Abstract
The American Society of Hypertension is publishing a series of Position Papers in their official journals throughout the 2008 ‐ 2009 years. The following Position Paper originally appeared: JASH. 2008;2(6):484–494.
Hypertension complicates 5% to 7% of all pregnancies. A subset of preeclampsia, characterized by new‐onset hypertension, proteinuria, and multisystem involvement, is responsible for substantial maternal and fetal morbidity and is a marker for future cardiac and metabolic disease. This American Society of Hypertension, Inc (ASH) position paper summarizes the clinical spectrum of hypertension in pregnancy, focusing on preeclampsia. Recent research breakthroughs relating to etiology are briefly reviewed. Topics include classification of the different forms of hypertension during pregnancy, status of the tests available to predict preeclampsia, and strategies to prevent preeclampsia and to manage this serious disease. The use of antihypertensive drugs in pregnancy, and the prevention and treatment of the convulsive phase of preeclampsia, eclampsia, with intravenous magnesium sulfate is also highlighted. Of special note, this guideline article, specifically requested, reviewed, and accepted by ASH, includes solicited review advice from the American College of Obstetricians and Gynecologists.
Hypertension, complicating 5% to 7% of all pregnancies, is a leading cause of maternal and fetal morbidity, particularly when elevated blood pressure (BP) is due to preeclampsia, either alone (pure) or “superimposed” on chronic vascular disease. 1 , 2 Preeclampsia is a major cause of preterm birth and an early marker for future cardiovascular and metabolic diseases, whereas preterm delivery is associated with immediate neonatal morbidity and has been linked to remote cardiovascular and metabolic disease in the newborns. 2 , 3 , 4 , 5 , 6 This bleak clinical picture and its large economic burden has been known for decades. Still, even in the current millennium, the hypertensive disorders of pregnancy remain among the most understudied areas and one of the lowest recipients of research funds compared with other diseases in terms of disability‐adjusted life‐years. 7 This dearth of research progress is a major factor underscoring decades of controversies that surrounded the classification, diagnosis, and management of the hypertensive disorders of pregnancy. More recently, we have witnessed an upsurge of investigative interest and achievements, mainly in regard to preeclampsia. In addition, national working groups have presented consensus documents aimed at achieving consistency in diagnosis and management of these diseases. 8 , 9 , 10 , 11 One example is the National High Blood Pressure Education Program (NHBPEP) report, last updated in 2000, 10 and coordinated with more recent practice bulletins of the American College of Obstetricians and Gynecologists (ACOG). 12
This American Society of Hypertension, Inc (ASH) position paper presents a précis of the hypertensive disorders complicating pregnancy, including whether they can be predicted and/or prevented, and guidelines for their management. It also incorporates solicited input from the ACOG.
Cardiovascular and Volume Changes in Normal Gestation
Striking alterations in both cardiovascular function and volume homeostasis occur during normal pregnancy. Knowledge of these normal adaptations is requisite to the early detection and optimal management of preexisting or new‐onset disease. 13 , 14 Large increments in cardiac output, accompanied by marked increases in intravascular and extracellular volume, occur rapidly during the first half of pregnancy, then plateau or rise more slowly thereafter. BP falls, with decrements starting in early gestation and reaching a nadir near mid‐pregnancy (Figure). The decrease in pressure is modest compared with the increases in cardiac output and intravascular volume, mainly because of a concurrent large increase in global vascular compliance. 15 Other changes include early renal vasodilatation and hyperfiltration and marked stimulation of the renin‐angiotensin‐aldosterone system (RAAS). 13 , 14 The latter is characterized by high levels of all measured elements of the RAAS chain, which react appropriately to volume‐change stimuli around new steady‐state set points. 14 There are also marked increases in free levels of other corticoids including those with both sodium retaining (eg, desoxycorticosterone) and natriuretic (eg, progesterone) potential. 14
Figure.
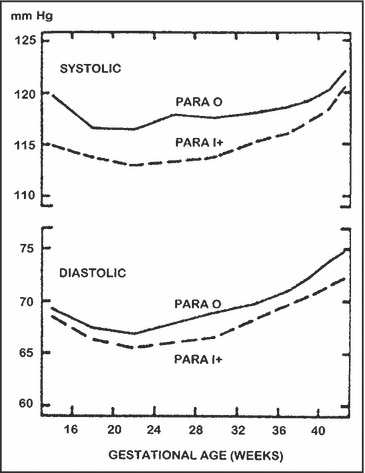
Systolic and diastolic blood pressures in relation to gestational age in 6000 white women 25 to 34 years of age who delivered single‐term infants. Reprinted with permission from Christianson. 69
Clinical relevance of these changes includes the following. Undiagnosed chronic hypertension may be masked in early pregnancy because of the initial decrease in pressure, then misdiagnosed as a gestation‐specific disorder when abnormal values appear later in pregnancy. Although hypertension in pregnancy remains defined as a BP ≥140 mm Hg systolic or 90 mm Hg diastolic, diastolic levels of 75 mm Hg in the first and 85 mm Hg in the second trimester or systolic values of 120 mm Hg in mid‐pregnancy and 130 mm Hg in late pregnancy may be abnormally elevated for some women. 16 , 17 In this respect, data from 2 studies (totaling >30,000 women) suggest that diastolic pressures >85 mm Hg or mean arterial pressures ≥90 mm Hg at any stage of gestation are associated with significant increases in fetal mortality. 16 , 18 Another caveat is that the rise in glomerular filtration rate (GFR) that normally occurs in pregnancy results in lower levels of creatinine and urea nitrogen. Failure to appreciate this (eg, failure to appreciate that creatinine levels of 0.9 or 1 mg/dL are abnormal in gestation) may lead one to miss evidence of preexisting nephrosclerosis or other renal diseases; the latter disorders are associated with higher incidences of superimposed and often severe preeclampsia. Finally, the marked stimulation of the RAAS in normal pregnancy combined with few published data to differentiate between the normally or excessively high aldosterone levels in gestation makes diagnoses of primary aldosteronism difficult. 14
Measurement of BP
Previous methodologic controversies have been resolved, with the current consensus being that BP during pregnancy is best measured with the woman sitting quietly for several minutes, the arm cuff at heart level, and diastolic pressure designated at the 5th Korotkoff sound. It is now apparent that the lower levels associated with measurements recorded when patients are positioned in lateral recumbence merely reflect differences in hydrostatic pressure when the cuff is positioned substantially above the left ventricle (reviewed elsewhere). 15 Older views suggesting that gravid women manifest large differences between the 4th Korotkoff (muffling) and 5th Korotkoff (disappearance), with the latter occasionally approaching zero because of their hyperdynamic circulations, have been disproved, and 5th Korotkoff has been established as the sound closest to true diastolic pressure. 14 , 19
Hypertension is defined as levels that are ≥140 mm Hg systolic or ≥90 mm Hg diastolic (preferably confirmed by 2 readings 4 to 6 hours apart). 11 , 12 Previously, an increase of 15 mm Hg diastolic and 30 mm Hg systolic, respectively, even if the final value ≥140/90 mm Hg was also included in the definition. However, data demonstrating that outcomes are similar irrespective of the magnitude of rise when values remain <140/90 mm Hg, have led consensus groups to delete this latter definition. Nevertheless, the NHBPEP consensus report 11 stressed that patients with BPs below the 140/90 mm Hg cutoff who have experienced a 30‐ or 15‐mm Hg rise in systolic and diastolic levels, respectively, be managed as high‐risk patients. Of interest, these differences in defining hypertension are one reason for discordant findings in areas such as epidemiology and outcome research, which are now hopefully resolved.
Classifying Hypertension in Pregnancy
Caregivers have been and continue to be confused by the multiple terminologies, some complex and detailed, used to classify the hypertensive disorders of pregnancy. For example, the terms toxemia, gestosis, pregnancy‐induced hypertension, and preeclamptic toxemia have each been used to classify the disorder we will label preeclampsia. The same term might have different meanings depending on the schema in which it was published. For example, pregnancy‐induced hypertension could signify both gestational hypertension and preeclampsia to some, whereas others require pregnancy‐induced hypertension plus proteinuria to signify preeclampsia. The terminology used here is that recommended by the NHBPEP working group 11 and is concise and practical. In it, BP in pregnancy is considered in only 4 categories:
-
•
Preeclampsia‐eclampsia
-
•
Chronic hypertension of any cause
-
•
Preeclampsia superimposed on chronic hypertension
-
•
Gestational hypertension
Preeclampsia, pure or superimposed (categories 1 and 3), is the disorder most often associated with severe maternal‐fetal‐neonatal complications (including fatalities). Most women in category 2 have essential hypertension, mostly mild (≤105 mm Hg) in intensity, and their pregnancies usually (but not invariably) uncomplicated. On occasion, the high BP is secondary, from known causes including endocrine tumors, renal artery stenosis, and renal disease, and some of these pregnancies do poorly. Pheochromocytoma, although rare, may present for the first time during pregnancy and is especially fatal when unsuspected, but if diagnosed it can be managed to a successful outcome, either surgically or pharmacologically, depending on the stage of gestation. 20 , 21 Cushing syndrome, also rare, has been associated with exacerbations of hypertension during pregnancy and poor fetal outcomes 20 , 22 and anecdotal reports of serious and fatal complications in pregnant women with scleroderma and periarteritis nodosa, particularly when these latter disorders involve the kidneys. 14 On the other hand, pregnancy may diminish the kaliuresis and BP rise associated with primary aldosteronism, perhaps related to the increase in circulating progesterone levels, hypertension, and hypokalemia represented postpartum when progesterone levels decline. 20 , 23 Finally, angioplasty and stent placement have been successfully performed on pregnant women with renal artery stenosis. 20
Gestational hypertension is characterized by mild to moderate elevation of BP after mid‐gestation but without abnormal proteinuria, usually near term (although more severe forms of hypertension have been described, and some of these patients are actually preeclamptics who shortly thereafter manifest other signs and symptoms of that disorder). Although the cause of gestational hypertension is unclear, this entity appears to identify women destined to develop essential hypertension later in life (analogous to the relationship of gestational diabetes to the subsequent development later in life of type 2 diabetes mellitus). 24 , 25 BP returns to normal during the immediate puerperium (at which point some relabel the entity “transient hypertension”). Many of these women are hypertensive in one, some, or all of their subsequent pregnancies.
There is an entity termed late postpartum hypertension that describes women with normotensive gestations who develop high BP (usually mild) several weeks to 6 months after delivery that normalizes by the end of the first postpartum year. 14 Little is known about this entity, although it may also predict essential hypertension later in life. Finally, a very rare group of patients harbor activating mineralocorticoid receptor mutations that result in an exaggerated sensitivity to the usually weak effect of progesterone. 26 These women manifest early salt‐sensitive hypertension, coincident with the rapid rise in progesterone production during the initial trimester.
The Clinical Spectrum of High BP in Pregnancy
Most women with chronic hypertension have uneventful gestations as long as their BP remains at (or is controlled to) levels considered “mild to moderate.” In contrast, preeclampsia is associated with many serious complications. Thus, early and accurate recognition and differentiation of preeclampsia from other causes of high BP in pregnancy has important implications regarding management. A precise diagnosis, however, is not always possible, in which case it is best to manage the woman as if she has preeclampsia, which is the more serious disorder with a broad clinical spectrum.
Preeclampsia, a protean disorder that involves many organ systems, is primarily characterized by hypertension and proteinuria. The latter is defined by excretion of ≥300 mg/24 h, a urine protein/creatinine ratio of ≥0.3, or a qualitative 1+ dipstick reading. The dipstick value of 1+ has many false‐positive and false‐negative results and is the least useful. 11 , 19 Accurate, timed urine collections are very difficult to obtain during pregnancy, and, theoretically, a urine creatinine/protein ratio eliminates such errors. However, the accuracy of this test is still being investigated.
Preeclampsia may also be accompanied by rapid weight gain and edema, appearance of coagulation or liver function abnormalities, and occurs most often in nulliparas, usually after gestational week 20, and most frequently near term. Attempts have been made to categorize preeclampsia as “mild” or “severe” (Table I). 11 , 27 The latter is often defined on the basis of BP levels (≥110 mm Hg diastolic and 160 mm Hg systolic), the appearance of nephrotic range proteinuria, sudden oliguria, neurologic symptoms (eg, headache, hyperreflexia), and laboratory test results demonstrating thrombocytopenia (defined as <100,000/μL), hemolysis, or abnormal liver function (including presence of schistocytes, hyperbilirubinemia, or elevated aspartate aminotransferase and lactic acid dehydrogenase levels), although the magnitude of proteinuria alone as a predictor of severity has been questioned. 27 , 28 Because a woman with seemingly mild disease (eg, a teenage gravida with a BP of 140/90 mm Hg and minimal proteinuria) can suddenly convulse, designations such as mild and severe can be misleading. In fact, de novo hypertension alone occurring after mid‐gestation in a nullipara is sufficient reason to manage the patient as if she were preeclamptic.
Table I.
Preeclampsia: Judging Severitya
| Less Severe | More Severe | |
|---|---|---|
| Presentation | ≥Gestational week 34 | ≥Gestational week 35 |
| Diastolic BP | <100 mm Hg | >110 mm Hg |
| Headache | Absent | Present |
| Visual disturbances | Absent | Present |
| Abdominal pain | Absent | Present |
| Oliguria | Absent | Present |
| Creatinine (GFR) | Normal | Elevated (decreasing) |
| LDH and AST proteinuria | Normal mild to moderate | Elevated nephrotic range (>3 g/24 h)b |
| Nonreassuring fetal testingc | Absent | Present |
The American College of Obstetrics and Gynecology bulletins utilize the terms mild and severe for our preferred less and more severe, so as to underscore diligence for any form of preeclampsia. Abbreviations: AST, aspartate aminotransferase; BP, blood pressure; GFR, glomerular filtration rate; LDH, lactic acid dehydrogenase. aPresence of convulsions (eclampsia), congestive heart failure, or pulmonary edema are always very ominous signs. bDegree of proteinuria alone may not indicate seriousness unless accompanied by other ominous sign or symptom. cGrowth restriction and adverse signs during periodic fetal testing including electronic monitoring and Doppler ultrasound.
Early preeclampsia (onset <34 weeks’ gestation) is associated with greater morbidity than when the disorder presents at term. In this respect, some suggest subdividing preeclampsia into 2 groups by time of onset because of differences in prognosis and management. 29 Such a distinction may be misleading, however, because all preeclampsia is potentially explosive.
The eclamptic convulsion, a dramatic and life‐threatening complication of preeclampsia, was once associated with a maternal mortality of 30%. 13 , 14 More recently, and primarily in developed nations, improved and aggressive obstetric management has decreased the occurrence of convulsions and made maternal deaths unusual. 1 , 13 , 14 , 30 Eclampsia is often preceded by premonitory signs including headache, visual disturbances, epigastric pain, constricting sensations in the thorax, apprehension, excitability, and hyperreflexia. However, convulsions can occur suddenly and without warning in a seemingly stable patient with no apparent or only minimal elevations of BP. 31 In fact, the capricious nature of this disorder makes early hospitalization of women with suspected preeclampsia advisable. Most eclamptic convulsions occur prepartum, intrapartum, or within 48 hours postpartum, but there is an unusual entity labeled “late postpartum eclampsia” that occurs from 48 hours to several weeks after delivery. 32
One complication, affecting approximately 5% of women with preeclampsia that can progress rapidly to a life‐threatening condition, is the HELLP syndrome, which is characterized by all or some of the following signs: hemolysis, abnormal elevation of liver enzyme levels (aspartate aminotransferase and lactic dehydrogenase may increase quickly, the latter to >1000 IU/dL), and low platelet counts (also evolving rapidly and decreasing to <40,000/mL), with schistocytes present on the blood smear. 13 , 14 , 33 The HELLP syndrome may at first appear deceptively benign, with initial enzyme elevations and thrombocytopenia of borderline severity. Such presentations require inpatient management, often termination of the pregnancy if the disease progresses, and, although postpartum recovery is usually rapid, the disease may persist for almost a week.
Pathogenic Mechanism in Preeclampsia
Preeclampsia has been dubbed the disease of theories, but recent progress concerning pathogenesis of its clinical phenotypes suggests breakthroughs that may lead to accurate prediction, prevention, and better treatments. Discussion of all etiologic theories (ie, altered cell and molecular biology of the placenta, antioxidants, the systemic inflammatory response, humeral and immune factors, and cardiovascular maladaptations to gestation) is beyond the scope of this article and reviewed in detail by others. 8 , 14 , 34 The most plausible theories focus on the placenta and describe the disorder in 2 stages. In the first, the initiating cause results in the placenta‐producing factors (eg, specific proteins, trophoblastic debris) that enter the maternal circulation. The second stage, called maternal, is overt disease that depends not only on the action of these circulating factors, but also the health of the mother, including diseases that may affect the vasculature (preexisting cardiorenal, metabolic, and genetic factors and obesity). A promising research area in 2008 involved elucidation of the role of antiangiogenic factors produced by the placenta in the pathogenesis of preeclampsia phenotypes. 8 , 14 , 34 , 35
Placentas of women destined to develop preeclampsia overproduce at least 2 antiangiogenic proteins that reach abnormally high levels in the maternal circulation. One soluble Fms‐like tyrosine kinase 1 (sFlt‐1) is a receptor for placental growth (PiGF) and vascular endothelial growth factors (VEGF). Increased maternal sFlt‐1 levels decrease circulating free PiGF and VEGF concentrations leading to endothelial dysfunction. The second antiangiogenic protein, soluble endoglin (sEng) may impair the binding of transforming growth factor‐1 to endothelial receptors, thereby decreasing endothelial nitric oxide–dependent vasodilatation. Simultaneous introduction of adenoviruses encoding both sFlt‐1 and sEng into pregnant rats produces severe hypertension, heavy proteinuria, elevated liver enzyme levels, and circulating schistocytes—in essence creating a powerful rodent model that simulates most of the protean manifestations of preeclampsia in humans and has obvious implications for the study of mechanisms and subsequent therapy of this disease. 36 , 37
The cause of placental overproduction of these proteins, however, remains an enigma. Research is currently focusing on immunologic mechanisms (eg, HLAG, natural killer cells, autoantibodies agonistic to the angiotensin I receptor), oxidative stress, mitochondrial pathology, and hypoxia genes. 8 , 14 , 34 In essence, research in this area, dormant for decades, is now quite promising.
The Multisystemic Pathophysiology and Pathology of Preeclampsia
BP and the Cardiovascular System
Hypertension in preeclampsia is due primarily to marked vasoconstriction, because both cardiac output and arterial compliance are reduced. 14 , 15 , 19 There is a reversal of the normal circadian rhythm, with the highest BP now at night, and a loss of the normal pregnancy‐associated refractoriness to pressor agents; the sensitivity to infused Ang II increasing weeks before overt disease. 14 Explanations for the increased reactivity to Ang II include up‐regulation of receptor sensitivity, synergy with circulating autoantibodies agonistic to the angiotensin type 1 receptor, 14 , 34 , 38 , 39 and decreases in the level of circulating Ang 1–7. Increases in insulin resistance and sympathetic nervous system tone also occur and have been implicated in the vasoconstriction characteristic of preeclampsia. 14
Kidney
As noted, renal hemodynamics increase markedly in normal gestation. Renal plasma flow (RPF) and GFR decrease in preeclampsia (∼25%); thus, values may still be above or at those measured in the nonpregnant state. 14 The decrement in RPF is attributable to vasoconstriction, whereas the fall in GFR relates both to the decrement of RPF and the development of a glomerular lesion termed glomerular endotheliosis (detailed elsewhere). 14 , 24 , 34 , 40
Placenta
Shallow and abnormal placentation is a hallmark of preeclampsia, highlighted by a failure of the normal trophoblastic invasion of the spiral arteries, these vessels failing to remodel and dilate. 41 This aberration underlies theories that restriction of placental blood flow leads to a relatively hypoxic uteroplacental environment, with subsequent events mediated through hypoxemia‐induced genes resulting in the release of factors (eg, antiangiogenic proteins) that enter the mother’s circulation and initiate the maternal syndrome.
Brain
The best descriptions of the gross and microscopic brain pathology in eclampsia can be found in the extensive autopsy series of Sheehan and Lynch, 42 because most of these necropsies were performed within 2 hours of death, thereby eliminating the rapid autolytic postmortem changes that might confound interpretation. They noted little evidence of brain edema and postulated that brain swelling was a late rather than a causal event. The major findings, however, were both gross and microscopic evidence of bleeding.
Previous controversy regarding the pathogenesis of eclampsia centered on whether it was a unique entity, due mainly to severe vasoconstriction (occasionally localized in the cerebral circulation) or more akin to hypertensive encephalopathy appears to have been resolved. Studies using sophisticated imaging techniques reveal increased cerebral blood flow in preeclamptic women, whereas data derived from animal models suggest that eclamptic women have increased perfusion pressures, perhaps exceeding the cerebral circulation’s autoregulatory capacity and that their vessels “leak” at perfusion pressures lower than what would be expected in nonpregnant women. 13 , 14 , 43 , 44 Reports based on computed axial tomography and magnetic resonance imaging describe transient abnormalities consistent with localized hemorrhage or edema, 45 with the latter described as vasogenic and fully reversible, but occasionally “cytotoxic” accompanied by infarction with lesions that persist.
Liver and Coagulation Abnormalities
Preeclampsia is associated with activation of the coagulation system, with thrombocytopenia (usually mild) as the most commonly detected abnormality. There is increased platelet activation and size, plus decrements in their lifespan. The hypercoagulability of normal pregnancy is accentuated (eg, reduced antithrombin III, protein S, and protein C) even when platelet counts appear normal. 14 , 46 However, occasionally, the coagulopathy can be severe, as detailed in the ominous HELLP syndrome discussed previously.
Preeclampsia also affects the liver. 13 , 14 Manifestations include elevated aspartate aminotransferase and lactic dehydrogenase levels, with the increments usually small, except when the HELLP syndrome supervenes. The gross hepatic changes in preeclampsia, also detailed in the autopsy series of Sheehan and Lynch, 42 are petechiae ranging from occasional to confluent areas of infarction, as well as subcapsular hematomas, some having ruptured and caused death. Hematomas were, however, unusual in a later study whose investigators assessed the liver laparoscopically. 47 The characteristic microscopic lesion is periportal, manifesting as hemorrhage into the hepatic cellular columns and at times concurrent infarction. Material obtained by laparoscopic‐guided biopsies show substantial intracellular fatty changes in all patients with preeclampsia, regardless of the severity of the disease. 46 However, autopsy and laparoscopy studies are by their nature quite selective.
Prediction and Prevention of Preeclampsia
Prediction
Numerous studies have evaluated tests to predict preeclampsia or to distinguish it from more benign hypertensive complications. They include evaluation of circulating or urinary markers and imaging techniques. In one large systematic literature review, the authors concluded that none of the screening methods tested through 2004 were clinically useful predictors of preeclampsia, and that analyzing combinations of tests might prove more valuable. 48 That review did not include a more recent literature assessing circulating or urinary antigenic and antiangiogenic proteins. The more recent studies have generated hope that combinations of sFlt‐1, sEng, and PlGF will provide the sensitivities and likelihood ratios required for prediction of preeclampsia and may prove useful in its differential diagnosis as well. 49 Several of these studies demonstrated prediction with very high sensitivities, especially combinations of serum sFlt‐1, sEng, and PiGF, but the vast majority of these data come from retrospective analyses of banked specimens from earlier trials. By early 2008, there were several ongoing prospective observational studies in progress.
Prevention
Numerous interventions have been proposed to prevent preeclampsia, usually predicated on theories that administration of a drug, mineral, or vitamin will inhibit or reverse a presumed causal mechanism. Systematic reviews through early 2008, however, identified only 2 interventions that have some minimal protective effects. 1 , 50 , 51 Low‐dose aspirin may reduce the incidence of preeclampsia by approximately 10%, but the numbers needed to treat to avoid adverse outcomes are large. 52 Calcium supplementation has a small effect in populations with low dietary calcium intake (<600 mg/d). 51 In these latter populations, the incidence of the disorder does not decrease, but there are small but significant decrements in serious adverse advents, including fetal demise. Supplementation with the antioxidant vitamins C and E has had no effect to date, and has even proved harmful in certain high‐risk populations, although the largest of these trials (by the National Institute of Child Health and Development [NICHD] Maternal Fetal Medicine Trials Network) was completed in late 2008 and is scheduled to be reported in early 2009. 53 , 54
Management
There are several unresolved controversies regarding treatment of the hypertensive disorders of pregnancy, and the hypertensive expert called to consult should be aware of them. If disagreements occur, it is prudent to note that it is the obstetrician who has been managing the pregnancy for months, who is responsible for both the mother’s and fetus’ outcomes and who may be required to defend bad outcomes to official committees and boards.
Preeclampsia‐Eclampsia
Suspicion of preeclampsia is sufficient reason to recommend hospitalization, given the disease’s potential to accelerate rapidly. 11 , 13 , 14 , 55 This approach will minimize diagnostic error, diminish the incidence of convulsions, and improve fetal outcome. Because delivery remains the only known “cure,” and maternal and fetal disease status may change rapidly, we recommend the following. Near term, induction of labor is the therapy of choice, whereas attempts to temporize should be made if pregnancy is at an earlier stage. If the latter decision is made, and BP rises to unacceptable levels, several antihypertensive agents considered safe in pregnancy are available and are discussed in the following sections (Table II). Delivery is indicated at any stage of pregnancy if severe hypertension remains uncontrolled for 24 to 48 hours or at the appearance of certain “ominous” signs such as clotting or liver abnormalities, decreasing renal function, signs of impending convulsions (headache, epigastric pain, and hyperreflexia), or the presence of severe growth retardation or nonreassuring fetal testing (Table I). Preeclampsia remote from term is a special situation in which the patients should be hospitalized and closely monitored in tertiary obstetric care centers (preferably those with prenatal close observation units), facilities not readily available to many practitioners. 56 Gestation is permitted to continue as long as BP is controlled, no ominous signs of life‐threatening maternal complications occur, and in the absence of signs of nonreassuring fetal testing.
Table II.
Drugs for Chronic Hypertension in Pregnancy
| Drug (Food and Drug Administration Risk)a | Dose | Concerns or Comments |
|---|---|---|
| Methyldopa (B) | 0.5–3.0 g/d in 2 divided doses | Drug of choice according to NHBEP working group; safety after first trimester well documented, including 7‐year follow‐up evaluation of offspring |
| Labetalol (C)b | 200–1200 mg/d in 2 or 3 divided doses | Gaining in popularity as concerns relating to growth restriction and neonatal bradycardia do not seem to have materialized |
| Nifedipine (C) | 30–120 mg/d of a slow‐release preparation | May inhibit labor and have synergistic interaction with magnesium sulfate; small experience with other calcium‐entry blockers |
| Hydralazine (C) | 50–300 mg/d in 2–4 divided doses | Few controlled trials, long experience with few adverse events documented, useful only in combination with sympatholytic agent; may cause neonatal thrombocytopenia |
| β‐Receptor blockers (C) | Depends on specific agent 25 mg/d | May cause fetal bradycardia and decrease uteroplacental blood flow, this effect may be less for agents with partial agonist activity; may impair fetal response to hypoxic stress; risk for growth retardation when started in first or second trimester (atenolol) |
| Hydrochlorothiazide (C) | Majority of controlled studies in normotensive pregnant women rather than hypertensive patients, can cause volume depletion and electrolyte disorders; may be useful in combination with methyldopa and vasodilator to mitigate compensatory fluid retention | |
| Contraindicated ACE inhibitors and angiotensin II type 1 receptor antagonists (D)c | Use associated with major anomalies plus fetopathy, oligohydramnios, growth restriction, and neonatal anuric renal failure, which may be fatal |
Note: No antihypertensive drug has been proven safe for use during the first trimester of pregnancy. Drug therapy is indicated for uncomplicated chronic hypertension when diastolic blood pressure is −100 mm Hg (Korotkoff V). Treatment at lower levels may be indicated for patients with diabetes mellitus, renal disease, or target organ damage. Abbreviations: ACE, angiotensin‐converting enzyme; NHBEP, National High Blood Pressure Education Program. aUS Food and Drug Administration classification. bWe omitted some agents (eg, clonidine, α‐blockers) because of limited data on use for chronic hypertension in pregnancy. cWe would classify in category X during second and third trimesters. Reprinted with permission from Lindheimer et al. 14
Sudden Escalating Hypertension and Imminent or Frank Eclampsia
Controversies remain as to whether and at what level to treat rapidly rising BP near term or during delivery (a phenomenon often indicating the appearance of pure or superimposed preeclampsia). There is further debate on how aggressively to lower the BP. The NHBPEP recommendations 11 state that diastolic levels >105 mm Hg require treatment (although some contemporary texts still recommend >110 mm Hg), with some reservations. Circumstances, such as a teenager whose recent diastolic levels were ≤70 mm Hg, or patients demonstrating signs of cardiac decompensation or cerebral symptoms such as excruciating headache, confusion, or somnolence, warrant treatment at lower levels. 11 , 13 , 14
Management of eclamptic convulsions requires parenteral magnesium sulfate administration, which is shown to be superior to either diazepam or phenytoin for both prevention and treatment. 13 , 14 , 52 , 57 However, there is no unanimity as to when and who to treat prophylactically. Intravenous magnesium is not without hazard, and some contend its risks outweigh those associated with “mild” preeclampsia and that it should be reserved for women with severe disease. 58 Trials to settle these questions are still needed.
Chronic Hypertension
Most pregnant women with chronic hypertension have the “essential” variety, with their disease mild in nature and of recent origin. The majority of these gestations are uncomplicated, although outcomes are worse than in women with normotensive pregnancies. 13 , 14 , 20 Chronic hypertension is associated with increased incidences of placental abruption, acute renal failure, cardiac decompensation, and cerebral accidents in the mother and of growth retardation and unexplained mid‐trimester fetal death. Such events are mainly associated with superimposed preeclampsia, whose incidence in chronic hypertensives is ≥20%. 59 Risk for complications correlates with the age of the mother, the duration and degree of control of her high BP, and the presence of end‐organ damage. Extremely obese women with chronic hypertension are at special risk for cardiac decompensation near term, and especially if volume loaded during labor. Echocardiography performed earlier in pregnancy may alert the physician to patients at risk with early evidence of ventricular dysfunction.
The approach to treatment of women with chronic hypertension is also controversial. Although all physicians would treat women with severe hypertension, opinions vary as to whether to treat mild hypertension. In this respect, systemic reviews of randomized studies to date suggest that treatment of mild to moderate hypertension does not prevent superimposed preeclampsia or decrease adverse outcomes and may even result in smaller fetuses. 60 Treatment does appear to decrease hospitalization of the mother, especially related to loss of BP control. However, it also appears that many of the trials reviewed were incomplete and flawed; therefore, comparing them is difficult because of obvious heterogeneity. Better‐designed, more definitive trials are needed to resolve this issue.
Given these limitations, the NHBPEP and ACOG guidelines 11 , 12 accept withholding antihypertensive drugs unless diastolic levels are >100 mm Hg (but support treatment at lower levels if there is evidence of end‐organ damage or specific risk factors such as underlying renal disease). In what may reflect the vagaries of consensus, they noted “end points” for reinstating treatment include exceeding threshold BPs of 150 to 160 mm Hg systolic and 100 to 110 mm Hg diastolic. However, subsequent retrospective analyses suggest that cerebral vascular accidents in women, especially with superimposed preeclampsia, may occur when systolic levels exceed 150 (and definitely 160) mm Hg and endorse the more firm suggestion that systolic levels be treated when they exceed 160 mm Hg. 31 , 61
Antihypertensive Therapy
The reader is referred further to several reviews that include systematic analysis of trials and detailed discussions of when and how to treat hypertension during pregnancy. 13 , 14 , 49 , 62 , 63 To summarize, clinicians considering the prescription of antihypertensive drugs to pregnant women should be aware of several points. There have been only a few large, randomized multicenter trials. Most studies have been limited in scope, and many therapies were started after mid‐gestation, when virtually all the risks of provoking congenital malformations have passed. Further, there are no rigorous animal testing requirements to be met before human trials are undertaken, including standardized means of evaluating the drug effect on the fetus’ ability to withstand hypoxic stress or more complex analyses of morphologic and physiologic variables in newborn animal models. This state of affairs should be kept in mind when reviewing the literature on antihypertensive therapy in pregnancy. II, III summarize the status of antihypertensive drugs during gestation, including their pregnancy risk categories (A to D, through X) as defined by the US Food and Drug Administration.
Table III.
Drugs for Urgent Control of Severe Hypertension in Pregnancy
| Drug (Food and Drug Administration Risk)a | Dose and Rate | Concerns or Commentsb |
|---|---|---|
| Labetalol (C) | 20 mg IV, then 20–80 mg every 20–30 min, up to a maximum of 300 mg; or constant infusion of 1–2 mg/min | Experience in pregnancy less than with hydralazine; probably less risk for tachycardia and arrhythmia than with other vasodilators |
| Hydralazine (C) | 5 mg IV or IM, then 5–10 mg every 20–40 min; or constant infusion of 0.5–10 mg/h | Drug of choice according to NHBEP working group; long experience of safety and efficacy |
| Nifedipine (C) | Tablets recommended only; 10–30 mg orally, repeat in 45 minutes if needed | Possible interference with labor |
| Relatively contraindicated nitroprusside (C)c | Constant infusion of 0.5–10 g/kg/min | Possible cyanide toxicity; agent of last resort |
Note: Indicated for acute increase of diastolic blood pressure ≥105 mm Hg; goal is a gradual reduction to 90/100 mm Hg. C indicates that either studies in animals have revealed adverse effects on the fetus (teratogenic, embryocidal, or other), that there are no controlled studies in women, or studies in women and animals are not available. Drugs should be given only if the potential benefits justify the potential risk to the fetus. Abbreviations: IM, intramuscularly; IV, intravenously; NHBEP, National High Blood Pressure Education Program. aUS Food and Drug Administration classification. bAdverse effects for all agents, except as noted, may include headache, flushing, nausea, and tachycardia (primarily caused by precipitous hypotension and reflex sympathetic activation). cWe would classify as category D; there is positive evidence of human fetal risk, but the benefits of use in pregnant women may be acceptable despite the risk (eg, if the drug is needed in a life‐threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective). Reprinted with permission from Lindheimer et al. 14
Briefly, the NHBPEP report 11 designated the central adrenergic inhibitor methyldopa as the “preferred” drug of choice based on 20+ years of postmarketing surveillance, several controlled trials, and the longest follow‐up (7.5 years) in neonates. Adrenergic‐blocking agents are associated with an increased incidence of fetal growth restriction although the effects are minimal, and many clinicians use the combined β‐ and adrenergic‐blocker labetalol. 14 , 62 Theoretically, there may be synergism between magnesium sulfate and calcium channel–blocking agents leading to precipitous decreases in BP and even respiratory arrest, but this has not been borne by systematic review. 64 Other comments concerning these agents can be found in II, III. Both angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers should not be prescribed to pregnant women. Until recently their class D, “black box” warning focused primarily on their association with fetopathy, including renal failure and death in the neonates. Because the fetal problems occurred related to events in the last 2 trimesters, some suggested the drug could be used through conception or the initial trimester in situations such as in chronic hypertensives where discontinuing the ACE inhibitor or receptor blocker might result in critical difficulties in reestablishing control with perhaps early pregnancy loss (eg, a hypertensive class C diabetic receiving the drug at conception). However, it is now more apparent that these drugs are also associated with serious fetal anomalies 65 and should not be used early in gestation either.
Information on use of antihypertensive drugs during lactation remains limited. Drugs with high protein binding are preferred (eg, labetalol or propranolol over atenolol and metoprolol). 11 , 62 ACE inhibitors are important for treating proteinuric and diabetic patients and can be quickly restarted. Diuretics may decrease breast milk production and should be withheld.
Other Management Considerations
Obstetrics management, including the current status of tests to monitor the fetus (eg, electronic fetal heart, monitoring, Doppler assessment of the uteroplacental circulation) is beyond the scope of this article and is discussed in the obstetric literature, including periodic bulletins issued by ACOG.
Remote Prognosis
Results of several large epidemiologic studies demonstrate that women whose pregnancies were complicated by preeclampsia have more remote cardiovascular and metabolic diseases later in life than women who were normotensive during all of their pregnancies. 6 , 13 , 14 , 66 , 67 It also appears that those women most likely to develop cardiovascular or metabolic diseases have had early preeclampsia (<34 weeks). 68 On the other hand, the few studies comparing the remote prognosis of previous preeclamptics with age‐ and sex‐matched populations in the general population find minimal or no such increases. 14 The best interpretation of these findings is that preeclampsia is a risk marker of patients predestined to have future cardiovascular or metabolic disease. Such women, therefore, should have more frequent health checkups and should be advised that lifestyle and dietary changes may minimize such problems in the future.
References
- 1. Ness RB, Roberts JM. Epidemiology of Hypertension. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Stamford, CT: Appleton & Lange; 1999:43–65 (3rd edition revision in press, May 2009, Elsevier). [Google Scholar]
- 2. Villar J, Say L, Gulmezoglu AM, et al. Pre‐eclampsia Eclampsia: a Health Problem for 2000 years. In: Critchly H, MacLean A, Poston L, Walker J, eds. Pre‐eclampsia. London, England: RCOG Press; 2003:189–207. [Google Scholar]
- 3. Zandi‐Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–508. [DOI] [PubMed] [Google Scholar]
- 4. Sibai BM. Preeclampsia as a cause of preterm and late preterm (near‐term) births. Semin Perinatol. 2006;13:16–19. [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Villar J, Sun W, et al. Blood pressure dynamics during pregnancy and spontaneous preterm labor. Am J Obstet Gynecol. 2007;197:162e1–162e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harskamp RE, Zeeman GG. Preeclampsia: at risk for remote cardiovascular disease. Am J Med Sci. 2007;334: 291–295. [DOI] [PubMed] [Google Scholar]
- 7. Gross CP, Anderson CF, Rowe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340:1881–1887. [DOI] [PubMed] [Google Scholar]
- 8. Davison JM, Lindheimer MD. New developments in preeclampsia. Semin Nephrol. 2004;24:537–625. [DOI] [PubMed] [Google Scholar]
- 9. Moutquin JM, Garner PR, Burrows RF, et al. Report of the Canadian Hypertension Society Consensus Conference 2. Non‐pharmacologic management and prevention of hypertensive disorders of pregnancy. CMAJ. 1997;157: 907–919. [PMC free article] [PubMed] [Google Scholar]
- 10. Brown MA, Hague WM, Higgins J, et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust N Z J Obstet Gynaecol. 2000;40:133–138. [DOI] [PubMed] [Google Scholar]
- 11. High Blood Pressure Education Program Working Group . Report of the National High Blood Pressure Education Program Working Group on high blood in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 12. ACOG . ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. [DOI] [PubMed] [Google Scholar]
- 13. Cunningham FG, Leveno KL, Bloom SL, et al. Williams Obstetrics, 22nd ed. New York, NY: McGraw‐Hill Co; 2005. [Google Scholar]
- 14. Lindheimer MD, Conrad KP, Karumanchi SA. Renal Physiology and Disease in Pregnancy. In: Alpern RJ, Hebert SC, eds. Seldin and Giebisch’s The Kidney; Physiology and Pathophysiology, 4th ed. San Diego, CA: Academic Press, Elsevier; 2008:2339–2398. [Google Scholar]
- 15. Hibbard JU, Shroff SG, Lang RM. Cardiovascular changes in preeclampsia. Semin Nephrol. 2004;24:580–587. [DOI] [PubMed] [Google Scholar]
- 16. Friedman EA, Neff RK. Pregnancy, Hypertension: a Systematic Evaluation of Clinical Diagnostic Criteria. Littleton, MA: PSG Publishing; 1977. [Google Scholar]
- 17. Sibai BM, Caritis SN, Thon E, et al. Prevention of preeclampsia with low‐dose aspirin in healthy nulliparous women. The National Institute of Child Health and Human Development network of maternal‐fetal medicine Units. N Engl J Med. 1993;39:1213–1218. [DOI] [PubMed] [Google Scholar]
- 18. Page EW, Christianson RE. The mean impact of mean arterial pressure in the middle trimester on the outcome of pregnancy. Am J Obstet Gynecol. 1976;125:740–746. [DOI] [PubMed] [Google Scholar]
- 19. Shennan AH, Waugh J. The Measurement of Blood Pressure and Proteinuria in Pregnancy. In: Critchly H, MacLean A, Poston L, Walker J, eds. Pre‐eclampsia. London, England: RCOG Press; 2003:305–324. [Google Scholar]
- 20. August P, Lindheimer M. Chronic Hypertension and Pregnancy. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy, 2nd ed. Stamford, CT: Appleton & Lange; 1999:605–633. [Google Scholar]
- 21. Dugas G, Fuller J, Singh S, et al. Pheochromocytoma and pregnancy: a case report and review of anesthetic management. Can J Anaesth. 2004;51:134–138. [DOI] [PubMed] [Google Scholar]
- 22. Blanco C, Maqueda E, Rubiio JA, et al. Cushing’s syndrome during pregnancy secondary to adrenal adenoma: metyrapone treatment and laparoscopic adrenalectomy. J Endocrinol Invest. 2006;29:164–167. [DOI] [PubMed] [Google Scholar]
- 23. Lindheimer M, Richardson DA, Ehrlich EN, et al. Potassium homeostasis in pregnancy. J Reprod Med. 1987;32:517–522. [PubMed] [Google Scholar]
- 24. Fisher KA, Luger A, Spargo BH, et al. Hypertension in pregnancy: clinical pathological correlations and remote prognosia. Medicine (Baltimore). 1981;60:267–276. [PubMed] [Google Scholar]
- 25. Villar J, Carroli G, Wojdyla D, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921. [DOI] [PubMed] [Google Scholar]
- 26. Geller DS, Fahri A, Pinkerton N, et al. Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science. 2000;289:119–123. [DOI] [PubMed] [Google Scholar]
- 27. Menzies J, Magee LA, MacNab YC, et al. Current CHS and NHBPEP criteria for severe preeclampsia do not uniformly predict adverse maternal or perinatal outcomes. Hypertens Pregnancy. 2007;26:447–462. [DOI] [PubMed] [Google Scholar]
- 28. Schiff E, Friedman SA, Kao L, et al. The importance of urinary protein excretion during conservative management of severe preeclampsia. Am J Obstet Gynecol. 1996;175:1313–1316. [DOI] [PubMed] [Google Scholar]
- 29. Von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–148. [DOI] [PubMed] [Google Scholar]
- 30. Mattar F, Sibai BM. Eclampsia. VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182: 307–312. [DOI] [PubMed] [Google Scholar]
- 31. Zeeman GG, Vollaard ES, Alexander JM, et al. “Delta preeclampsia”– a hypertensive encephalopathy in “normotensive” women. Am J Obstet Gynecol. 2007;197: S140. [Google Scholar]
- 32. Hirshfeld‐Cytrin J, Lam C, Karumanchi SA, et al. Late postpartum eclampsia: examples and review. Obstet Gynecol Survey. 2006;61:471–480. [DOI] [PubMed] [Google Scholar]
- 33. Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981–991. [DOI] [PubMed] [Google Scholar]
- 34. Hladunewich M, Karumanch SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2:543–549. [DOI] [PubMed] [Google Scholar]
- 35. Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Ann Rev Med. 2007;59:61–78. [DOI] [PubMed] [Google Scholar]
- 36. Lindheimer MD, Umans JG. Explaining and predicting preeclampsia (editorial). N Engl J Med. 2006;355:1056–1058. [DOI] [PubMed] [Google Scholar]
- 37. Li Z, Zhang Y, Ying Ma J, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. [DOI] [PubMed] [Google Scholar]
- 38. Dechend R, Homuth V, Wallukat G, et al. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Invest. 2006;13:79–86. [DOI] [PubMed] [Google Scholar]
- 39. Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor‐activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Conrad KP, Lindheimer MD. Renal and Cardiovascular Alterations. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy, 2nd ed. Stamford, CT: Appleton & Lange, 1999:263–326 (3rd edition revision in press, May 2009, Elsevier). [Google Scholar]
- 41. McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol. 2004;24:540–547. [DOI] [PubMed] [Google Scholar]
- 42. Sheehan HL, Lynch JP. The Pathology of Toxemia. Baltimore, MD: Wilkins and Wilkins; 1973. [Google Scholar]
- 43. Zeeman GG, Hatab MR, Twickler DM. Increased cerebral blood flow in preeclampsia with magnetic resonance imaging. Am J Obstet Gynecol. 2004;191:1425–1429. [DOI] [PubMed] [Google Scholar]
- 44. Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50:14–24. [DOI] [PubMed] [Google Scholar]
- 45. Zeeman GG, Fleckenstein GL, Twinckler DM, et al. Cerebral infarction in preeclampsia. Am J Obstet Gynecol. 2004;190:714–720. [DOI] [PubMed] [Google Scholar]
- 46. Baker PN, Cunningham FG. Platelet and Coagulation Abnormalities. In:Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy, 2nd ed. Stamford, CT: Appleton & Lange, 1999:349–375 (3rd edition revision in press, May 2009, Elsevier). [Google Scholar]
- 47. Dani R, Mendes GS, Medeiros Jde L, et al. Study of the liver changes occurring in preeclampsia and their possible pathogenetic connection with acute fatty liver of pregnancy. Am J Gastroenterol. 1996;91:292–294. [PubMed] [Google Scholar]
- 48. Conde‐Agudelo A, Villar J, Lindheimer MD. WHO systematic review of screening tests for prediction of preeclampsia. Obstet Gynecol. 2004;104:1367–1914. [DOI] [PubMed] [Google Scholar]
- 49. Widmer M, Villar J, Benigni A, et al. Mapping the theories of preeclampsia and the role of angiogenic factors. Obstet Gynecol. 2007;109:168–180. [DOI] [PubMed] [Google Scholar]
- 50. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat preeclampsia. Evidence from randomized controlled trials. Semin Nephrol. 2004;24: 607–615. [DOI] [PubMed] [Google Scholar]
- 51. Villar J, Abdel‐Aleem H, Merialdi M, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol. 2006;194:639–649. [DOI] [PubMed] [Google Scholar]
- 52. Askie LM, Duley L, Henderson‐Smart DJ, et al. PARIS Collaborative Group Antiplatelet agents for prevention of pre‐eclampsia: a meta‐analysis of individual patient data. Lancet. 2007;369:1765–1766. [DOI] [PubMed] [Google Scholar]
- 53. Poston L, Briley A, Seed P, et al. The vitamins in pre‐eclampsia (VIP) Trial Consortium vitamin C and vitamin E in pregnant women at risk for pre‐eclampsia (VIP trial): randomised placebo‐controlled trial. Lancet. 2006;367: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 54. Rumbold A, Crowther C, Haslam R, et al. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806. [DOI] [PubMed] [Google Scholar]
- 55. Von Dadelszen P, Menzies J, Gilgoff S, et al. Evidence‐based management for preeclampsia. Front Biosci. 2007;12:2876–2889. [DOI] [PubMed] [Google Scholar]
- 56. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514. [DOI] [PubMed] [Google Scholar]
- 57. Duley L, Henderson‐Smart DJ, Meher S, et al. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;2:CD004659. [DOI] [PubMed] [Google Scholar]
- 58. Sibai BH. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520–1526. [DOI] [PubMed] [Google Scholar]
- 59. Gilbert WM, Young AL, Danielson B. Pregnancy outcome in women with chronic hypertension: a population based study. J Reprod Med. 2007;52:1046–1051. [PubMed] [Google Scholar]
- 60. Von Dadelszen P, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. J Obstet Gynaecol Can. 2002;24:941–945. [DOI] [PubMed] [Google Scholar]
- 61. Martin JN Jr, Thigpen BD, Moore RC, et al. Stroke and severe preeclampsia, and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–254. [DOI] [PubMed] [Google Scholar]
- 62. Podymow T, August P, Umans JG. Antihypertensive therapy in pregnancy. Semin Nephrol. 2004;24:616–625. [DOI] [PubMed] [Google Scholar]
- 63. Abalos E, Duley L, Steyn DW, et al. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Review. Cochrane Database Syst Rev. 2007;1:CD002252. [DOI] [PubMed] [Google Scholar]
- 64. Magee LA, Miremadi S, Li J, et al. Therapy with both magnesium sulfate and nifedipine does not increase the risk of serious magnesium‐related maternal side effects in women with preeclampsia. Am J Obstet Gynecol. 2005;193:153–163. [DOI] [PubMed] [Google Scholar]
- 65. Cooper WO, Hemandez‐Diaz S, Arbogast PD, et al. Major congenital anomalies after first‐trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451. [DOI] [PubMed] [Google Scholar]
- 66. Jonsdottir LS, Arngrimsson R, Geirsson RT, et al. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776. [DOI] [PubMed] [Google Scholar]
- 67. Funai EF, Friedlander Y, Paltiel O, et al. Long‐term mortality after preeclampsia. Epidemiology. 2005;16:206–215. [DOI] [PubMed] [Google Scholar]
- 68. Irgens HU, Reisaeter L, Irgens LM, et al. Long‐term mortality of mothers and fathers after preeclampsia: population‐based cohort study. BMJ. 2001;323:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Christianson RE. Studies on blood pressure during pregnancy. I. Influence of parity and age. Am J Obstet Gynecol. 1976;125:509–513. [DOI] [PubMed] [Google Scholar]


