma until the patient developed a cyclic BP crisis. Such periodicity is uncommon and there are only a few case reports in which it is described. 3 The pathophysiologic basis for this periodicity is unclear. A majority of the previous cases with cyclic BP occurred in the setting of predominantly epinephrine‐secreting tumors. One hypothesis links the periodicity to the intermittent release of catecholamines from the tumor. First, high levels of catecholamines stimulate β‐adrenergic receptors, thus leading to hypertension. Then, eventual washout of catecholamines from the body leads to down‐regulated adrenergic receptors. This decreases the vascular tone and results in hypotension and reflex tachycardia. As demonstrated in Figure 1, the complex interplay of the autonomic nervous system and the vascular baroreceptor reflex leads to an inverse relationship between the BP and heart rate. While secretion of epinephrine from tumors can cause vasodilatation in volume‐depleted patients, that would be unlikely in this patient who is volume‐replete. A more unusual aspect of the periodic BP in our case was the long cycle length. The BP cycle between hypertension to hypotension averaged 30 minutes. Previous case reports have noted a much shorter cycle length, usually ranging from 4 to 20 minutes.
(3)
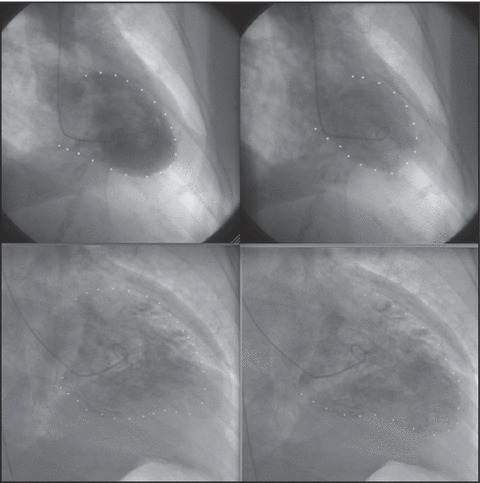
[ Takotsubo cardiomyopathy pattern in systole and diastole. The top row shows typical takotsubo cardiomyopathy (diastole and systole). The apex is akinetic and the bases are hypercontractile. Inverted takotsubo pattern in systole and diastole. The bottom row shows inverted takotsubo cardiomyopathy (systole and diastole). The bases are akinetic and the apex is hypercontractile. ]
One possible reason for the longer cycle length in our patient is that the use of exogenous vasopressors may have stabilized the catecholamine‐related fluctuations. Two case reports describe the resolution of this periodicity, one with administration of an α‐blocker and the other with norepinephrine. However, in our patient, the mean arterial pressure was as low as 40 mm Hg, thus requiring concomitant use of intravenous fluids and vasoconstrictor agents.
The tumor‐induced high catecholamine state leads to many acute and chronic cardiac changes. The fluctuating catecholamine levels, the differential distribution of the cardiac adrenergic receptors, and their variable response to catecholamines lead to different forms of stress cardiomyopathy. In response to the catecholaminergic state, contraction‐band necrosis may occur in the myocardium. In recent case reports, patients with pheochromocytoma crisis have been noted to present with an inverted takotsubo pattern. 6 , 13 Our case supports this link between a high catecholaminergic state and inverted takotsubo cardiomyopathy. The underlying mechanism for this specific linkage is not clear. Therefore, although any pattern such as a takotsubo pattern or diffuse hypokinesis can occur presumably due to catecholamine excess, the inverted takotsubo pattern appears to be the most prevalent presentation. It is important to consider pheochromocytoma in any patient with acute nonischemic cardiomyopathy and may be especially so if an inverted takotsubo pattern is observed. It has been suggested that perhaps differences in β‐adrenergic receptor density in the heart account for the inverted takotsubo pattern with pheochromocytoma. 14
There are an increasing number of reports 4 , 6 , 13 suggesting that in presentations of inverted takotsubo, pheochromocytoma should be considered as an underlying pathology. Sanchez‐Recalde and colleagues 7 report that they had 3 cases with pheochromocytomas who presented with acute coronary syndrome–like symptoms with inverted takotsubo pattern. Thus, it is reasonable to investigate pheochromocytoma in patients with inverted takotsubo cardiomyopathy pattern on presentation.
Our patient’s ECG findings a few months prior to this presentation showed evidence of LV hypertrophy, usually suggesting chronic hypertension in the absence of other causes of LV hypertrophy, such as valvular diseases. The patient had severely reduced LVEF at the time of admission, with an admission BP of >180/100 mm Hg. His echocardiographic results confirmed the presence of underlying LV hypertrophy and increased LV mass. Removal of the pheochromocytoma (with subsequent normalization of catecholamines) led to normalization of his LVEF in less than 1 week. Interestingly, the ECG obtained after resection of the pheochromocytoma showed resolution of the evidence of LV hypertrophy. The echocardiogram showed persistence of LV hypertrophy but resolution of left atrial enlargement. These findings suggest that the excess sympathetic tone leads to increased afterload and depressed cardiac index. Even though signs of diastolic dysfunction remain in this patient, his heart failure resolved with treatment of the hypertension. Interestingly, takotsubo cardiomyopathy, which is hypothesized to be a catecholamine‐related cardiomyopathy as well, resolved once sympathetic tone was reversed.
The nonmalignant adrenal mass probably developed over many years in this patient and some acute event led to the crisis. From the pathology, it is reasonable to speculate that spontaneous adrenal hemorrhage precipitated the acute event. 15 , 16 In the care of these patients it is important to realize that radiocontrast can lead to catecholamine release from the medulla, 17 although no significant changes in BP were noted after contrast in this case.
We conclude that this patient had underlying pheochromocytoma as a cause of his chronic hypertension with crisis, most likely precipitated by possible hemorrhage. Resection of this tumor was curative, but long‐term changes include the effects of a chronic high catecholamine state. Anatomic as well as physiologic changes in the cardiovascular system may take months to years to completely normalize.
Conclusions
Pheochromocytoma is a rare yet fascinating cause of secondary hypertension and hypertensive crises. The presentation and management of these patients is challenging. A high index of suspicion is needed to diagnose pheochromocytoma. We note unique features of cyclic BP and inverted takotsubo as important clues to alert the clinician to this entity. Prompt treatment of the pheochromocytoma with resultant normalization of BP and catecholamines reverses this unusual cardiomyopathy.
Acknowledgments
Acknowledgment: The authors would like to acknowledge Dr Hiroshi Miyamoto for his assistance with the pathology figures.
References
- 1. Bravo EL, Tagle R. Pheochromocytoma: state‐of‐the‐art and future prospects. Endocr Rev. 2003;24(4):539–553. [DOI] [PubMed] [Google Scholar]
- 2. Kobal SL, Paran E, Jamali A, et al. Pheochromocytoma: cyclic attacks of hypertension alternating with hypotension. Nat Clin Pract Cardiovasc Med. 2008;5(1):53–57. [DOI] [PubMed] [Google Scholar]
- 3. Guzik P, Wykretowicz A, Wesseling IK, et al. Adrenal pheochromocytoma associated with dramatic cyclic hemodynamic fluctuations. Int J Cardiol. 2005;103(3):351–353. [DOI] [PubMed] [Google Scholar]
- 4. Zegdi R, Parisot C, Sleilaty G, et al. Pheochromocytoma‐induced inverted Takotsubo cardiomyopathy: a case of patient resuscitation with extracorporeal life support. J Thorac Cardiovasc Surg. 2008;135(2):434–435. [DOI] [PubMed] [Google Scholar]
- 5. Lassnig E, Weber T, Auer J, et al. Pheochromocytoma crisis presenting with shock and tako tsubo‐like cardiomyopathy. Int J Cardiol. 2008. Jun 23. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. Sanchez‐Recalde A, Costero O, Oliver JM, et al. Images in cardiovascular medicine. Pheochromocytoma‐related cardiomyopathy: inverted Takotsubo contractile pattern. Circulation. 2006;113(17):e738–e739. [DOI] [PubMed] [Google Scholar]
- 7. Sanchez‐Recalde A, Iborra C, Costero O, et al. Takotsubo cardiomyopathy ‐ A new variant and widening disease spectrum. “Inverted Takotsubo” pattern related to catecholamine‐toxicity. Int J Cardiol. 2007. Dec 26. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST‐segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858–865. [DOI] [PubMed] [Google Scholar]
- 9. Darze ES, Von Sohsten RL. Pheochromocytoma‐induced segmental myocardial dysfunction mimicking an acute myocardial infarction in a patient with normal coronary arteries. Arq Bras Cardiol. 2004;82(2):178–180, 175–177. [PubMed] [Google Scholar]
- 10. Menke‐van der Houven van Oordt CW, Twickler TB, Van Asperdt FG, et al. Pheochromocytoma mimicking an acute myocardial infarction. Neth Heart J. 2007;15(7–8):248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3(2):92–102. [DOI] [PubMed] [Google Scholar]
- 12. Sayer WJ, Moser M, Mattingly TW. Pheochromocytoma and the abnormal electrocardiogram. Am Heart J. 1954;48(1):42–53. [DOI] [PubMed] [Google Scholar]
- 13. Di VM, Balestra GM, Christ M, et al. Inverted Takotsubo cardiomyopathy due to pheochromocytoma. Eur Heart J. 2008;29(6):830. [DOI] [PubMed] [Google Scholar]
- 14. Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine‐induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5(1):22–29. [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi T, Iwai A, Takahashi R, et al. Spontaneous rupture of adrenal pheochromocytoma: review and analysis of prognostic factors. J Surg Oncol. 2005;90(1):31–35. [DOI] [PubMed] [Google Scholar]
- 16. Sumino Y, Tasaki Y, Satoh F, et al. Spontaneous rupture of adrenal pheochromocytoma. J Urol. 2002;168(1):188–189. [PubMed] [Google Scholar]
- 17. Elian D, Harpaz D, Sucher E, et al. Reversible catecholamine‐induced cardiomyopathy presenting as acute pulmonary edema in a patient with pheochromocytoma. Cardiology. 1993;83(1–2):118–120. [DOI] [PubMed] [Google Scholar]