Abstract
Hypertensive disorders of pregnancy (HDP), including pre‐existing hypertension, gestational hypertension, and preeclampsia, further complicate already high‐risk pregnancies in women with diabetes mellitus (DM). Women with both pre‐existing and gestational diabetes are at increased risk for HDP, leading to higher maternal and fetal morbidity. Further, particularly in diabetic women and women with a history of gestational diabetes, HDP significantly increases the risk for future cardiovascular events. For clinicians, women with hypertension and diabetes during pregnancy pose a management challenge. Specifically, preconception management should stress strict control of glycemia, blood pressure, and prevention of diabetic complications, specifically nephropathy, which specifically increases the risk for preeclampsia. During gestation, clinicians must be aware of potential maternal and fetal complications associated with various anti‐hypertensive therapies, including known fetotoxicity of ACE inhibitors and ARBs when given in the 2nd or 3rd trimester, and the risks and benefits of expectant management versus delivery in cases of severe gestational hypertension or preeclampsia. Indeed, diabetic women must be followed closely prior to conception and throughout gestation to minimize the risk of HDP and its associated complications. J Clin Hypertens (Greenwich). 2011;13:275–284. © 2011 Wiley Periodicals, Inc.
Hypertensive disorders complicate 5% to 10% of all pregnancies and are a leading cause of maternal and fetal morbidity and mortality. Hypertension during pregnancy frequently leads to preterm delivery, intrauterine growth restriction (IUGR), placental abruption, and small‐for‐gestational‐age (SGA) infants. 1 In women with diabetes mellitus (DM), hypertension adds further risk to that posed by diabetes alone. Health care providers are thus challenged with controlling maternal glucose levels and blood pressure (BP) in ways that optimize both maternal and fetal outcome.
Hypertensive disorders of pregnancy (HDP) are classified as either chronic (pre‐existing) hypertension, gestational hypertension (GH), preeclampsia, or the superimposition of preeclampsia on chronic hypertension 2 (Table I). Chronic hypertension, affecting approximately 3% of pregnancies, is defined as BP >140/90 mm Hg with onset prior to pregnancy or that is recognized by 20 weeks’ gestation. This is likely an underestimate, as many cases go unrecognized when BP is not measured prior to pregnancy and physiologic pregnancy‐induced vasodilation may lower BP into the normal range for nonpregnant individuals (<120/80 mm Hg) in early gestation. Advanced maternal age, African American race, and obesity are important risk factors for essential hypertension complicating pregnancy. 3 GH, on the other hand, is hypertension with initial onset during gestation, defined as hypertension occurring after 20 weeks’ gestation (although GH typically presents late in the 3rd trimester) with resolution by 6 to 12 weeks postpartum. 2 , 4 GH occurs in the absence of proteinuria, whereas preeclampsia is diagnosed when proteinuria (≥300 mg/24 hour urine) is also present. Diagnostic uncertainty sometimes results when the onset of proteinuria lags behind that of hypertension, sometimes by several weeks. 5 Preeclampsia complicates approximately 5% to 8% of all pregnancies. 3 Not surprisingly, women with underlying chronic hypertension or pre‐gestational DM are at increased risk for preeclampsia compared with normotensive and nondiabetic women, with rates of preeclampsia of approximately 25% and 20%, respectively. 6 The rate of preeclampsia is lower (<20%) and more variable in women with gestational DM (GDM), due to variations in degree of insulin resistance as well as comorbid risk factors, particulary maternal body mass index (BMI). 7
Table I.
Defining HTN Disorders of Pregnancy
| HTN Disorder | Onset | Proteinuria (>300 mg/d)? Yes/No | Prevalence |
|---|---|---|---|
| Chronic HTN | Prior to pregnancy, recognized <20 wk gestation | No | 3% of all pregnancies |
| Gestational HTN | ≥20 wk gestation; usually presents in 3rd trimester | No | 6%–17%, nulliparous 2%–4%, multiparous women |
| Preeclampsia | >20 wk gestation; usually presents in 3rd trimester | Yes (Onset of HTN and proteinuria are not necessarily concurrent) | 5%–8% of all pregnancies; 20% among diabetic women |
| Preeclampsia superimposed on chronic HTN | Chronic HTN: ≤20 wk gestation PE: >20 wk gestation | Yes | 25% of women with chronic HTN develop superimposed preeclampsia |
Abbreviations: HTN, hypertension; PE, preeclampsia.
In this review, we discuss hypertensive disorders of pregnancy in women with DM, including those who develop GDM. Poor glycemic control, pre‐existing hypertension, and diabetic renal disease all increase a diabetic woman’s risk for HDP and associated maternal and fetal complications. 8 Here, we specifically address epidemiology, pathophysiology, management strategies, and future cardiovascular disease (CVD) risk with respect to HDP among diabetic women. Management of postpartum hypertension, including hypertension during lactation, is beyond the scope of this review.
Hypertension in Diabetic Pregnancies: Epidemiology
Compared with nondiabetics, women with diabetes are at considerably higher risk for HDP. 8 , 9 , 10 , 11 At least 20% of pregnant diabetic women will develop GH and/or preeclampsia, with the most at‐risk patients being those with underlying microvascular complications, pre‐existing hypertension, or poor glycemic control. 8 , 11 Glycemic control, particularly during the first half of pregnancy, is a strong predictor of preeclampsia in women with type 1 diabetes mellitus (T1DM). 9 , 10 , 12 In addition to glycemic control, microvascular complications, specifically retinopathy and nephropathy, are independent predictors for preeclampsia in diabetic women, with nephropathy being the most important. 10 , 11 , 13 In a Finnish cohort, preeclampsia was 5 times more frequent and GH was twice as frequent in women with T1DM without nephropathy compared with nondiabetic controls. On the other hand, diabetic women with known nephropathy were approximately 6‐fold more likely than those without nephropathy and approximately 30‐fold more likely than nondiabetic women to develop HDP. 10 Pre‐existing microalbuminuria also significantly increases the risk for preeclampsia; thus, monitoring for microalbuminuria in this population is an important component of preconception care. Furthermore, among women with diabetic nephropathy, preexisting hypertension is a significant predictor of adverse pregnancy outcomes, including increased likelihood of super‐imposed preeclampsia, preterm delivery, and IUGR. 14 Of course, diagnostic difficulty arises when underlying proteinuria due to diabetic nephropathy confuses the distinction between GH and preeclampsia. It does not appear that this diagnostic dilemma would be resolved even if assays of circulating (anti‐)angiogenic factors were to become routinely available. 15 In these cases, it is important to be aware that the potential for preeclampsia is greatly increased among women with underlying diabetic renal disease; thus assuming preeclampsia exists when the distinction cannot be made will provide the most conservative approach to care.
Gestational diabetes also increases a woman’s risk for HDP. 16 , 17 After adjustment for maternal age and BMI, GDM confers an approximately 1.5‐fold increased risk of GH or preeclampsia. 16 Similar to the risk for essential hypertension, risk for HDP is much more striking in African American women compared with all other ethnic groups. Indeed, African American women with GDM have a 3‐ to 4‐fold increased risk of HDP compared with women who do not develop GDM, suggesting African American race and GDM additively increase a woman’s risk for HDP. 16
Pathophysiology of Hypertensive Disorders of Pregnancy
The pathophysiologic mechanisms resulting in GH and preeclampsia have not been fully elucidated but are certainly multifactorial, with vascular, immune, genetic, and placental factors hypothesized to play important roles, among others. Evidence suggests that GH and preeclampsia are caused by distinct underlying pathophysiologic mechanisms; thus, these two conditions should not be thought of as abnormalities along a continuum of severity, but as distinct disorders. Importantly for diabetic pregnant women, both endothelial dysfunction and insulin resistance likely have important roles in DM, HDP, and overall CVD risk.
Endothelial dysfunction is characteristic of preeclampsia as well as many predisposing conditions such as essential hypertension and is hypothesized to contribute to its pathophysiology. Elevated circulating levels of endothelial activators (such as fibronectin, E‐selectin, and others) have been demonstrated in women with preeclampsia. Further, during early gestation, women who go on to develop preeclampsia have abnormal uterine artery flow dynamics, indicative of pre‐existing endothelial dysfunction. Pre‐existing hypertension, metabolic syndrome, and renal disease, which also involve endothelial dysfunction and are more commonly seen in diabetic patients, increase a woman’s risk for both preeclampsia and future CVD. Together, these findings suggest that dysfunctional vascular endothelium is a common manifestation of preeclampsia, CVD, and diabetes. 18
Insulin resistance may also contribute to the development of HDP in diabetic women, as well as in women without overt DM but with prediabetes (impaired glucose tolerance and/or impaired fasting glucose). 19 A degree of insulin resistance is characteristic of normal pregnancy due to multiple physiologic hormonal changes, including production of placental lactogen, placental growth hormone, and high levels of estradiol, progesterone, and cortisol. 20 Women with higher fasting insulin levels prior to pregnancy are at increased risk for developing HDP, 21 and, during pregnancy, there is a temporal relationship between peak maternal insulin resistance and the first manifestation of GH or preeclampsia, both of which occur most commonly in the 3rd trimester. Further, women with the metabolic syndrome, polycystic ovary syndrome, and obesity—conditions associated with reduced insulin sensitivity—are at increased risk for HDP. 21 Among nondiabetic women, a positive correlation exists between both fasting glucose and subclinical abnormalities in response to glucose challenge and preeclampsia. 17 , 22 In fact, nondiabetic women in the highest quartile of post‐challenge glucose have a 2‐fold increased risk for preeclampsia compared with those in the lowest quartile. 17 Interestingly, nondiabetic women with hypertension during pregnancy also have a 3‐fold elevated risk for developing T2DM in the future, 23 and preeclamptic nondiabetics have been shown to have residual insulin resistance after delivery that may persist for years. 24 , 25 Together, these observations suggest that insulin resistance may play a causal role in the development of HDP, particularly preeclampsia. Indeed, measures to improve insulin sensitivity may help prevent HDP as well as attenuate the associated increase in CVD risk. 19
Diabetic Renal Disease and Hypertension in Pregnancy
Management of diabetic renal disease prior to conception and during pregnancy is both complex and controversial. In nonpregnant individuals, the mainstay of management of diabetic nephropathy is inhibition of the renin‐angiotensin system with angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). 26 , 27 However, both ACE inhibitors and ARBs have well‐established fetotoxic effects when used during the 2nd and 3rd trimesters of pregnancy: renal failure and anuria, with resultant oligohydramnios and pulmonary hypoplasia; skeletal/calvarial abnormalities; and fetal growth restriction. 28 There is conflicting evidence regarding the potential teratogenicity of ACE inhibitors or ARBs when used during the 1st trimester, with one study showing an increased risk of fetal cardiac valve and central nervous system defects after 1st trimester ACE inhibitor exposure 29 but other studies showing no increased teratogenic risk specific to this drug class. 30 , 31 , 32 Further complicating management, pregnancy itself may accelerate progression of clinically significant diabetic nephropathy, particularly when there is already a significant decrease in renal function prior to conception, which may result in progression to end‐stage renal disease earlier than predicted based on the natural course of diabetic nephropathy in nonpregnant patients. 11 , 33 , 34 In addition, pre‐existing maternal nephropathy is associated with significant maternal and fetal morbidity, including preeclampsia, premature delivery, low birth weight, and increased incidence of respiratory distress syndrome and perinatal infant death. 35 The prevalence of fetal complications increases with increasing severity of maternal renal disease. 36 For these reasons, it was previously common practice to discourage pregnancy in women with known diabetic nephropathy. On the other hand, after careful consideration of the possible increased teratogenic risk of limited 1st trimester exposure to ACE inhibitors, 29 , 30 , 31 , 32 the maternal and fetal benefits conferred by their renal‐protective effects may far outweigh the potential risks of short‐term exposure in reliable patients who will discontinue them in early gestation.
Several studies have shown that treating diabetic women who desire pregnancy with an ACE inhibitor prior to conception, then stopping the medication as soon as pregnancy is known, decreases proteinuria and improves maternal outcome without increased risk to the fetus. In a small uncontrolled study of women with insulin‐dependent diabetes (IDDM) and known nephropathy, 6 to 12 months of treatment with the ACE inhibitor captopril prior to conception appeared to improve maternal‐fetal outcome. 37 Specifically, pre‐conception captopril decreased proteinuria prior to pregnancy, and despite discontinuation of the medication early in the 1st trimester, progression of nephropathy was minimal in most cases and proteinuria reverted back to prepregnancy levels within 3 months postpartum. Neonatal outcome was also improved relative to what would be expected in diabetic mothers with severe proteinuria, with only 1 of 8 births being delivered preterm, 1 SGA, and none with congenital anomalies. 37 A similar study by the same group, in women with IDDM and mild nephropathy (rather than severe nephropathy as in the prior study), half of whom also had pre‐existing hypertension, suggested long‐term renal protection following treatment with captopril for 6 months prior to conception along with strict glycemic control. Moreover, the complications that occurred, including preeclampsia, IUGR, and preterm delivery, occurred at lower rates than expected in pregnancies complicated by diabetic nephropathy. In fact, pre‐existing hypertension was the only significant predictor of these complications. Likewise, none of the offspring from these women exhibited any congenital abnormality despite maternal ACE inhibitor use early in the 1st trimester. 38
Women with diabetic nephropathy should be counseled about the risks of pregnancy and made aware that tight control of glycemia and BP are paramount to minimizing pregnancy complications, including progression of microvascular disease. Ideally, close medical follow‐up should allow these women to continue ACE inhibitor or ARB use as renal‐sparing agents in anticipation of pregnancy, with immediate discontinuation as soon as pregnancy is achieved, to optimize outcomes. Specifically, minimizing progression of diabetic nephropathy during pregnancy is critical to reduce the risk of GH or preeclampsia and associated adverse maternal/fetal outcomes.
Management of Hypertension in Diabetic Women During Pregnancy
Chronic (pre‐existing) hypertension is normally well‐tolerated in pregnancy and frequently requires no treatment or discontinuation of preconception antihypertensive medications due to physiologic vasodilation and subsequent BP reduction that occur in early pregnancy. Pharmacotherapy is generally reserved for severe hypertension in pregnancy, with recommendations for threshold and goal BP varying among professional societies (Table II). On average, consideration of medical management begins with BP of 140 to 150 mm Hg systolic/90 to 100 mm Hg diastolic (and slightly lower thresholds for high‐risk patients), with a goal of preventing maternal cerebrovascular or cardiovascular events. 4 It is important to note that maternal stroke, which is typically hemorrhagic and often fatal, is related to elevated systolic (not diastolic) BP and begins to occur at systolic BP ≥155 mm Hg. 39
Table II.
BP Goals and Treatment Thresholds During Pregnancy
| Professional Society | Threshold BP for Medical Treatment | Target Maternal BP |
|---|---|---|
| JNC (nonpregnant) 62 | BP >140/90 mm Hg | SBP ≤120 mm Hg DBP ≤80 mm Hg |
| NHBPEP 63 | BP ≥160/105 mm Hg Consider treatment if high‐risk patient with BP >140/90 mm Hg and 30‐mm Hg or 15‐mm Hg increase in SBP or DBP, respectively | None |
| ASH 64 | Chronic hypertension: DBP ≥100 mm Hg or at lower levels in patients with DM, renal disease or end‐organ damage; acute severe hypertension: DBP ≥105 mm Hg | Gradual reduction to DBP 90–100 mm Hg |
| ACOG 65 | DBP >105–110 mm Hg or DBP >100 mm Hg if chronic hypertension or clinical judgement if end‐organ damage or renal disease present | None |
| Australia 66 | BP ≥160/90 mm Hg | SBP <160 and DBP ≤110 mm Hg |
| Canadian Hypertension Society 67 | BP ≥140/90 mm Hg | DBP 80–90 mm Hg |
| European Society of Cardiology 46 | Consider treatment to prevent progression if BP 140–160 mm Hg/90–110 mm Hg Close monitoring and clinical judgement in high‐risk patients (BP >160/110 mm Hg or end‐organ damage or DM or renal disease or collagen vascular disease) | None |
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; ASH, American Society of Hypertension; BP, blood pressure; DM, diabetes mellitus; DBP, diastolic blood pressure; JNC, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; NHBPEP, National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy; SBP, systolic blood pressure.
The only definitive treatment for GH or preeclampsia is delivery. Delaying delivery to maximize fetal development when maternal hypertension manifests remote from term is attempted to improve fetal outcomes, as long as risk for maternal morbidity is low. Expectant management to prolong pregnancy, with individualized decision‐making based on close monitoring of both maternal and fetal well‐being, may often improve outcomes in carefully selected gravidas at 25 to 34 weeks. 40 Severe preeclampsia or GH prior to 25 weeks’ gestation is especially threatening and frustrating due to poor fetal outcomes associated with extreme prematurity and the likelihood of severe maternal morbidity in many cases of expectant management. 41 In cases of hemolysis, elevated liver enzymes, and low platelet count (HELLP) prior to 34 weeks gestation, there is no benefit to expectant management, with fetal outcome being poor at the expense of high risk to maternal health. 42 On the other hand, in women with either GH or preeclampsia that manifests ≥36 weeks’ gestation, delivery results in improved maternal outcome compared with expectant management, primarily due to decreased progression to severe hypertension or eclampsia, with no difference in neonatal outcome. 43 Unfortunately, these, and most other, studies either excluded or failed to account for patients with diabetes and/or underlying renal disease, which further complicate hypertensive pregnancies and must also be considered when deciding between expectant vs active management in clinical practice.
Clinicians will usually try to limit drug use during pregnancy, motivated by concerns related to teratogenicity or fetotoxicity, while balancing these risks against those of untreated or undertreated maternal disease. Antihypertensive therapy is often necessary, as severe maternal hypertension (BP ≥155–160 mm Hg/110 mm Hg), if left untreated, may lead to maternal stroke (particularly systolic BP ≥155 mm Hg) and even maternal death. 44 That being said, a clear benefit to treating mild to moderate hypertension in pregnancy (BP >140/90 mm Hg, <155/110 mm Hg) has not been clearly demonstrated, and, theoretically, the beneficial decrease in incidence of more severe forms of maternal hypertension may be offset by an increased incidence of SGA infants due to decreased uteroplacental perfusion, with no consistent advantages to overall perinatal outcome. 44 However, to date, no prospective controlled studies of targeted BP control in pregnancy and relation to perinatal outcome have been performed, making any such recommendations entirely speculative. Without sound evidence in favor of treating mild to moderate hypertension, clinicians will still agree on the need to control more severe hypertension (BP ≥155/110 mm Hg), or hypertension accompanied by nephropathy, or other high‐risk features 4 (Figure).
Figure.
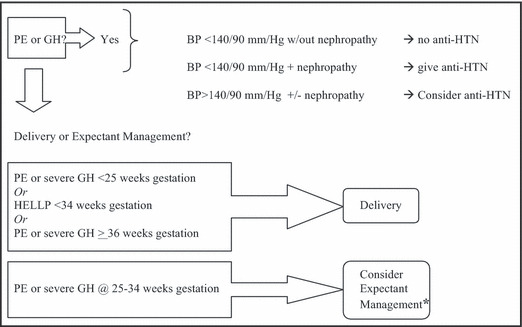
Algorithm for the management of hypertensive (HTN) disorders of pregnancy in diabetic women. *With immediate delivery for any progression of maternal renal, neurologic, hepatic or hematologic abnormalities. PE indicates preeclampsia; GH, gestational hypertension; anti‐HTN, antihypertensive medication; HELLP, hemolysis, elevated liver enzymes, low platelet count.
Clinical decision‐making becomes more challenging when a woman with long‐standing diabetes presents with elevated BP and albuminuria during pregnancy, making it difficult to distinguish preeclampsia from GH or undiagnosed pregestational hypertension in the setting of diabetic nephropathy. Indeed, both diabetes and renal disease significantly increase a woman’s risk for developing preeclampsia, with the severity of diabetic renal disease prior to conception being positively associated with risk for worsening albuminuria and preeclampsia. 45 When a distinction cannot be made, it is best to manage patients conservatively, with the assumption that preeclampsia—the condition associated with more fetal and maternal morbidity—has developed.
When antihypertensive therapy is warranted, one should avoid medications that may be teratogenic or fetotoxic (Table III [recommendations on antihypertensive pharmacotherapy during pregnancy]). ACE inhibitors and ARBs have well‐established fetotoxicity during the 2nd and 3rd trimesters, 28 , 31 and a controversial literature suggests that they may be teratogenic when used during the 1st trimester. 29 , 30 , 31 , 32 Use of these medications is therefore avoided when possible, especially after very early pregnancy. Diuretics, although considered third‐line treatment in pregnancy, especially in the setting of comorbid renal disease or heart failure, should generally be avoided due to potential electrolyte abnormalities and to avoid volume contraction, which may limit amniotic fluid volume and fetal growth. Diuretics are irrational choices in preeclampsia, where hypertension is characterized by systemic vasoconstriction and some degree of central volume contraction. 46 Of note, the potassium‐sparing diuretic spironolactone should be avoided because of additional anti‐androgenic effects. Indeed, women with polycystic ovary syndrome, who have an increased risk of GDM and HDP, 47 are often treated with spironolactone and should be educated about the potential dangers of continuing this drug if pregnancy is desired or possible.
Table III.
Pharmacologic Treatment of Hypertensive Disorders of Pregnancy
| Medication Class | 1st, 2nd, 3rd‐Line, Treatment or Contraindicated | Teratogenicity/Fetotoxicity | Formulations | Considerations in DM Patients |
|---|---|---|---|---|
| α‐Adrenergic agonists | 1st | None | Methyldopa, PO/IV Clonidine, PO | No maternal renal protection |
| β‐Blockers | 1st or 2nd | Fetal growth restriction, fetal bradycardia | Labetolol, PO/IV Atenolol, PO | Maternal hypoglycemia unawareness (labetalol) |
| Vasodilators | 2nd (IV)a 3rd (PO) | Neonatal thrombocytopenia (hydralazine) Cyanide toxicity if used >4 hour (nitroprusside) | Hydralazine, PO/IV; Nitroprusside, IV | |
| Calcium channel blockers | 2nd | None | Verapamil, PO Diltiazem, PO Nifedipine, PO Nicardipine, IV | Potential decreased maternal albuminuria and CVDb |
| ACE inhibitors/ARBs | Contraindicated in 2nd and 3rd trimesters; Unknown in 1st trimester | Renal failure, anuria; pulmonary hypoplasia; skeletal/calvarial abnormalitiesc Cardiac and CNS defects due to 1st trimester exposure (?)d | eAny | May slow progression of DM renal disease when given prior to pregnancy and discontinued early in 1st trimester |
| Diuretics | 3rd Avoid when possible | Decreased uteroplacental flow due to volume contraction | Lasix, PO HCTZ, PO | Avoid aldosterone antagonists (spironolactone) due to anti‐androgenic effect on fetus; avoid completely in preeclampsia |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CNS, central nervous system; CVD, cardiovascular disease; DM, diabetes mellitus; HCTZ, hydrochlorothiazide; IV, intravenous; PO, oral; aIV formulations used for hypertensive urgency. bHas been demonstrated in diabetic women but not during pregnancy. cProven teratogenic effects due to exposure during the 2nd and 3rd trimesters only. dPossible effect of 1st trimester exposure, although data are conflicting. eCaptopril, PO, has been shown to slow progression of DM nephropathy during pregnancy without teratogenic effects when used pre‐conception and stopped as soon as pregnancy is diagnosed.
Controversy has surrounded the use of β‐blockers during pregnancy due to evidence suggesting an increased risk for pre‐term delivery, IUGR, and SGA infants when these agents are used. 48 , 49 There are conflicting data among various studies, with some showing worse pregnancy outcome in the setting of any antihypertensive therapy, with no effect attributable specifically to β‐blockers. 49 Currently, β‐blockers are considered safe in pregnancy and are recommended as first‐ or second‐line agents. The dual α‐/β‐antagonist labetalol is generally preferred to β‐selective formulations such as the β1‐selective antagonist atenolol because, although no β‐blocker has been shown to be teratogenic, atenolol has been associated with fetal growth restriction in some studies. With respect to diabetic women, labetalol may reduce hypoglycemia awareness; thus, this should also be taken into consideration when choosing a specific β‐blocking agent for diabetic pregnant women.
In addition to β‐blockers, acceptable and commonly prescribed medication options during pregnancy include methyldopa, calcium channel blockers (CCBs), and hydralazine. Methyldopa, a central α‐adrenergic agonist, is considered first‐line treatment in the treatment of HDP due to its long history of use without evidence of adverse fetal effects and with proven efficacy in reducing progression to severe maternal hypertension. The nondihydropyridine CCBs (diltiazem and verapamil), when used as alternatives or adjuncts to ACE inhibitors or ARBs among diabetics, may reduce CVD risk and decrease albuminuria, 50 although these benefits have not been demonstrated in pregnancy. Hydralazine, a direct vasodilator, is considered a third‐line oral agent and a second‐line intravenous formulation for the urgent treatment of severe hypertension, although β‐blockers or central α‐antagonists should be used concomitantly to avoid reflex tachycardia and achieve a full antihypertensive effect. Other formulations that may be used in hypertensive urgency or emergency during pregnancy include intravenous labetalol (first‐line), oral nifedipine, and intravenous nicardipine. When urgently treating severe hypertension in pregnancy, avoiding even transient hypotension is paramount to avoid fetal distress due to decreased uteroplacental perfusion. 4
How Does Hypertension During Pregnancy Impact Overall CVD Risk in Diabetic Women?
Independent of the presence of diabetes, hypertension during pregnancy has been shown to significantly increase a woman’s risk for subsequent chronic hypertension 51 and for subsequent cardiovascular events, including stroke, thromboembolic disease, congestive heart failure, and ischemic heart disease. 23 , 52 In diabetic women, preeclampsia is associated with remote progression of diabetic nephropathy and increased lifetime risk of developing end‐stage renal disease, as well as with premature death. 53 As hypertension increases in severity, the risk for maternal cardiovascular morbidity also increases, suggesting that HDP may be viewed as an independent risk factor for future CVD. 23 Compared with women who have had uncomplicated pregnancies, women with a history of preeclampsia have a 3‐fold increased risk for developing chronic hypertension 54 and a higher average BP and decreased insulin sensitivity when followed for up to 2 decades postpartum. 25 Further, a diagnosis of preeclampsia increases a woman’s risk for ischemic heart disease within the next 20 years by approximately 2‐fold, independent of whether chronic hypertension exists. 55 Among premenopausal women who have experienced an acute myocardial infarction (AMI), the risk for AMI is approximately 3‐fold higher in women with a history of preeclampsia, independent of whether chronic hypertension has been diagnosed. 56 Not only is cardiovascular mortality higher among women who have a history of hypertension complicating pregnancy compared with those who remained normotensive, but the severity of hypertension also positively correlates with CVD risk, with women with severe preeclampsia having an 8‐fold increased risk of death from ischemic heart disease in older age. 57 , 58 Although HDPs identify women at high risk for future cardiovascular events, it is not clear whether the HDP itself alters maternal vasculature to increase future CVD risk, or whether shared underlying pathophysiologic mechanisms or risk factors contribute to both HDP and CVD.
Diabetes during pregnancy is also a significant predictor of future CVD. A diagnosis of diabetes significantly increases a woman’s risk for ischemic heart disease, with diabetic women having a 14‐fold increased risk of AMI in one series. 56 Among women with GDM, approximately 50% will develop T2DM, a well‐established independent cardiovascular risk factor, within 10 years postpartum. 59 Further, GDM itself, independent of whether T2DM develops, increases the risk for future CVD by approximately 70%. 60 , 61 Compared with a control population, women with a history of GDM who have not developed T2DM have elevated BP, increased peripheral vascular resistance, and decreased cardiac output, as well as decreased insulin sensitivity, higher fasting triglycerides, and increased rates of metabolic syndrome, all of which contribute to overall CVD risk. These effects are independent of BMI and persist for at least 1 year postpartum in the absence of frank T2DM. 61
Given the clear increased CVD risk associated with HDP, particularly in diabetic women, it is important that physicians inquire about a history of hypertension and diabetes in pregnancy when assessing a woman’s overall cardiovascular risk profile in order to guide more aggressive CVD risk assessment and intervention. Specifically, diabetic women who have experienced an HDP should be counseled regarding their increased CVD risk and actively practice preventative lifestyle habits, such as caloric limitation and regular exercise to prevent or attenuate obesity, and maintain compliance with medications prescribed to control blood glucose, BP, and plasma lipids.
Conclusions
Hypertension increases the risk for both maternal and fetal complications in already high‐risk diabetic pregnancies. Furthermore, women with diabetes and those predisposed to develop gestational diabetes are at significantly increased risk for HDP. Minimizing both maternal and fetal morbidity in hypertensive diabetic pregnancies is often a challenge to medical professionals. Increased understanding of the pathophysiology of hypertension in pregnancy, as well as advances in medical therapy so that risks of fetal toxicity and teratogenicity are minimized, will improve our ability to prevent and treat hypertension in pregnancy. It is clear that in diabetic women, complications of diabetes, particularly diabetic nephropathy and poor glycemic control, are independent risk factors for HDPs. Additionally, diabetic women with a history of HDP experience a higher cardiovascular mortality later in life. Thus, all diabetic women contemplating pregnancy need to be counseled regarding the importance of prevention during the preconception period and require close medical attention during gestation and postpartum.
References
- 1. Ness RB, Sibai BM. Shared and disparate components of the pathophysiolgies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–49. [DOI] [PubMed] [Google Scholar]
- 2. Surya P. National High Blood Pressure Education Program Working Group. Report on high blood pressure in pregnancy. Am J Obstet Gyncol. 1990;163:1689–1712. [DOI] [PubMed] [Google Scholar]
- 3. Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–969. [DOI] [PubMed] [Google Scholar]
- 4. Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens. 2008;2:484–494. [DOI] [PubMed] [Google Scholar]
- 5. Wolf M, Shah A, Jimenez‐Kimble R, et al. Differential risk of hypertensive disorders of pregnancy among Hispanic women. J Am Soc Nephrol. 2004;15:1330–1338. [DOI] [PubMed] [Google Scholar]
- 6. Caritis S, Sibai B, Hauth J, et al. Low‐dose aspirin to prevent preelampsia in women at high risk. N Engl J Med. 1998;338:701–705. [DOI] [PubMed] [Google Scholar]
- 7. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol. 2010;202:255.e1–255.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garner PR, D’Alton ME, Dudley DK, et al. Preeclampsia in diabetic pregnancies. Am J Obstet Gynecol. 1990;163:505–508. [DOI] [PubMed] [Google Scholar]
- 9. Hanson U, Persson B. Epidemiology of pregnancy‐induced hypertension and preeclampsia in type 1 (insulin‐dependent) diabetic pregnancies in Sweden. Acta Obstet Gynecol Scand. 1998;77:620–624. [DOI] [PubMed] [Google Scholar]
- 10. Hiilesmaa V, Suhonen L, Teramo K. Glycaemic control is associated with preeclampsia but not with pregnancy‐induced hypertension in women with Type 1 diabetes mellitus. Diabetologia. 2000;43:1534–1539. [DOI] [PubMed] [Google Scholar]
- 11. Bond MJ, Umans JG. Microvascular complications and the diabetic pregnancy. Curr Diab Rep. 2006;6:291–296. [DOI] [PubMed] [Google Scholar]
- 12. Rosenn B, Miodovnik M, Combs CA, et al. Poor glycemic control and antepartum obstetric complications in women with insulin‐dependent diabetes. Int J Gynaecol Obstet. 1993;43:21–28. [DOI] [PubMed] [Google Scholar]
- 13. Combs CA, Rosenn B, Kitzmiller JL, et al. Early‐pregnancy proteinuria in diabetes related to preeclampsia. Obstet Gynecol. 1993;82:802–807. [PubMed] [Google Scholar]
- 14. Bar J, Ben‐Rafael Z, Padoa A, et al. Prediction of pregnancy outcome in subgroups of women with renal disease. Clin Neprhol. 2000;53:437–444. [PubMed] [Google Scholar]
- 15. Powers RW, Jeyabalan A, Clifton RG, et al. Soluble fms‐like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PIGF) in preeclampsia among high risk pregnancies. PLoS ONE. 2010;5:e13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bryson CL, Ioannou GN, Rulyak SJ, et al. Association between gestational diabetes and pregnancy‐induced hypertension. Am J Epidemiol. 2003;158:1148–1153. [DOI] [PubMed] [Google Scholar]
- 17. Joffe GM, Esterlitz JR, Levine R, et al. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Am J Obstet Gynecol. 1998;179:1032–1037. [DOI] [PubMed] [Google Scholar]
- 18. Ness R, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:1–15. [DOI] [PubMed] [Google Scholar]
- 19. Seely EW, Solomon CG. Insulin resistance and its potential role in pregnancy‐induced hypertension. J Clin Endocrinol Metab. 2003;88:2393–2398. [DOI] [PubMed] [Google Scholar]
- 20. Barbour LA, McCurdy CE, Hernandez TL, et al. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–S119. [DOI] [PubMed] [Google Scholar]
- 21. Solomon CG, Carroll JS, Okamura K, et al. Higher cholesterol and insulin levels are associated with increased risk for pregnancy‐induced hypertension. Am J Hypertens. 1999;12:276–282. [DOI] [PubMed] [Google Scholar]
- 22. HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 23. Lykke JA, Langhoff‐Roos J, Sibai BM, et al. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. [DOI] [PubMed] [Google Scholar]
- 24. Fuh MMT, Yin CS, Pei D, et al. Resistance to insulin‐mediated glucose uptake and hyperinsulinemia in women who had preeclampsia during pregnancy. Am J Hypertens. 1995;8:768–771. [DOI] [PubMed] [Google Scholar]
- 25. Laivori H, Tikkanen MJ, Ylikorkala O. Hyperinsulinemia 17 years after preeclamptic first pregnancy. J Clin Endocrinol Metab. 1996;81:2908–2911. [DOI] [PubMed] [Google Scholar]
- 26. Parving HH, Andersen AR, Smidt UM, et al. Effect of antihypertensive treatment on kidney function in diabetic neprhopathy. Br Med J. 1987;294:1443–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ravid M, Savin H, Jutrin I, et al. Long‐term stabilizing effect of angiotensin‐converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive Type II diabetic patients. Ann Intern Med. 2000;118:577–581. [DOI] [PubMed] [Google Scholar]
- 28. Quan A. Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum Dev. 2006;82:23–28. [DOI] [PubMed] [Google Scholar]
- 29. Cooper WO, Hernandez‐Diaz S, Arbogast PG, et al. Major congenital malformations after first‐trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2500. [DOI] [PubMed] [Google Scholar]
- 30. Lennestal R, Olausson PO, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65:615–625. [DOI] [PubMed] [Google Scholar]
- 31. Nakhai‐Pour HR, Rey E, Berard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small‐for‐gestational‐age newborns. Birth Defects Res B Dev Reprod Toxicol. 2010;89:147–154. [DOI] [PubMed] [Google Scholar]
- 32. Caton AR, Bell EM, Druschel CM, et al. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009;54:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biesenbach G, Stroger H, Zazgornik J. Influence of pregnancy on progression of diabetic nephropathy and subsequent requirement of renal replacement therapy in female type 1 diabetic patients with impaired renal function. Nephrol Dial Transplant. 1992;7:105–109. [DOI] [PubMed] [Google Scholar]
- 34. Purdy LP, Hantsch CE, Molitch ME, et al. Effect of pregnancy on renal function in patients with moderate‐to‐severe diabetic renal insufficiency. Diabetes Care. 1996;19:1067–1074. [DOI] [PubMed] [Google Scholar]
- 35. Biesenbach G, Grafinger P, Zazgornik J, et al. Perinatal complications and three‐year follow up of infants of diabetic mothers with diabetic nephropathy stage IV. Ren Fail. 2000;22:573–580. [DOI] [PubMed] [Google Scholar]
- 36. Khoury JC, Miodovnik M, LeMasters G, et al. Pregnancy outcome and progression of diabetic nephropathy. What’s next? J Matern Fetal Neonatal Med. 2002;11:238–244. [DOI] [PubMed] [Google Scholar]
- 37. Hod M, van Dijk DJ, Karp M, et al. Diabetic nephropathy and pregnancy: the effect of ACE inhibitors prior to pregnancy on fetomaternal outcome. Nephrol Dial Transplant. 1995;10:2328–2333. [DOI] [PubMed] [Google Scholar]
- 38. Bar J, Chen R, Schoenfeld A, et al. Pregnancy outcome in patients with insulin dependent diabetes mellitus and diabetic nephropathy treated with ACE inhibitors before pregnancy. J Pediatr Endocrinol Metab. 1999;12:659–665. [DOI] [PubMed] [Google Scholar]
- 39. Martin JN, Thigpen BD, Moore RC, et al. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–254. [DOI] [PubMed] [Google Scholar]
- 40. Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190:1590–1597. [DOI] [PubMed] [Google Scholar]
- 41. Budden A, Wilkinson L, Buksh MJ, et al. Pregnancy outcome in women presenting with pre‐eclampsia at less than 25 weeks’ gestation. Aust N Z J Obstet Gynaecol. 2006;46:407–412. [DOI] [PubMed] [Google Scholar]
- 42. Abramovici D, Friedman SA, Mercer BM, et al. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation: does HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221–225. [DOI] [PubMed] [Google Scholar]
- 43. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild preeclampsia after 36 weeks’ gestation (HYPITAT): a multi‐centre, open‐label randomized controlled trial. Lancet. 2009;374:979–988. [DOI] [PubMed] [Google Scholar]
- 44. Yankowitz J. Pharmacological treatment of hypertensive disorders during pregnancy. J Perinat Neonatal Nurs. 2004;18:23040. [DOI] [PubMed] [Google Scholar]
- 45. Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. Am J Obstet Gynecol. 1997;177:1003–1010. [DOI] [PubMed] [Google Scholar]
- 46. Eur Soc Cardiology Task Force . Expert consensus document on management of cardiovascular disease during pregnancy. Eur Heart J. 2003;24:761–781. [DOI] [PubMed] [Google Scholar]
- 47. Iavazzo C, Vitoratos N. Polycystic ovarian syndrome and pregnancy outcome. Arch Gynecol Obstet. 2010;282:235–239. [DOI] [PubMed] [Google Scholar]
- 48. Magee LA, Duley L. Oral beta‐blockers for mild to moderate hypertension during pregnancy (Cochrane Review). Cochrane Database Syst Rev. 2000;4:CD002863. [DOI] [PubMed] [Google Scholar]
- 49. Ray JG, Vermeulen MJ, Burrows EA, et al. Use of antihypertensive medications in pregnancy and the risk of adverse perinatal outcomes: McMaster Outcome Study of Hypertension in Pregnancy 2 (MOS HIP 2). BMC Pregnancy Childbirth. 2001;1:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weber MA, Bakris GL, Jamerson K, et al. Cardiovascular events during differing hypertension therapies in patients with diabetes. J Am Coll Cardiol. 2010;56:77–85. [DOI] [PubMed] [Google Scholar]
- 51. Adams EM, Cantab MA, Aberd MD, et al. Long‐term effect of pre‐eclampsia on blood‐pressure. Lancet. 1961;23:1373–1375. [Google Scholar]
- 52. Bellamy L, Casas JP, Hingorani AD, et al. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systemic review and meta‐analysis. BMJ. 2007;335:974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sandvik MK, Iversen BM, Irgens LM, et al. Are adverse pregnancy outcomes risk factors for development of end‐stage renal disease in women with diabetes? Nephrol Dial Transplant. 2010;25:3600–3607. [DOI] [PubMed] [Google Scholar]
- 54. Sibai BM, el‐Nazer A, Gonzalez‐Ruiz A. Severe preeclampsia‐eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011–1016. [DOI] [PubMed] [Google Scholar]
- 55. Smith GCS, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. [DOI] [PubMed] [Google Scholar]
- 56. Mann JI, Doll R, Thorogood M, et al. Risk factors for myocardial infarction in young women. Br J Prev Soc Med. 1976;30:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arnadottir GA, Geirsson RT, Arngrimsson R, et al. Cardiovascular death in women who had hypertension in pregnancy: a case‐control study. BJOG. 2005;112:286–292. [DOI] [PubMed] [Google Scholar]
- 58. Jonsdottir LS, Arngrimsson R, Geirsson RT, et al. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776. [DOI] [PubMed] [Google Scholar]
- 59. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30:s251–s2560. [DOI] [PubMed] [Google Scholar]
- 60. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31:1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Heitritter SM, Solomon CG, Mitchell GF, et al. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2005;90:3983–3988. [DOI] [PubMed] [Google Scholar]
- 62. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 63. Surya P . Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 64. Lindheimer MD, Taler SJ, Cunningham FG. ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. ACOG . ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. [DOI] [PubMed] [Google Scholar]
- 66. Brown MA, Hague WM, Higgins J, et al. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust N Z J Obstet Gynaecol. 2000;40:139–155. [DOI] [PubMed] [Google Scholar]
- 67. Helewa ME, Burrows RF, Smith J, et al. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ. 1997;157:715–725. [PMC free article] [PubMed] [Google Scholar]


