Abstract
Patients with hypertension exhibit changes in vessel conductance and resistance. The aim of this study was to evaluate the effect of the angiotensin receptor blocker irbesartan on coronary microvascular function. Thirty‐six hypertensive patients without coronary artery or systemic disease were examined. Coronary flow velocity reserve (CFR) was measured using transthoracic Doppler echocardiography in 18 men (54±9 years) before and after 3 months of treatment with 600 mg/d of irbesartan and in 18 controls (55±11 years). Carotid intima‐media thickness (IMT) was evaluated with high‐resolution echocardiography. Baseline CFR did not differ between groups. CFR significantly improved in the irbesartan group (from 2.87±.42 to 3.78±.32; P<.001), but remained unchanged in controls (from 2.94±.61 to 3.06±.72; P=not significant). CFR improved with treatment independent of associated risk factors. BP decreased from 150±18 mm Hg to 129±25 mm Hg (P<.001) during treatment, whereas IMT and left ventricular mass index showed no significant differences at the end of the follow‐up period in both groups. Three‐month irbesartan treatment significantly increased CFR in patients with hypertension. This improvement is attributed to blockade of the renin‐angiotensin system. Coronary microvascular function was shown to improve independent of hypertrophy regression. Patients with lower baseline CFR tended to show a more pronounced CFR response. J Clin Hypertens (Greenwich). 2011;13:155–161. © 2010 Wiley Periodicals, Inc.
In patients with systemic hypertension, microvascular damage occurs early and prior to the development of stenotic lesions. The resulting microvascular dysfunction may lead to signs and symptoms of myocardial ischemia despite angiographically normal coronary arteries. 1 Coronary flow reserve (CFR) is considered an important physiologic parameter in the coronary circulation, reflecting the function of large epicardial arteries and the microcirculation in the absence of epicardial coronary stenosis. Moreover, in normal coronary arteries or in those with only minor arteriosclerosis, CFR is an independent predictor of long‐term outcome. 2
Structural and functional alterations of the vasculature may contribute to complications of hypertension. The pathophysiologic mechanisms leading to microvascular alterations, angina, ischemia, and reduced CFR in patients without coronary artery disease (CAD) are not fully understood. The renin‐angiotensin system (RAS) plays an important role early in the development of hypertension‐induced vascular alterations such as changes in vascular tone and inflammation, cell growth, cardiac hypertrophy, remodeling, and apoptosis. 3
Because angiotensin II (Ang II) may be pivotal in some of these vascular abnormalities, angiotensin‐converting enzyme (ACE) inhibitors and Ang II type I receptor blockers (ARBs) are likely to influence these microvascular alterations, not only by improving loading conditions, but also by direct action on the vascular wall. ARBs specifically block the RAS and inhibit effects of Ang II such as vasoconstriction and cell growth, without affecting Ang II type 2 receptor–mediated effects of Ang II, such as vasodilatation and inhibition of cell growth. Thus, in addition, ARBs seem to have anti‐inflammatory, metabolic, and vascular effects, albeit independent of their BP‐lowering effect. Clinical studies showed that the anti‐inflammatory effect of ARBs could be related to the dosage and/or the length of the treatment. 4 Histopathologic data from animal studies also show a reduction of interstitial and perivascular fibrosis under treatment with ARBs. 5 Therefore, clinical effects of ARBs on the coronary microvascular perfusion in humans could be of prognostic significance.
CFR can be assessed in a reliable, reproducible, and noninvasive manner using high‐resolution transthoracic Doppler echocardiography (TTDE). Several studies have demonstrated TTDE‐derived CFR to be of similar excellent quality as CFR measured by invasive methods or by using positron emission tomography. 6 , 7 , 8 , 9
ACE inhibitors have been shown in several studies to improve CFR in arterial hypertension. 10 , 11 Our objective was to evaluate the effect of a high‐dose, long‐acting ARB, irbesartan, on CFR in patients with arterial hypertension without relevant left ventricular hypertrophy.
Methods
Patients and Study Design
Thirty‐six consecutive male patients with hypertension underwent 24‐hour ambulatory blood pressure (BP) monitoring for initial patient screening. Hypertension was diagnosed in 18 patients with an average daytime BP >135/85 mm Hg and an average nighttime BP >125/75 mm Hg. These 18 patients constituted the study population. The control patients included 18 hypertensive age‐matched male volunteers. Each patient was screened by clinical history, physical examination, electrocardiography, echocardiography, stress echocardiography, and routine chemical analysis to rule out any other systemic disease.
Exclusion criteria for the treatment group were current treatment with ACE inhibitors or ARBs, relevant left ventricular hypertrophy (septal thickness >14 mm), and evidence of overt atherosclerotic disease (CAD, peripheral vascular disease, and stroke). Significant CAD was excluded on the basis of clinical history, ergometry, and baseline echocardiography. A dynamic stress echocardiogram positive for ischemia was considered an exclusion criterion. The same criteria were applied to the control group, with the exception that pretreatment with ACE inhibitors or ARBs for more than 1 year was not an exclusion criterion in this group.
ARB treatment with irbesartan was started at a dose of 150 mg/d for 1 week, and subsequently increased continuously until 300 mg twice a day was reached after 4 weeks of treatment. Treatment was continued over 3 months. Previous concomitant treatment was maintained. The treatment of the control group was not changed during the study period. Written informed consent was obtained from each participant, and the institutional ethics committee approved the study protocol.
Echocardiography
Each participant was examined with an electronic phased‐array ultrasound system. Left ventricular diastolic diameter and septal and posterior wall thickness were assessed in M‐mode images of parasternal long‐ and short‐axis views. Left ventricular mass index (LVMI) was calculated according to the recommendations of the American Society of Echocardiography. 12 Transthoracic M‐mode echocardiographic measurements were taken by one investigator blinded to the patient data.
Measurement of CFR
In all patients, TTDE evaluation of CFR was performed in the left anterior descending artery by one experienced echocardiographer blinded to the patient data. Measurements were performed at baseline and at the 3‐month follow‐up. Two‐dimensional images were recorded with a 7.0‐MHz transducer at 3.5 MHz and coronary flow was visualized using high‐frequency 5.0‐MHz color Doppler technique with a Nyquist limit of 12 cm/s to 16 cm/s. Coronary flow velocity was measured using PW Doppler technique at 3.5 MHz with minimal angle correction. Stop frames and clips were digitally recorded and stored on magneto‐optical disks. All measurements were performed off‐line using an ultrasound machine–incorporated analysis and calculation package.
Visualization of Coronary Flow
A systematic approach was made to visualize the supra‐apical portion of the distal left anterior descending artery (LAD). This portion of the LAD was chosen to reliably perform both CFR measurements at the same segment of the vessel. For this purpose, the transducer was shifted from the typical apical 4‐chamber view position 3 cm to 4 cm medially and cranially and rotated into a sagittal plane to obtain a modified 2‐chamber view of the apical left ventricle and the anterior groove. LAD was identified by systolic‐diastolic color blood flow seen in the region of the anterior groove of the left ventricle. For the following PW Doppler measurement the transducer was carefully rotated to minimize angle correction.
Echocardiographic Measurement of CFR
The intake of xanthin‐containing food or beverages was stopped the day before the examination. PW Doppler recording of coronary flow was guided by color Doppler. Flow velocity recordings were performed with stable transducer position at rest and maximal hyperemia, which was induced by administration of intravenous adenosine (140 μg/kg/min). CFR was calculated using the ratio of hyperaemia‐induced systolic‐diastolic coronary flow to coronary flow at rest (Figure 1). The values of 3 consecutive beats were averaged to calculate the average peak velocity. Off‐line analysis of spectral Doppler tracings required consensus of 2 examiners.
Figure 1.
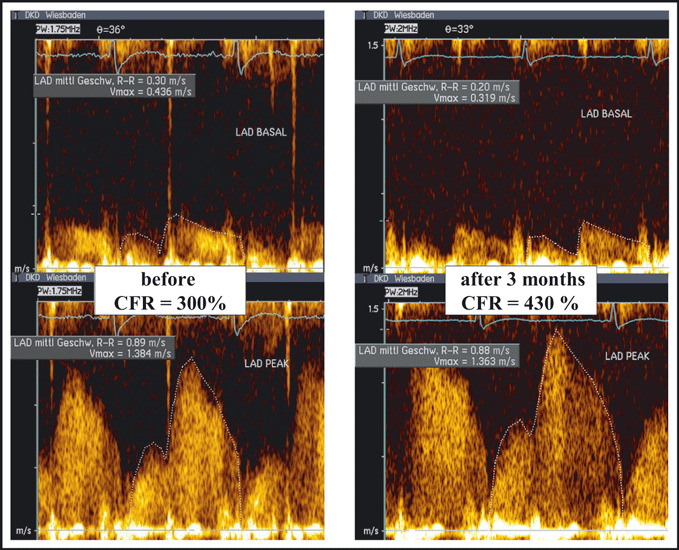
Assessment of coronary flow reserve in a hypertensive patient before (left panel) and after (right panel) treatment with irbesartan. CFR indicates coronary flow reserve.
Measurement of Carotid Intima‐Media Thickness
An 8.0‐MHz linear array transducer was used. Based on the recommendations of the American Society of Echocardiography, 13 a longitudinal scan of the common carotid artery of the patient in the supine position was recorded. Zoom was deployed on the carotid bifurcation and the cranial portion of the common carotid artery. Carotid intima‐media thickness (IMT) was measured between the bifurcation and 1 cm proximal to the bifurcation. At least 3 points were measured in the studied segment. The maximum IMT of both carotid arteries was recorded for subsequent follow‐up.
Statistical analysis
Data were analyzed with SPSS version 9.0 (SPSS Inc, Chicago, IL). Quantitative variables were expressed as means and standard deviations, and discrete variables as frequencies and percentages. Comparisons of continuous variables were carried out with Student t test. To evaluate the effect of therapy, a paired t test was used. Statistical significance was established at a P value <.05.
Results
Clinical and echocardiographic patient characteristics of both groups were similar (Table I). CFR in the treatment and control groups were 2.87±.42 and 2.94±.61, respectively.
Table I.
Baseline Clinical Characteristics and Medication of the Study Population
| Variable | Irbesartan (n=18) | Control (n=18) | P Value |
|---|---|---|---|
| Age, % | 54±9 | 55±11 | ns |
| Systolic BP, mm Hg | 150±18 | 147±22 | ns |
| Diastolic BP, mm Hg | 88±11 | 89±13 | ns |
| LVMI, g/m2 | 135±35 | 134±29 | ns |
| IMT, mm | 1.058±.34 | 1.003±.46 | ns |
| CFR | 2.87±.42 | 2.94±.61 | ns |
| Comorbid conditions, No. (%) | |||
| Diabetes | 3 (16.7) | 4 (22.2) | ns |
| Dyslipidemia | 7 (38.9) | 6 (33.3) | ns |
| Concomitant medication, No. (%) | |||
| ASA | 7 (38.9) | 9 (50.0) | ns |
| Diuretic | 8 (44.4) | 10 (55.5) | ns |
| β‐Blocker | 5 (27.8) | 6 (33.3) | ns |
| CCB | 5 (27.8) | 6 (33.3) | ns |
| ARB/ACE inhibitor | 0 (0) | 5 (27.8) | <.01 |
| Statin | 5 (27.8) | 6 (33.3) | ns |
Values are mean ± standard deviation unless otherwise indicated. Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin II type I receptor blocker; BP, 24‐h ambulatory blood pressure; CCB, calcium channel blocker; CFR, coronary flow velocity reserve; IMT, intima‐media thickness; LVMI, left ventricular mass index; ns, not significant.
CFR was increased in all irbesartan‐treated patients after 3 months (from 2.87±.42 to 3.78±.32; P<.001 [31.5% increase]) (Figure 2, Table II), whereas no change in CFR was observed in the control group (2.94±.61 to 3.06±.72; P=not significant).
Figure 2.
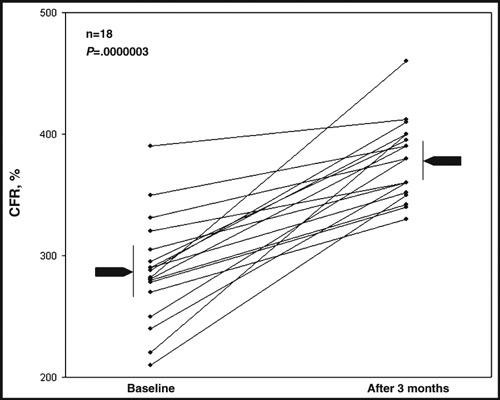
Coronary flow reserve values before and after treatment. CFR indicates coronary flow reserve.
Table II.
Baseline and Final Values of BP, IMT, CFR, and LVMI
| Irbesartan | Control | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 Month | P Value | Baseline | 3 Month | P Value | |
| SBP, mm Hg | 150±18 | 129±25 | .001 | 145±23 | 147±22 | ns |
| DBP, mm Hg | 88±11 | 81±12 | .003 | 89±13 | 87±16 | ns |
| IMT, mm | 1.06±.34 | 1.04±.30 | .225 | 1.00±.46 | .97±.52 | ns |
| CFR | 2.87±.42 | 3.78±.32 | .0001 | 2.94±.61 | 3.06±.72 | ns |
| LVMI, g/m2 | 135±35 | 131±37 | ns | 134±29 | 132±33 | ns |
Abbreviations: CFR, coronary flow velocity reserve; DBP, diastolic 24‐h ambulatory blood pressure; IMT, intima‐media thickness; LVMI, left ventricular mass index; ns, not significant; SBP, systolic 24‐h ambulatory blood pressure.
The increase in CFR was correlated with basal CFR (R=−.43, P=.03). Patients with lowest baseline CFR responded better to therapy.
Irbesartan led to a significant reduction in systolic and diastolic BP (150±18 mm Hg to 129±25 mm Hg, P=.001 and 88±11 mm Hg to 81±12 mm Hg; P=.03) in the treatment group. BP remained unchanged in the control group (Table II).
Target organ damage measured as IMT was mild at baseline in both groups, and IMT was not significantly modified at the end of the follow‐up (1.058±.34 to 1.038±.30; P=.225).
Discussion
Numerous trials have demonstrated that antihypertensive therapy is effective in reducing major vascular events, including stroke and coronary heart disease. 14 However, several forms of specific end organ damage, which primarily involve the microcirculation, are thought to be secondary to hypertension, including nephropathy, retinopathy, lacunar infarction, and microvascular angina. Thus, it is to be expected that there will be additional benefits from targeting the microcirculation during antihypertensive therapy in terms of the prevention of or reduction in end organ damage.
The aim of this study was to noninvasively evaluate the effect of the ARB irbesartan on coronary microvascular function and thus on CFR in hypertensive patients. We found a significant improvement in CFR as measured by TTDE in the irbesartan treatment group compared with the control group. Irbesartan treatment also led to a significant reduction in systolic and diastolic BP, whereas BP remained unchanged in the control group.
CFR is considered a marker of coronary microcirculatory function if epicardial stenosis is not present, and is significantly lower in patients with prehypertension than in normotensive patients. Decrease in CFR is found to be even more pronounced in patients with systemic hypertension. 15 This observation supports the premise that pharmacologic improvement of CFR is of prognostic relevance for patients with hypertension.
Impairment of CFR may independently occur long before left ventricular hypertrophy is detected in hypertensive patients, leading to perivascular fibrosis and subsequent ischemia. 15 , 16 Reduction in CFR is seen as a result of increased microvascular coronary resistance. Several studies support the notion that structural changes in the coronary vasculature significantly contribute to a reduction in CFR and that these effects are independent of vascular tone. 16 , 17 , 18 , 19 Animal studies of Ang II–dependent hypertension have shown that cardiac fibrosis was associated with left ventricular hypertrophy, both of which were prevented by treatment with the selective AT1 receptor antagonist irbesartan. 5 Furthermore, structural remodeling of the intramyocardial coronary arterioles and the accumulation of fibrillar collagen were found to be causative for a reduced coronary dilatory capacity in patients with arterial hypertension and angina pectoris in the absence of relevant coronary artery stenoses. 19 CFR thus enables detection of alterations in the coronary microcirculation in a noninvasive manner, with the objective to prevent further microvascular damage by pharmacologic intervention.
Because lower CFR of hypertensive patients is presumably caused, at least in part, by structural and functional abnormalities within the coronary microcirculation, Ang II is likely to play a role in the development of the vascular alternations, since it is known to cause oxidative stress, vasoconstriction, inflammation, and vascular thrombosis and remodelling. 20 , 21 , 22 , 23 Blocking this system by treatment with an ACE inhibitor was previously shown to cause remodelling regression, as well as improvement in CFR and endothelial function. 10 , 11 , 24 Studies on the effect of ARBs on CFR are scarce. Kitakaze and colleagues 25 report that infusion of an ARB into canine coronary arteries causes an increase in coronary blood flow, and that the combination of an ACE inhibitor and an ARB mediates an even greater increase in coronary blood flow. A study by Hinoi and colleagues 26 demonstrates that antihypertensive therapy with telmisartan (ARB), but not with nifedipine (calcium channel blocker), had a beneficial effect on coronary microcirculation and insulin resistance among essential hypertensive patients. Further, Goette and colleagues 27 were able to show, in a model of pacing‐induced microvascular flow abnormalities, that these are in part related to the induction of oxidative stress and that this induction was related to the presence of Ang II. On the other hand, the addition of irbesartan to these experiments abolished the majority of pacing‐induced alterations with an improvement in microvascular flow and a reduction in oxidative stress. In accordance with our results, Tomás and colleagues 3 found an improvement in CFR of hypertensive patients treated with the ARB candesartan, and, similar to our findings, patients with lower baseline CFR responded better to therapy, suggesting that histopathologic changes are more pronounced in patients with more depressed CFR, thus making beneficial effects of ARB treatment more significant. AT1 receptor blockade by treatment with irbesartan (ARB) was also shown to improve peripheral but not coronary endothelial dysfunction in patients with CAD. 28
The impact of the RAS on hypertension thus extends beyond the increase in arterial pressure to encompass several aspects of hypertensive heart disease, including left ventricular hypertrophy, coronary insufficiency, endothelial dysfunction, and occlusive coronary artery disease. 29 The potential mechanism explaining the improvement in vasodilator reserve are multiple and include hemodynamic changes secondary to BP lowering, the regression of pathologic left ventricular hypertrophy, or vascular remodelling.
The ARB‐induced reduction of interstitial perivascular fibrosis documented in several animal studies seems to be responsible for at least part of the beneficial effect of CFR improvement. 5 , 30 , 31 As a consequence, an improvement of vasodilation capacity can be expected under ARB treatment with irbesartan, explaining at least part of the CFR improvement.
Conclusions
Microvascular changes are hallmarks of the long‐term complications of hypertension and diabetes; however, it is now clear that microvascular changes occur very early in these conditions and may be important in their pathogenesis and progression. It is noteworthy that microvascular changes that result from one risk factor could predispose to other risk factors.
Three‐month treatment with the ARB irbesartan for hypertensive patients without relevant arteriosclerosis and without significant left ventricular hypertrophy resulted in significant improvement in CFR as a marker of coronary microcirculatory function. Since impairment of CFR indicates an elevated risk for cardiovascular morbidity, this finding may add valuable information for first‐line treatment of systemic hypertension.
Acknowledgments
Disclosure: This work was supported by an unrestricted grant by Sanofi Aventis Deutschland GmbH. Peter Bramlage and Heinz Lambertz have been receiving honoraria for lectures and research support from Sanofi Aventis and other companies that produce ARBs.
References
- 1. Houghton JL, Frank MJ, Carr AA, et al. Relations among impaired coronary flow reserve, left ventricular hypertrophy and thallium perfusion defects in hypertensive patients without obstructive coronary artery disease. J Am Coll Cardiol. 1990;15:43–51. [DOI] [PubMed] [Google Scholar]
- 2. Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long‐term outcome. Coron Artery Dis. 2004;15:259–264. [DOI] [PubMed] [Google Scholar]
- 3. Tomas JP, Moya JL, Barrios V, et al. Effect of candesartan on coronary flow reserve in patients with systemic hypertension. J Hypertens. 2006;24:2109–2114. [DOI] [PubMed] [Google Scholar]
- 4. Barra S, Vitagliano A, Cuomo V, et al. Vascular and metabolic effects of angiotensin II receptor blockers. Expert Opin Pharmacother. 2009;10:173–189. [DOI] [PubMed] [Google Scholar]
- 5. Seccia TM, Belloni AS, Kreutz R, et al. Cardiac fibrosis occurs early and involves endothelin and AT‐1 receptors in hypertension due to endogenous angiotensin II. J Am Coll Cardiol. 2003;41:666–673. [DOI] [PubMed] [Google Scholar]
- 6. Caiati C, Montaldo C, Zedda N, et al. New noninvasive method for coronary flow reserve assessment: contrast‐enhanced transthoracic second harmonic echo Doppler. Circulation. 1999;99:771–778. [DOI] [PubMed] [Google Scholar]
- 7. Hozumi T, Yoshida K, Akasaka T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32:1251–1259. [DOI] [PubMed] [Google Scholar]
- 8. Lethen H, Tries HP, Brechtken J, et al. Comparison of transthoracic Doppler echocardiography to intracoronary Doppler guidewire measurements for assessment of coronary flow reserve in the left anterior descending artery for detection of restenosis after coronary angioplasty. Am J Cardiol. 2003;91:412–417. [DOI] [PubMed] [Google Scholar]
- 9. Lethen H, Tries P, Kersting S, et al. Validation of noninvasive assessment of coronary flow velocity reserve in the right coronary artery. A comparison of transthoracic echocardiographic results with intracoronary Doppler flow wire measurements. Eur Heart J. 2003;24:1567–1575. [DOI] [PubMed] [Google Scholar]
- 10. Motz W, Strauer BE. Improvement of coronary flow reserve after long‐term therapy with enalapril. Hypertension. 1996;27:1031–1038. [DOI] [PubMed] [Google Scholar]
- 11. Schwartzkopff B, Brehm M, Mundhenke M, et al. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. 2000;36:220–225. [DOI] [PubMed] [Google Scholar]
- 12. Devereux RB. Detection of left ventricular hypertrophy by M‐mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987; 9(2 pt 2):II19–II26. [DOI] [PubMed] [Google Scholar]
- 13. Roman MJ, Naqvi TZ, Gardin JM, et al. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006;19:943–954. [DOI] [PubMed] [Google Scholar]
- 14. Levy BI, Ambrosio G, Pries AR, et al. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. [DOI] [PubMed] [Google Scholar]
- 15. Erdogan D, Yildirim I, Ciftci O, et al. Effects of normal blood pressure, prehypertension, and hypertension on coronary microvascular function. Circulation. 2007;115:593–599. [DOI] [PubMed] [Google Scholar]
- 16. Laine H, Raitakari OT, Niinikoski H, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol. 1998;32:147–153. [DOI] [PubMed] [Google Scholar]
- 17. Brilla CG, Janicki JS, Weber KT. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ Res. 1991;69:107–115. [DOI] [PubMed] [Google Scholar]
- 18. Panza JA, Quyyumi AA, Brush JE Jr, et al. Abnormal endothelium‐dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. [DOI] [PubMed] [Google Scholar]
- 19. Schwartzkopff B, Motz W, Frenzel H, et al. Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation. 1993;88:993–1003. [DOI] [PubMed] [Google Scholar]
- 20. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 21. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. [DOI] [PubMed] [Google Scholar]
- 22. Dzau VJ. Theodore Cooper lecture: tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. [DOI] [PubMed] [Google Scholar]
- 23. Kern MJ, Lerman A, Bech JW, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. [DOI] [PubMed] [Google Scholar]
- 24. Buus NH, Bottcher M, Jorgensen CG, et al. Myocardial perfusion during long‐term angiotensin‐converting enzyme inhibition or beta‐blockade in patients with essential hypertension. Hypertension. 2004;44:465–470. [DOI] [PubMed] [Google Scholar]
- 25. Kitakaze M, Asanuma H, Funaya H, et al. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers synergistically increase coronary blood flow in canine ischemic myocardium: role of bradykinin. J Am Coll Cardiol. 2002;40:162–166. [DOI] [PubMed] [Google Scholar]
- 26. Hinoi T, Tomohiro Y, Kajiwara S, et al. Telmisartan, an angiotensin II type 1 receptor blocker, improves coronary microcirculation and insulin resistance among essential hypertensive patients without left ventricular hypertrophy. Hypertens Res. 2008;31:615–622. [DOI] [PubMed] [Google Scholar]
- 27. Goette A, Bukowska A, Dobrev D, et al. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor‐mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J. 2009;30:1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warnholtz A, Ostad MA, Heitzer T, et al. AT1‐receptor blockade with irbesartan improves peripheral but not coronary endothelial dysfunction in patients with stable coronary artery disease. Atherosclerosis. 2007;194:439–445. [DOI] [PubMed] [Google Scholar]
- 29. Frohlich ED, Apstein C, Chobanian AV, et al. The heart in hypertension. N Engl J Med. 1992;327:998–1008. [DOI] [PubMed] [Google Scholar]
- 30. Kumagai K, Nakashima H, Urata H, et al. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41:2197–2204. [DOI] [PubMed] [Google Scholar]
- 31. Li D, Shinagawa K, Pang L, et al. Effects of angiotensin‐converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing‐induced congestive heart failure. Circulation. 2001;104:2608–2614. [DOI] [PubMed] [Google Scholar]


