Abstract
This 4‐week randomized, double blind, placebo‐controlled study (N=240), 1‐year open label trial (N=233), and single‐dose pharmacokinetic study (N=22) evaluated candesartan cilexetil (3 doses) in hypertensive children aged 6 to 17 years. Seventy‐one percent were 12 years of age or older, 71% were male, and 47% were black. Systolic (SBP)/diastolic (DBP) blood pressure declined 8.6/4.8–11.2/8.0 mm Hg with candesartan and 3.7/1.8 mm Hg with placebo (P<.01 compared to placebo for SBP and for the mid and high doses for DBP; placebo‐corrected 4.9/3.0–7.5/6.2 mm Hg). The slopes for dose were not, however, different from zero (P>.05). The response rate (SBP and DBP <95th percentile) after 1 year was 53%. The pharmacokinetic profiles in 6‐ to 12‐ and 12‐ to 17‐year‐olds were similar and were comparable to adults. Eight candesartan patients discontinued treatment because of an adverse event. Candesartan is an effective, well‐tolerated antihypertensive agent for children aged 6 to 17 years and has a pharmacokinetic profile that is similar to that in adults.
With the establishment of normative blood pressure (BP) data for children and the definition of age‐specific criteria for the diagnosis of hypertension, it has become apparent that pediatric hypertension is more prevalent than previously believed. 1 , 2 Although it is not yet established that hypertensive children will suffer the same long‐term cardiovascular consequences as hypertensive adults, such as myocardial infarction and stroke, it is not uncommon for the hypertensive child to exhibit some vascular abnormalities. 2 , 3 , 4 For example, left ventricular hypertrophy may be noted in approximately one‐third of hypertensive children, and carotid intima‐media thickness in hypertensive children exceeds that in normotensive children. 2 , 5 , 6 Given these considerations, expert committees recommend lowering elevated BP in children and specifically recommend pharmacologic therapy when lifestyle changes are inadequate or when the hypertension is symptomatic or accompanied by end organ changes. 2 , 7
While dosing guidelines for children have been established for a number of antihypertensive agents, many of the recommendations have simply been derived from adult doses, modified by trial and error. More recently, controlled clinical trials accompanied by pharmacokinetic (PK) studies have been undertaken. 7 The results of these studies are often consistent with the antihypertensive effects described in adults, but there have been some exceptions. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Thus, careful study of antihypertensive agents is important to clarify the use of medications for treating the hypertensive child.
Candesartan is an angiotensin receptor–blocking agent that selectively inhibits the angiotensin II, type 1 receptor by binding tightly to and dissociating slowly from the receptor site. 17 It lowers BP with once‐daily dosing in adults with the dose range of 2 to 32 mg. 18 The tablet for oral administration is candesartan cilexetil, which is hydrolyzed to candesartan upon absorption from the gastrointestinal tract. The tablets are relatively small (7–9.5 mm in diameter) and are palatable for children. 19
The Candesartan in Children With Hypertension (CINCH) program set out to evaluate the use of candesartan tablets as treatment for hypertensive children aged 6 to 17 years and a candesartan liquid (suspension) formulation for hypertensive children aged 1 to 6 years. The program was specifically designed to comply with a US Food and Drug Administration (FDA) written request. As directed by the FDA, the program excluded children younger than 1 year, given the potential for inhibitors of the renin‐angiotensin‐aldosterone system to impair renal maturation in the incompletely developed kidney. 20 , 21 The current report describes the results of studies in 6‐ to 17‐year‐old hypertensives: a 4‐week dose‐ranging study, a 52‐week clinical experience study, and a single‐dose PK substudy. A study in children 1 to 6 years of age is ongoing and will be reported separately.
Methods
Study Design
The 4‐week dose‐ranging study was randomized, double‐blind, placebo‐controlled, and parallel‐group in design and was conducted in the United States and Europe (total of 42 sites). The study permitted enrollment of children aged 6 to 17 years with both newly diagnosed and previously diagnosed hypertension. Potentially eligible patients first entered a 1‐week single‐blind placebo period, after which those found to be study‐eligible (systolic blood pressure [SBP] or diastolic blood pressure [DBP] greater than the population 95th percentile height‐corrected age/sex BP distributions but not exceeding the 95th percentile by >20/10 mm Hg) were randomly allocated to treatment with placebo or 1 of 3 dose levels of candesartan in a 1:2:2:2 ratio. For children weighing <50 kg, the candesartan doses were 2, 8, and 16 mg once daily, and for those ≥50 kg, they were 4, 16, and 32 mg. The highest dose in each weight panel was administered as a “half‐dose” for 1 week before blinded escalation to the assigned dose. Clinic visits for BP and safety assessments occurred at weekly intervals. The study protocol excluded persons with known secondary hypertension (eg, coarctation of the aorta, endocrinopathies), bilateral renal artery stenosis, uncompensated nephrotic syndrome, and insulin‐dependent diabetes mellitus and those thought to be pregnant (positive pregnancy test result). Patients with an estimated glomerular filtration rate <50 mL/min/1.73 m2 were also excluded. 22
The longer‐term clinical experience study enrolled patients who had completed participation in the 4‐week dose‐ranging study as well as a limited number of patients who would have otherwise met the dose‐ranging study eligibility criteria. In the 1‐year study, investigators began open‐label candesartan at 4 or 8 mg (depending on the patient’s weight) but could then adjust doses between 2 and 32 mg with the goal of controlling the BP. Other antihypertensive agents, with the exception of other angiotensin receptor blockers, were permitted, if added to candesartan 32 mg (or the highest tolerated dose). The follow‐up visit schedule was every 4 weeks to week 16 then every 8 weeks to week 48; the final visit was at week 52. Investigators were also to query postmenarche females monthly as to changes in menstrual pattern and to conduct necessary pregnancy testing.
The clinical experience study included PK and neurocognition substudies. In the PK study, patients weighing at least 25 kg abstained from candesartan dosing for at least 48 hours, then received a single 16‐mg dose and had predosing and serial postdosing blood samples collected over 24 hours for determination of plasma candesartan concentrations. They then resumed (or started) candesartan treatment. The neurocognition substudy included a baseline and 1‐year follow‐up Wechsler Intelligence Scale for Children, 4th Edition, conducted by or under the supervision of a licensed, certified psychologist.
In both the dose‐ranging and clinical experience study, BP was measured with patients in a resting, sitting position, 24 hours postdosing with an appropriately sized cuff. SBP was taken as the first and DBP as the 5th Korotkoff sounds. Each BP determination represented the mean of 3 measurements.
Laboratory test (hematology, chemistry, and urinalysis) and electrocardiography results were collected at the screening visit and at week 4 in the dose‐ranging study, and laboratory test results were collected at weeks 24 and 52 in the clinical experience study.
Study participants (parents or guardians) provided written informed consent/assent prior to participating in the study, and the study was approved by each investigator’s ethics committee or institutional review board as per local regulations.
Statistical Methods
In the dose‐ranging study, placebo‐corrected change in sitting SBP (SiSBP) served as the primary efficacy measure; the primary analysis evaluated the change as a function of dose (excluding placebo) by multiple linear regression with the two weight panels pooled (low dose, 2/4 mg; medium dose, 8/16 mg; high dose, 16/32 mg) and with assigned relative dose values of 1:4:8. Secondary analyses included a similar analysis for sitting DBP (SiDBP) as well as pairwise comparisons in ANCOVA models with baseline BP as a covariate and treatment group as a factor.
The dose‐ranging study was originally planned for 210 participants and was based on assumed standard deviations of the predictor variable (dose) of 10.1 and of residuals of 12 (α=.05 [two‐sided]) and a 20% dropout rate. (The corresponding power was 84%.) Subsequent to a revised FDA‐written request, which specified statistical criteria for excluding a minimum treatment effect, the target sample size was increased to 238 participants. No formal sample size estimates were done for the clinical experience study as it simply allowed for the enrollment of patients who were recruited from the dose‐ranging study as well as for an additional 10% who could enroll directly.
For both studies, therapeutic response was defined as both an SiSBP and a SiDBP less than the 95th percentile and was expressed as a response rate, which was the simple proportion of responders. Growth (change in height and weight) was based on Z‐scores (age‐ and sex‐specific standard deviation scores based on the US Centers for Disease Control national growth standards).
For the PK substudy, a sample size of 16 patients (8 aged 6–12 years and 8 aged 12–17 years) was projected to adequately describe the PK parameters given the variability in plasma concentrations observed in prior adult studies. The PK parameters included maximum plasma concentration (Cmax), time to Cmax (tmax), area under the plasma concentration curve extrapolated to infinity determined by trapezoidal rule (AUC), and terminal elimination half‐life (t1/2 calculated as 0.693/terminal elimination rate constant).
The neurocognition substudy projected a need for approximately 24 participants (12 aged 6–12 years and 12 aged 12–17 years) to detect a 10‐point decline in the full‐scale intelligence quotient (FSIQ), assuming a mean score of 100 and an SD of 15 (α=.05 [one‐sided]; power, 80%).
Laboratory Methods
Hematology, chemistry, and urinalysis tests (including microalbumin/creatinine ratio) were performed by a central laboratory (Quintiles, Smyrna, GA), and plasma candesartan concentration samples were analyzed by Quintiles AB, Sweden, based on established methods. 23
Results
Four‐Week Dose‐Ranging Study
The study investigators randomized a total of 240 patients, all of whom were included in the intent‐to‐treat analysis group. For 9 patients, a week 4 BP value was imputed with the last available observation. Eleven patients discontinued treatment in the dose‐ranging study: 3 for study eligibility violations, 3 for adverse events, and 5 for other reasons.
Most of the children were aged 12 years or older, were male, weighed ≥50 kg, and had a body mass index exceeding the 95th percentile. There were approximately equal proportions of patients who were black compared to nonblack, and about a third were preadolescent (Tanner sexual maturity score <3). The majority of the patients were discovered to be hypertensive within the prior year, and the majority had isolated systolic hypertension; about a third had systolic/diastolic hypertension, and most were naive to pharmacologic therapy (Table). The treatment groups were well balanced with regard to these patient characteristics. Twenty‐two patients had a medical or surgical history of a renal or urologic abnormality, and two of these had chronic kidney disease with elevated serum creatinine levels (estimated glomerular filtration rates, 61 and 136 mL/min/1.73 m2). Twenty patients had a baseline urinary albumin/creatinine ratio >30 mg/g.
Table.
Demographic and Baseline Characteristics
| 4‐Week Double‐Blind Phase Total (n=240) | 52‐Week Open‐Label Phase Total (n=233) | |
|---|---|---|
| Age, y, No. (%) | ||
| <12 | 70 (29.2) | 68 (29.2) |
| ≥12 | 170 (70.8) | 165 (70.8) |
| Sex, No. (%) | ||
| Male | 170 (70.8) | 166 (71.2) |
| Female | 70 (29.2) | 67 (28.8) |
| Race, No. (%) | ||
| White | 108 (45.0) | 111 (47.6) |
| Black | 113 (47.1) | 102 (43.8) |
| Other | 19 (7.9) | 20 (8.6) |
| Tanner score, No. (%) | ||
| <3 | 82 (34.2) | 75 (32.2) |
| ≥3 | 158 (65.8) | 140 (60.1) |
| Unknown | NA | 18 (7.7) |
| Weight at screening, kg, No. (%) | ||
| <50 | 31 (12.9) | 34 (14.6) |
| ≥50 | 209 (87.1) | 199 (85.4) |
| Body mass index percentile at screening, No. (%) | ||
| <95 | 75 (31.3) | 77 (33.0) |
| ≥95 | 165 (68.8) | 156 (67.0) |
| Duration of hypertension, y, No. (%) | ||
| <1 | 154 (64.2) | 151 (64.8) |
| 1 to <2 | 35 (14.6) | 32 (13.7) |
| 2 to <3 | 23 (9.6) | 25 (10.7) |
| 3 to <4 | 14 (5.8) | 12 (5.2) |
| ≥4 | 14 (5.8) | 13 (5.6) |
| Type of hypertensiona | ||
| None | 14 (5.8) | 9 (3.9) |
| Diastolic only | 16 (6.7) | 14 (6.0) |
| Systolic only | 125 (52.1) | 122 (52.4) |
| Systolic and diastolic | 85 (35.4) | 88 (37.8) |
aType of hypertension is defined as diastolic hypertension ≥95th percentile; systolic hypertension ≥95th percentile. The no hypertension group represents an apparent difference in the study site’s determination of the 95th percentile (using blood pressure charts) vs the percentiles calculated by a central program. Tanner score assesses sexual maturity.
Although study‐qualifying BP levels were specific to each patient (based on 95th percentile), baseline mean BP values were nearly identical across treatment groups (mean SiSBP, 133–135 mm Hg; mean SiDBP, 78–80 mm Hg).
BP declined with all active treatments, with adjusted mean reductions ranging from 8.6 to 11.2 mm Hg for SiSBP and from 4.8 to 8.0 mm Hg for SiDBP; the reduction with placebo was 3.7/1.8 mm Hg (placebo‐corrected declines, 4.9/3.0–7.5/6.2 mm Hg). While neither the slope for placebo‐corrected change in SiSBP nor the slope for placebo‐corrected change in SiDBP were significantly different from zero in the dose‐response model (P=.10 and P=.24, respectively), the tests for declines in BP compared to placebo yielded significant results: P<.01 for all 3 dose levels for SiSBP and P<.01 for the medium‐ and high‐dose levels for SiDBP (Figure 1).
Figure 1.
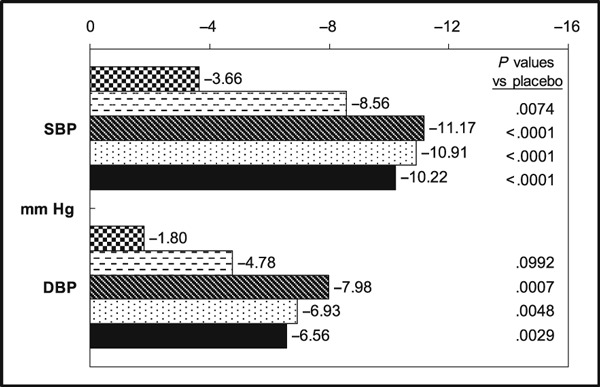
Least square mean changes from baseline to week 4. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.  Placebo,
Placebo,  Candesartan 2/4 mg.
Candesartan 2/4 mg.  Candesartan 8/16 mg.
Candesartan 8/16 mg.  Candesartan 16/32 mg.
Candesartan 16/32 mg.  All candesartan doses.
All candesartan doses.
The response rates (proportion of patients with both SiSBP and SiDBP <95th percentile) were 54%, 62%, and 65% for the low‐, medium‐, and high‐dose groups, respectively, which were significantly greater than the 31% for the placebo group (P<.05 for all doses vs placebo).
The decline in SiSBP appeared to be somewhat greater among patients in the lighter‐weight panel (<50 kg), but the same trend was not apparent for SiDBP. While candesartan lowered BP across all racial groups, the reduction was less in blacks than in nonblacks (eg, the placebo‐corrected reduction in SiSBP [all active doses pooled] was 4.8 mm Hg in blacks vs 7.9 mm Hg in nonblacks and 3.9 vs 6.7 mm Hg for SiDBP). There were no apparent differences in BP response based on age, sex, Tanner stage, or type of hypertension.
52‐Week Open‐Label Study
A total of 237 patients enrolled in the 52‐week clinical experience study; 213 had participated in the antecedent dose‐ranging study. In all, 235 took at least 1 dose of candesartan, and 233 could be included in the intent‐to‐treat population. As most of the patients entered the clinical experience study following participation in the dose‐ranging study, the characteristics of the two study populations were nearly identical (Table). By the end of the study, approximately one‐fourth of the patients were taking candesartan at either an 8‐, 16‐, or 32‐mg dose, while somewhat fewer were taking other doses. Twenty‐two patients (9.4%) were taking an additional antihypertensive agent, most often (n=13) a thiazide diuretic.
Over the course of the study, the response rate ranged from 50% to 63%, and at 1 year it was 52%. Consistent with the observation in the dose‐ranging study, the response rate was lower for blacks than for nonblacks (43% vs 61% at week 52, respectively).
Pharmacokinetics
Twenty‐two patients (12 younger than 12 years and 10 aged 12–17 years) participated in the single‐dose PK substudy. The PK parameters for the younger (6–12 years) and older (12–17 years) children were similar (Cmax, 334 vs 397 nmol/L; AUC, 2728 vs 3060 nmol*h/L; tmax, 4.3 hours for both; t½ 6.7 vs 5.7 hours). The plasma concentration time curves for the two age groups are illustrated in Figure 2. Furthermore, there was no significant correlation between age and either Cmax or AUC. Body weight correlated (negatively) with Cmax and AUC, but there was considerable variability and the associations were relatively weak (R=−0.528 and −0.557, respectively).
Figure 2.
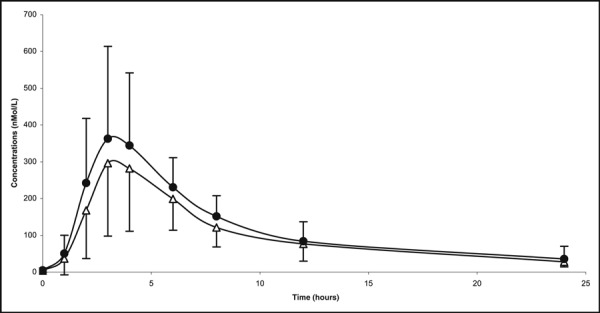
Mean (SD) candesartan plasma levels (nmol/L) in a single‐dose (16 mg) pharmacokinetic study. Δ indicates 6–12 years of age (n=12); • 12–17 years of age (n=10).
Safety
Candesartan was generally well tolerated: 3 patients discontinued treatment in the dose‐ranging study because of an adverse event, and 5 did so from the clinical experience study. These adverse events included hypotension, arm fracture, dizziness, headache, low white blood cell count, and progression of underlying renal disease (2 patients). None of the study participants became pregnant. The most commonly reported adverse events included headache, upper respiratory infection, dizziness, cough, and sore throat. There were no abnormalities of note in the laboratory or electrocardiographic examinations, and there was no consistent pattern as to change in urinary albumin/creatinine ratio. There was no notable change in height (mean [SD] Z‐score change to week 52, −0.05 [0.35]) or weight (mean [SD] change in Z‐score, −0.01 [0.31]).
In the neurocognition substudy, a total of 33 patients had both a baseline and 1‐year follow‐up evaluation. The mean (SD) baseline FSIQ was 95.0 (12.7) and the change to the end of the study was +2.6 (6.5). Three patients had an increase in FSIQ by >10 points, and 3 had a decline of ≥10 points (95% confidence interval, 0.0–18.9). In all 3 cases in which there was a decline, the baseline value was above “average” (100), and for all 3 the end‐of‐study score was within “normal” range.
Discussion
This pediatric hypertension treatment program describes the antihypertensive effects of an angiotensin receptor blocker, candesartan, in hypertensive children aged 6 to 17 years, in terms of the dose relationship and a long‐term treatment experience, and describes the single‐dose pharmacokinetics of this agent in the same population. The study population was consistent with the epidemiologic descriptions for hypertension in this age group: more common among males, strongly associated with obesity, and usually “primary” or “essential” in etiology. 1 , 2
The study’s primary objective focused on detecting a significant slope for reduction in BP across the dose levels of the drug tested. While candesartan induced significant reductions in BP at all 3 dose levels relative to placebo, the slopes across dose levels did not differ from zero. It would appear that the highest 2 doses studied were both toward the top of the dose‐response curve, thereby minimizing the ability to scribe a dose‐response curve with only 3 dose levels. In other words, the highest doses did not appear to produce much additional BP lowering. The observation is, however, not inconsistent with findings from adult studies in which BP reduction is dose‐related but the relationship is not particularly “steep” (8–12/4–8 mm Hg over 8–32 mg). 18 The findings are also consistent with the pediatric study of the angiotensin receptor blocker losartan in which the antihypertensive effect was essentially flat between a middle and high dose. 10 The selection of doses for study in the current program attempted to mimic the candesartan doses established for adult hypertensive persons by adjusting roughly for weight, but the approach was constrained by the available tablet strengths and the number of dose levels that could be studied. It is possible that a study with more dose levels over a wider range of doses would scribe a statistically significant dose‐response curve. In addition, this study highlights the potential vulnerability of the dose‐response design. 24 Perhaps concurrent placebo‐control designs, when feasible, may be preferable as they allow for direct quantitation of active treatment effects.
More important, the magnitude of the BP reductions obtainable with candesartan as observed in this study (8.6–11.2/4.8–8.0 mm Hg [placebo‐corrected 4.9/3.0–7.5/6.2 mm Hg]) compares favorably not only with the adult experience but with antihypertensive effects reported for other agents in children of the same general age; for example, 5.2–7.7/3.1–7.5‐mm Hg reductions with the β‐blocker metoprolol succinate extended release vs 1.9/2.1 mm Hg with placebo. 25 The results are also consistent with the findings reported for the angiotensin receptor blockers losartan and valsartan, 10 , 26 although some early studies with angiotensin‐converting enzyme inhibitors have indicated somewhat greater reductions (7.6–16.4‐mm Hg reduction in DBP with lisinopril). 9 In the case of candesartan in the current study, the BP reductions were sufficient to translate into hypertension control rates of about 50% to 60%; this was maintained with long‐term treatment.
The experience with renin‐angiotensin‐aldosterone system–inhibiting antihypertensive therapy in adults consistently indicates that black hypertensive patients respond less well to these agents than do nonblacks. 27 The experience in this study suggests that the same may be true in hypertensive children.
Also, consistent with the adult angiotensin receptor blocker treatment experience, candesartan was generally well tolerated in the current studies, although children with advanced renal impairment and those at high risk for significant adverse effects such as hyperkalemia were not included in the trials.
Together, the efficacy, safety, and PK data in the current study suggest that the antihypertensive effects of candesartan and its PK properties in children aged 6 years and older closely parallel those seen in hypertensive adults.
Other published reports of candesartan treatment in children are limited to one small study (n=17) in children aged 0.5 to 16 years with hypertension or proteinuria; this study reported a BP decline of 9/9 mm Hg, 28 and a study in 11 children suggested that ambulatory BP and clinic BP values are equally useful in assessing the antihypertensive response. 29
It should be noted that our study evaluated only 3 dose levels, which limits the precision with which the dose response can be characterized. The study also excluded children with severe hypertension and hypertension associated with causative etiologies, such as renovascular hypertension, and there were no renal transplant patients in the trial.
Perspectives
In keeping with the experience in adults, candesartan is effective and well tolerated in a pediatric hypertensive population. The same admonitions that accompany the use of candesartan in adults are also relevant for children: use caution to prevent hypotension in volume‐depleted patients and avoid administration if bilateral renal artery stenosis is suspected. Of particular importance for adolescent females is the admonition to avoid use of candesartan during pregnancy.
Together, this program of studies indicates that candesartan cilexetil (2–32 mg) administered once daily is an effective, well‐tolerated antihypertensive agent for children aged 6 to 17 years.
Acknowledgments
Acknowledgments and disclosures: The authors thank Michaelene Llewellyn, RN, for study conduct, oversight, and coordination. AstraZeneca LP funded the program of studies. HT reports having received research funding from and served as a consultant to AstraZeneca. JR reports serving as a consultant to AstraZeneca. JWH, JS, JMS, RT, and Michaelene Llewellyn are employees of AstraZeneca.
Investigators
Johan Vande Walle, MD, U.Z. Gent, Gent, Belgium; Laszio Szabo, MD, Miskolcc Mkh, Miskolc, Hungary; Tivadar Tulassay, MD, SEI. Gyerm Klin, Budapest, Hungary; Sandor Turi, MD, SZTE Gyerm Klin, Szeged, Hungary; Eva Marova, MD, Detska Kardiologicka ambulancia, Bratislava, Slovakia; Alexander Jurko, MD, Detska Kardiologicke ambulancia, Martin, Slovakia; Maria Horakova, MD, Detska Kardiologicka ambulancia, Tmava, Slovakia; Robert Achtel, MD, Sutter North Medical Foundation, Yuba City, CA; John Barcia, MD, University of Virginia, Charlottesville, VA; Donald Batisky, MD, Pediatric Clinical Trials International, Columbus, OH; Patrick Brophy, MD, University of Michigan Medical Center, Ann Arbor, MI; Bonita Falkner, MD, Thomas Jefferson University Hospital, Philadelphia, PA; Joseph Flynn, MD, Albert Einstein College of Medicine, Bronx, NY; Randall Jenkins, MD, Northwest Pediatric Kidney Specialist, Portland, OR; Vijay Kusnoor, MD, Southeast Texas Clinical Research Center, Beaumont, TX; Kenneth Miller, MD, Nephrology & Hypertension Consultants, Park Ridge, IL; Ana Paredes, MD, Miami Childrens Hospital; Miami, FL; Irene Restaino, MD, Children’s Hospital of The King’s Daughters, Norfolk, VA; Joseph Sherbotie, MD, University of Utah, Salt Lake City, UT; Gaston Zilleruelo, MD, University of Miami, Miami, FL; Myra Chiang, MD, CamCare Health Ed & Res Inst, Charleston, WV; Farahnak Assadi, MD, Rush‐Presbyterian‐St Luke’s Medical Center, Chicago, IL; Shashi Nagaraj, MD, Wake Forest University Health Sciences School of Medicine, Winston‐Salem, NC; Janice Sullivan, MD, Univ. of Louisville School of Medicine, Louisville, KY; Michael Aigbe, MD, Children’s Nephrology Clinic, Las Vegas, NV; Ronald Portman, MD, University of Texas Health Services Center, Houston, TX; Robert Mak, MD, Oregon Health Sciences University, Portland, OR; Michael Moritz, MD, Children’s Hospital of Pittsburgh, Pittsburgh, PA; Robert Williams, MD, Georgia Clinical Professional Group, Athens, GA; Juan Kupferman, MD, Maimonides Medical Center, Brooklyn, NY; Naomi Neufeld, MD, Neufeld Medical Group, Los Angeles, CA; Jimmy Stewart, MD, University of Mississippi Medical Center, Jackson, MS; Coral Hanevold, MD, Medical College of Georgia, Augusta, GA; Lydia Hazan, MD, IMPACT Clinical Trials, Beverly Hills, CA; Jeffery Blummer, MD, University of Cleveland, Cleveland, OH; Melissa Henshaw, MD, Medical University of South Carolina, Charleston, SC; David Headley, MD, Planters Clinic, Port Gibson, MS; Howard Trachtman, MD, Schneider Children’s Hospital, New Hyde Park, NY; William Primack, MD, Fallon Clinic, Auburn, MA; L. Richard Feldenberg, MD, Children's Hospital Central California, Medera, CA.
References
- 1. Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school‐aged children. Pediatrics. 2004;113:475–482. [DOI] [PubMed] [Google Scholar]
- 2. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. US Department of Health and Human Services . National Institute of Health; National Heart, Lung, and Blood Institute; 2005:1–48. [Google Scholar]
- 3. Hanevold C, Waller J, Daniels S, et al. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–333. [DOI] [PubMed] [Google Scholar]
- 4. Daniels SR, Loggie JM, Khoury P, et al. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97:1907–1911. [DOI] [PubMed] [Google Scholar]
- 5. Brady TM, Fivush B, Flynn JT, et al. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr. 2008;152:73–78. [DOI] [PubMed] [Google Scholar]
- 6. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness. A matched controlled study. Hypertension. 2006;48:40–44. [DOI] [PubMed] [Google Scholar]
- 7. Flynn JT, Daniels SR. Pharmacologic treatment of hypertension in children and adolescents. J Pediatr. 2006;149:746–754. [DOI] [PubMed] [Google Scholar]
- 8. Wells T, Frame V, Soffer B, et al. A double‐blind, placebo‐controlled, dose–response study of the effectiveness and safety of enalapril for children with hypertension. J Clin Pharmacol. 2002;42:870–880. [DOI] [PubMed] [Google Scholar]
- 9. Soffer B, Zhang Z, Miller K, et al. A double‐blind, placebo controlled, dose‐response study of the effectiveness and safety of lisinopril for children with hypertension. Am J Hypertens. 2003;16:795–800. [DOI] [PubMed] [Google Scholar]
- 10. Shahinfar S, Cano F, Soffer BA, et al. A double‐blind, dose‐response study of losartan in hypertensive children. Am J Hypertens. 2005;18:183–190. [DOI] [PubMed] [Google Scholar]
- 11. Flynn JT, Newburger JW, Daniels SR, et al. A randomized, placebo‐controlled trial of amlodipine in children with hypertension. J Pediatr. 2004;145:353–359. [DOI] [PubMed] [Google Scholar]
- 12. Sorof JM, Cargo P, Graepel J, et al. Beta‐blocker/thiazide combination for treatment of hypertensive children: a randomized double‐blind, placebo‐controlled trial. Pediatr Nephrol. 2002;17:345–350. [DOI] [PubMed] [Google Scholar]
- 13. Blowey DL, Monica I, Scolnik D, et al. The pharmacokinetics of extended release felodipine in children. Eur J Clin Pharmacol. 1996;50:147–148. [DOI] [PubMed] [Google Scholar]
- 14. Hogg RJ, Delucchi A, Sakihara G, et al. A multicenter study of the pharmacokinetics of lisinopril in pediatric patients with hypertension. Pediatr Nephrol. 2007;22:695–701. [DOI] [PubMed] [Google Scholar]
- 15. Trachtman H, Frank R, Mahan JD, et al. Clinical trial of extended‐release felodipine in pediatric essential hypertension. Pediatr Nephrol. 2003;18:548–553. [DOI] [PubMed] [Google Scholar]
- 16. Li JS, Berezny K, Kilaru R, et al. Is the extrapolated adult dose of fosinopril safe and effective in treating hypertensive children? Hypertension. 2004;44:289–293. [DOI] [PubMed] [Google Scholar]
- 17. Morsing P, Adler G, Brandt‐Eliasson U, et al. Mechanistic differences of various AT1‐receptor blockers in isolated vessels of different origin. Hypertension. 1999;33:1406–1413. [DOI] [PubMed] [Google Scholar]
- 18. Elmfeldt D, Olofsson B, Meredith P. The relationships between dose and antihypertensive effects of four AT‐1 receptory blockers. Differences in potency and efficacy. Blood Press. 2002;11:293–301. [DOI] [PubMed] [Google Scholar]
- 19. Meier CM, Simonetti GD, Ghiglia S, et al. Palatability of angiotensin II antagonists among nephropathic children. Br J Clin Pharmacol. 2006;63:628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chevalier RL. Developmental renal physiology of the low birth weight pre‐term newborn. J Urol. 1996;156:714–719. [DOI] [PubMed] [Google Scholar]
- 21. Lasaitiene D, Chen Y, Adams MA, et al. Further insights into the role of angiotensin II in kidney development. Clin Physiol Funct Imaging. 2006;26:197–204. [DOI] [PubMed] [Google Scholar]
- 22. Schwartz GJ, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am. 1987;34:571–590. [DOI] [PubMed] [Google Scholar]
- 23. Stenhoff H, Lagerstrom PO, Andersen C. Determination of candesartan cilexetil, candesartan and a metabolite in human plasma and urine by liquid chromatography and fluorometric detection. J Chromatogr B. 1999;73:411–417. [DOI] [PubMed] [Google Scholar]
- 24. Benjamin DK, Smith PB, Jadhav P, et al. Pediatric antihypertensive trial failures: Analysis of end points and dose range. Hypertension. 2008;51:834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batisky DL, Sorof JM, Sugg J, et al. Efficacy and safety of extended release metoprolol succinate in hypertensive children 6 to 16 years of age: a clinical trial experience. J Pediatr. 2007;150:134–139. [DOI] [PubMed] [Google Scholar]
- 26. Wells T, Blumer J, Litwin M, et al. Safety and effectiveness of valsartan (VAL) in hypertensive children ages 6 to 16 years. J Clin Hypertens. 2007;9(Supp A):79–80. [Google Scholar]
- 27. Association of Black Cardiologists (ABC) Candesartan Study Group . Evaluation of candesartan cilexetil in black patients with systemic hypertension: the ABC trial. Heart Dis. 2000;2:392–399. [PubMed] [Google Scholar]
- 28. Simonetti GD, Von Vigier RO, Konrad M, et al. Candesartan cilexetil in children with hypertension or proteinuria: preliminary data. Pediatr Nephrol. 2006;21(10): 1480–1482. [DOI] [PubMed] [Google Scholar]
- 29. Franks AM, O'Brien CE, Stowe CD, et al. Candesartan cilexetil effectively reduces blood pressure in hypertensive children. Ann Pharmacother. Pharmacotherapy. 2008;42(10):1388–1395. [DOI] [PubMed] [Google Scholar]


