Abstract
Ambulatory blood pressure monitoring (ABPM) allows determining of the nocturnal blood pressure fall (NBPF). An NBPF below 10% (nondipper pattern) has been related to increased cardiovascular risk, and it is a common finding in type 2 diabetic hypertensive patients. The authors evaluated the impact on 24‐hour blood pressure, NBPF, and albuminuria of olmesartan 40 mg, administered in a morning‐ vs a nocturnal‐based dosing scheme, in type 2 diabetic patients with newly diagnosed hypertension. Using a crossover design, 40 patients (42.1% men) received olmesartan 40 mg once daily at wake up or bedtime for 8 weeks. Patients underwent 24‐hour ABPM at baseline and at weeks 8 and 16, and albumin to creatinine ratio was measured at baseline and 8 weeks. Night systolic blood pressure (BP) (P=.007) and mean BP (P=.012) were significantly reduced following the bedtime dose, compared with morning dosing. Night BP fall (%) was significantly reduced by bedtime dosing, compared with morning dosing (P=.0001). No differences were seen for urinary albumin excretion between both arms at week 8. Without affecting 24‐hour BP control, night dosing of olmesartan increases nocturnal BP fall significantly more than conventional morning dosing, increasing the number of dipper diabetic hypertensive patients.
Blood pressure (BP) and heart rate in humans are characterized by cyclic changes during 24 hours that parallel the rest/activity state. 1 Along with this observation, it has been demonstrated that the extent of the nocturnal BP decline and the subsequent morning BP rate of rise along with the awakening and starting of diurnal activity are both independent risk factors for stroke and other cardiovascular (CV) events. 2
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 3 has stated that in persons in whom a 10% to 20% decrease of BP during the night is not present (referred to as “nondipper pattern”) are at increased risk for CV events. Therefore, CV risk could be influenced not only by BP elevation but also by the magnitude of the circadian BP variability.
Antihypertensive drugs either in monotherapy or in combination are traditionally administered together in the morning upon arising from bed. This is mainly because this approach has been applied in the vast majority of outcome trials that showed the benefits of treatment in reducing the risk of CV and renal disease. 3 Nevertheless, there is considerable evidence that the time of antihypertensive drug administration can modify the 24‐hour BP curve. Morning administration gives its full effect during daytime activities and a lesser effect during nighttime and the early morning hours, whereas bedtime administration has a larger effect during nighttime and the early morning hours. It might be argued that bedtime administration should be considered as an alternative strategy that has the potential to provide more effective CV and renal protection. 4 , 5 Several short‐term randomized controlled trials assessed the bedtime dosing of antihypertensive drugs compared with conventional morning dosing. Overall, nighttime administration of angiotensin‐converting enzyme inhibitors 6 , 7 and angiotensin II receptor blockers (ARBs) 8 , 9 results in a greater effect on nocturnal BP and a significant modification of the circadian profile of BP, although significant differences on 24‐hour BP have not been demonstrated.
Material and Methods
Patients
Outpatient type 2 diabetic patients were prospectively enrolled in this study after matching for the following inclusion criteria: (1) age between 18 and 75 years, (2) body mass index (BMI) between 20 and 40 kg/m2, (3) diagnosis of hypertension based on an office systolic BP (SBP) reading >130 mm Hg and/or a diastolic BP (DBP) reading >80 mm Hg, confirmed by further ambulatory BP monitoring (ABPM), and (4) no pharmacologic agent aimed to treat hypertension in the past 6 months, before initial visit.
Diabetes mellitus was defined as a fasting glucose level >126 mg/dL (7.8 mmol/L), a random nonfasting glucose level >200 mg/dL (11.1 mmol/L), a glycated hemoglobin A1c >6.2%, or the use of an oral hypoglycemic agent or insulin. Urinary albumin excretion was measured throughout the study by determination of albumin/creatinine ratio (ACR) measured at each visit in a first morning void urine specimen.
Office and 24‐Hour Ambulatory BP Readings
Office BP was measured 3 times after resting for at least 5 minutes in the sitting position and the average of the 2 latter readings was used. Diagnostic criteria for hypertension following office BP readings were based on American Diabetes Association 19 and JNC 7 recommendations. 20 Office readings were taken with an OMRON M10‐IT automatic device (OMRON Healthcare, Kyoto, Japan).
Patients meeting previous inclusion criteria underwent further screening with 24‐hour ABPM to confirm hypertensive status. Despite the fact that there are no clear‐cut thresholds for the diagnosis of hypertension using ABPM in a diabetic population, JNC 7 recommendations were followed and thus a daytime average BP ≥135 mm Hg (SBP) and/or ≥85 mm Hg (DBP) and/or a nighttime average BP ≥120 mm Hg (SBP) and/or ≥75 mm Hg (DBP) was considered consistent with the initial diagnosis of hypertension.
ABPM was performed on a weekday with 1 of 2 automatic devices (Model Spacelabs 90,217, Spacelabs HealthCare, Hertford, UK) that were set to record BP and heart rate every 30 minutes during daytime and every 60 minutes during nighttime, to complete a period of at least 24 hours. All devices were calibrated before and throughout the study every 10 tests. Patients who obtained <80% of either awake‐ or asleep‐valid BP readings were rescheduled for a new test within 1 week. Mean BP (MBP) was calculated for 24‐hour MBP, daytime MBP, and night MBP according to the following formula: MBP = DBP + (SBP−DBP) / 3.
Nighttime average SBP, DBP, and MBP, respectively were defined as the average value of SBPs, DBPs, and MBPs, respectively, from the time when the patient went to bed until the time he or she got out of bed, and daytime average SBP, DBP, and MBP were defined as the average of BPs and MBPs, respectively, recorded during the rest of the day. The nocturnal BP fall (NBPF) (%) was defined as the coefficient between nighttime MBP and daytime MBP. An NBPF between 10% and 20% was considered to correspond with a dipper pattern, while an NBPF <10% was considered to correspond with a nondipper pattern. 20
Study Design
Patients who met initial inclusion criteria were invited to participate in this study. After giving written informed consent, patients underwent a baseline 24‐hour ABPM measurement (baseline visit). Patients with either daytime SBP ≥135 mm Hg or daytime DBP ≥85 mm Hg or nighttime SBP ≥120 mm Hg or nighttime DBP ≥75 mm Hg entered the active study phase and were randomly assigned to receive olmesartan medoxomil at an initial dose of 40 mg that could eventually be reduced to 20 mg in cases of hypotension, either following a conventional morning dose regimen (drug administration at awakening, between 7 am and 9 am) or a bedtime dose regimen (between 10 pm and 12 am). After 8 weeks, patients underwent a second 24‐hour ABPM (visit 2) and then shifted from a morning to a bedtime dose regimen and vice versa (crossover design). After a second period of 8 weeks, patients underwent a final 24‐hour ABPM measurement (visit 3 and final). Prior to the baseline visit and visit 2, patients were instructed to collect a first morning void urine specimen in order to measure renal albumin excretion by determination of ACR (Figure). Study protocol was approved by the central ethics committee.
Figure.
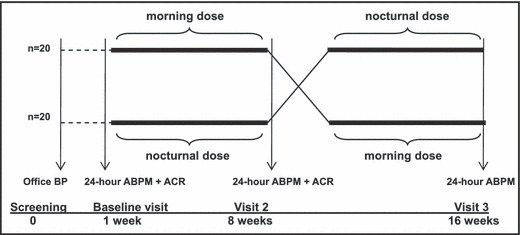
After initial office and baseline ambulatory blood pressure (BP) readings, patients were randomly assigned to either a morning or nocturnal dosing scheme in a crossover design. First morning void urine samples were collected at baseline and visit 2 to measure albumin excretion. ABPM indicates ambulatory blood pressure monitoring; ACR, albumin‐creatinine ratio.
Statistical Analysis
A descriptive statistical analysis was carried forward. Mean and dispersion measures were used for quantitative variables and absolute and relative frequency measures for categoric variables. In order to evaluate changes in BP evolution in each patient, a covariate analysis for repeated measures (analysis of covariance) was performed. Tukey correction model was applied. All statistical analyses were performed with a 2‐tailed, 5% level of significance using the SAS statistical package version 8.2 (European Biometrics Institute, Barcelona, Spain).
Results
A total of 51 patients were enrolled for a baseline visit between January and October 2007. After initial 24‐hour ABPM was performed, 11 patients were excluded due to either daytime ABPM <135/85 mm Hg or nighttime ABPM <120/75 mm Hg. Forty patients (23 women) started active treatment. Two patients were lost during follow‐up. After 12 weeks, data were successfully collected from 38 patients (22 women). Baseline demographic characteristics are presented in Table I. No differences were found for BP values in patients who were initially assigned to daytime vs nighttime administration of the study drug.
Table I.
Baseline Demographic Characteristics of Study Population (N=38)
| Characteristic | |
|---|---|
| Male, No. (%) | 16 (42.1) |
| Age, y | 53.7±12.4 |
| DM duration, y | 5.4±3.9 |
| HbA1c, % | 6.7±0.8 |
| Body mass index, kg/m2 | 27.9±3.5 |
| 24‐hour SBP, mm Hg | 138.48±9.27 |
| 24‐hour DBP, mm Hg | 87.47±8.36 |
| 24‐hour MBP, mm Hg | 104.3±7.99 |
| NBPF, % | 10.83±6.54 |
| Dip pattern, No. (%) | 26 (70) |
| Albumin/creatinine ratio, mg/g | 43.74±61.88 |
| Active smoking, % | 23 |
Values are expressed as mean ± standard deviation unless otherwise indicated. Abbreviations: DBP, diastolic blood pressure; DM, diabetes mellitus; HbA1c, glycated hemoglobin A1c; MBP, mean blood pressure; NBPF, nocturnal blood pressure fall; SBP, systolic blood pressure.
24‐Hour, Daytime, and Nighttime ABPM and Heart Rate
Both morning and nighttime administration of olmesartan resulted in a statistically significant reduction of all 24‐hour SBP, 24‐hour DBP, and 24‐hour MBP (Table II). This reduction was also maintained throughout both diurnal and nocturnal periods when compared with baseline values.
Table II.
Results of 24‐Hour, Daytime, and Nighttime ABPM and Heart Rate
| Mean± SD, mm Hg | Baseline | Olmesartan Morning Dose | Olmesartan Night Dose | P Valueb |
|---|---|---|---|---|
| 24‐hour SBP | 138.48±9.27 | 124.80±7.14a | 124.09±6.89a | .86 |
| 24‐hour DBP | 87.47±8.36 | 78.91±9.01a | 77.4±6.97a | .36 |
| 24‐hour MBP | 104.3±7.99 | 93.52±6.56a | 92.96±6.20a | .83 |
| 24‐hour HR | 82.16±10.21 | 76.57±10.44a | 76.49±10.39a | .99 |
| Day SBP | 142.16±11.73 | 128.65±8.47a | 129.52±8.19a | .83 |
| Day DBP | 89.53±9.02 | 81.35±8.17a | 81.06±7.42a | .95 |
| Day MBP | 106.97±9.26 | 96.46±6.89a | 97.22±6.62a | .73 |
| Day HR | 85.25±10.95 | 79.39±10.90a | 79.58±11.11a | .97 |
| Night SBP | 124.26±8.38 | 112.39±9.61a | 108.07±9.11a | .007 |
| Night DBP | 80.82±7.8 | 73.97±12.34a | 71.04±7.71a | .069 |
| Night MBP | 95.32±6.96 | 86.77±9.39a | 83.35±7.75a | .012 |
| Day HR | 72.12±9.99 | 67.78±10.80a | 66.90±10.60a | .47 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure; SD, standard deviation. a P<.0001 compared with baseline. bMorning vs nighttime dosing.
Nighttime administration of olmesartan resulted in a significantly greater reduction of nighttime SBP (11.87±10.26 vs 16.19±10.02; P=.007) and nighttime MBP (8.55±8.73 vs 11.97±8.12; P=.012). No significant differences were seen between morning and nighttime dosing, for all daytime values (daytime SBP, daytime DBP, and daytime MBP).
Olmesartan administration resulted in a significant reduction of heart rate compared with baseline, although no significant differences were seen between morning and nighttime dosing (Table II).
Nighttime BP Fall and Dip Status
Nighttime BP reduction was measured by nighttime ABPM/daytime ABPM ratio. Nighttime administration of olmesartan resulted in a significant increase in NBPF compared with baseline (7.37±6.11%; P<.0001) and with diurnal administration (2.21%±5.41% vs 7.37%±6.11%; P<.0001). Diurnal administration of olmesartan failed to significantly increase NBPF vs baseline (P=.0501).
Twenty six (68%) patients yielded a dipper pattern at baseline 24‐hour ABPM. Diurnal administration of olmesartan increased the number of dipper patients to 28 (74%), while nocturnal administration of the drug increased to 32 (82%). Only this strategy reached statistical significance compared with baseline (P=.012) (Table III).
Table III.
NBPF, Dip Status, and Albumin Excretion
| Results | Baseline | Olmesartan Morning Dose | Olmesartan Night Dose | P Valuea |
|---|---|---|---|---|
| NBPF, % | 10.89±6.61 | 12.78±8.48b | 17.94±6.41c | .001 |
| Dipper, % | 68 | 74 | 84 | |
| ACR, mg/g | 43.74±61.88 | 34.32±53.42c | 32.83±57.32c | .66 |
Values are expressed as mean ± standard deviation unless otherwise indicated. Abbreviations: ACR, albumin/creatinine ratio; NBPF, nocturnal blood pressure fall. aMorning vs nighttime dosing. b P=.0501. c P<.0001.
Albumin Excretion Rate
Albumin excretion rate was measured by ACR in a single day first morning void sample that was collected during baseline and second 24‐hour ABPM tests. Both morning and nighttime administration of olmesartan resulted in a similar and significant reduction of the ACR compared with baseline after 8 weeks of treatment, but no difference was seen between both schemes (9.42±11.67 mg/g morning time vs 10.91±11.3 mg/g nighttime; P=.669) (Table III).
Safety and Tolerability
Throughout the study no patient developed serious side events. As well, no patient needed further reduction of study drug due to hypotension or hyperkalemia. Patients underwent routine laboratory examinations both at baseline and study termination and no clinically significant deviations were observed in main laboratory values (data not shown). One patient was missed for follow‐up after first 24‐hour ABPM, and 1 patient withdrew after second 24‐hour ABPM. Both participants were excluded from final analysis.
Discussion
In this study, nighttime administration of the angiotensin AT1 receptor blocker olmesartan produced a significant reduction of night systolic and MBP and conversely a significantly greater NBPF from baseline, compared with daytime administration. Furthermore, this greater BP reduction paralleled an increased percentage of patients with a normal dipper pattern at the end of the study, although no significant differences were seen between both arms in terms of 24‐hour average BP values. These results are in accordance with other studies previously published where nocturnal dosing of antihypertensive drugs exert a greater nocturnal fall of BP without modifying 24‐hour average BP when compared with conventional morning dosing, 4 , 8 , 9 , 21 , 22 although this is the first study performed in diabetic patients. Furthermore, other studies have shown an improvement of the nondipper pattern after night‐based chronotherapy schemes. 23 In our study, nighttime administration of olmesartan reverted to a dipper pattern in 16% of patients with a baseline nondipper pattern, while conventional morning dose was associated with an 8% increase in patients with dipper BP profile. Finally, albumin excretion, as expected, was significantly reduced in both arms compared with baseline, although no differences could be proven in terms of a greater reduction following nighttime dosing of olmesartan.
Many studies have reported that type 2 diabetic patients tend to have higher rates of nondipper hypertension. 10 , 11 , 12 Even more, it has been demonstrated that blunted nocturnal hypertension, a common finding in type 2 diabetes, increases the risk of microvascular 10 , 13 and macrovascular 11 , 12 , 14 complications in these patients. Urinary albumin excretion is a strong and independent predictor of renal disease and CV mortality, both in type II diabetic patients and in the general population. 15 , 16 A study carried out in hypertensive type II diabetic male patients showed that a blunted NBPF is associated with higher urinary albumin excretion and increased prevalence of microalbuminuria. 10
ARBs have demonstrated to reduce urinary albumin excretion beyond their antihypertensive effect and to ameliorate glomerular filtration fall rate in type II diabetic patients. 17 , 18 , 19 Olmesartan medoxomil is an angiotensin II type 1 receptor antagonist that, administered once daily, inhibits the actions of angiotensin II on the renin‐angiotensin‐aldosterone system, which plays a key role in the pathogenesis of hypertension, especially in type 2 diabetic patients. The aim of this study was to evaluate the effect of two different chronotherapeutic schemes of administration of olmesartan, a conventional morning‐based regimen vs a bedtime regimen, on both 24‐hour BP control and night to day BP ratio on a population of type 2 diabetic patients with a recent diagnosis of hypertension. Additionally, first morning urine void was collected to evaluate the impact of both different schemes on albumin excretion.
Diabetic patients usually present with nondipper hypertension. 24 Furthermore, nondipper pattern has been associated in diabetic patients with increase albumin excretion, 14 renal impairment, and thus increased mortality. 12 The reason diabetic hypertensive patients more frequently show this blunted nocturnal fall is unknown, but it has been associated with increased nocturnal sympathetic activity in patients with diabetic neuropathy 13 and with insulin resistance, a common finding in type 2 diabetic patients. 25 ARBs reduce BP through competitive antagonism of angiotensin II type 1 angiotensin receptor, exerting their effect over 24 hours due to their long half‐life, but could further reduce nocturnal BP through an effect on insulin sensitivity 26 , 27 and a reduction in the noradrenergic system. 28 In our study, heart rate was reduced significantly by both morning and night administration of the drug and, despite a slightly higher reduction seen following nocturnal dosing, it was not significant.
Limitations
A major limitation of this study is the small population. Another limitation concerns urinary albumin excretion; a wider time lapse between baseline and visit 2 might have been associated with greater differences in ACR, as we know that ARBs’ effects on albuminuria are time‐ and dose‐dependent.
Conclusions
This small study has proven differences in terms of BP control in diabetic hypertensive patients following two different chronotherapeutic schemes, with a greater reduction of SBP and MBP after nocturnal administration of olmesartan, despite no differences seen on 24‐hour BP values. Whether these differences are significant in terms of CV outcomes warrants further investigation in order to evaluate whether antihypertensive medications should be given following a chronotherapeutic‐based strategy in type 2 diabetic patients, where nondipper hypertension is a common finding that carries an increased risk of cardiovascular events.
Disclosure: This study was funded with a nonrestrictive research grant from Pfizer Laboratories N. CT25‐SPA01‐06.
References
- 1. Lemmer B. Cardiovascular chronobiology and chronopharmacology. In: Touitou Y, Haus E, eds. Biologic Rhythms in Clinical and Laboratory Medicine. Berlin. Springer‐Verlag; 1992:418–427. [Google Scholar]
- 2. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
- 4. Hermida RC, Ayala DE, Calvo C, et al. Chronotherapy of hypertension: administration‐time‐dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev. 2007;59:923–939. [DOI] [PubMed] [Google Scholar]
- 5. Hassler C, Burnier M. Circadian variations in blood pressure: implications for chronotherapeutics. Am J Cardiovasc Drugs. 2005;5:7–15. [DOI] [PubMed] [Google Scholar]
- 6. Kuroda T, Kario K, Hoshide S, et al. Effects of bedtime vs. morning administration of the long‐acting lipophilic angiotensin‐converting enzyme inhibitor trandolapril on morning blood pressure in hypertensive patients. Hypertens Res. 2004;27:15–20. [DOI] [PubMed] [Google Scholar]
- 7. Myburgh DP, Verho M, Botes JH, et al. 24‐Hour pressure control with ramipril: comparison of once‐daily morning and evening administration. Curr Ther Res. 1995; 56: 1298–1306. [Google Scholar]
- 8. Hermida RC, Ayala DE, Fernandez JR, et al. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension. 2007;50:715–722. [DOI] [PubMed] [Google Scholar]
- 9. Hermida RC, Calvo C, Ayala DE, et al. Treatment of nondipper hypertension with bedtime administration of valsartan. J Hypertens. 2005;23:1913–1922. [DOI] [PubMed] [Google Scholar]
- 10. Fogari R, Zoppi A, Malamani GD, et al. Urinary albumin excretion and nocturnal blood pressure in hypertensive patients with type II diabetes mellitus. Am J Hypertens. 1994;7:808–813. [DOI] [PubMed] [Google Scholar]
- 11. Aronson D. Impaired modulation of circadian rhythms in patients with diabetes mellitus: a risk factor for cardiac thrombotic events? Chronobiol Int. 2001;18:109–121. [DOI] [PubMed] [Google Scholar]
- 12. Sturrock ND, George E, Pound N, et al. Non‐dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000;17:360–364. [DOI] [PubMed] [Google Scholar]
- 13. Spallone V, Bernardi L, Ricordi L, et al. Relationship between the circadian rhythms of blood pressure and sympathovagal balance in diabetic autonomic neuropathy. Diabetes. 1993;42:1745–1752. [DOI] [PubMed] [Google Scholar]
- 14. Farmer CK, Goldsmith DJ, Quin JD, et al. Progression of diabetic nephropathy – is diurnal blood pressure rhythm as important as absolute blood pressure level? Nephrol Dial Transplant. 1998;13:635–639. [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 16. Klausen K, Borch‐Johnsen K, Feldt‐Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. [DOI] [PubMed] [Google Scholar]
- 17. Brenner BM, Cooper ME, De Zeeuw D, et al; RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Eng J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 18. Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Eng J Med. 2001;345:851–860. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association . Treatment of hypertension in adults with diabetes. Diabetes Care. 2003;26(suppl 1):S80–S82. [DOI] [PubMed] [Google Scholar]
- 20. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. US Department of Health and Human Services. National Institutes of Health. National Heart, Lung, and Blood Institute. NIH Publication No. 03‐5233 December 2003.
- 21. Lemmer B. The importance of circadian rhythms on drug response in hypertension and coronary heart disease from mice and man. Pharmacol Ther. 2006;111:629–651. [DOI] [PubMed] [Google Scholar]
- 22. Langner B, Lemmer B. Circadian changes in the pharmacokinetics and cardiovascular effects of oral propranolol in healthy subjects. Eur J Clin Pharmacol. 1988;33:619–624. [DOI] [PubMed] [Google Scholar]
- 23. Hermida RC, Ayala DE, Fernandez JR, et al. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension. 2008;51:69–76. [DOI] [PubMed] [Google Scholar]
- 24. Kamoi K, Miyakoshi M, Soda S, et al. Usefulness of home blood pressure measurement in the morning in type 2 diabetic patients. Diabetes Care. 2002;25:2218–2223. [DOI] [PubMed] [Google Scholar]
- 25. Fallo F, Dalla Pozza A, Sonino N, et al. Nonalcoholic fatty liver disease, adiponectin and insulin resistance in dipper and nondipper essential hypertensive patients. J Hypertens. 2008;26:2191–2197. [DOI] [PubMed] [Google Scholar]
- 26. Marshall TG, Lee RE, Marshall FE. Common angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2b. Theor Biol Med Model. 2006;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kintscher U, Foryst‐Ludwig A, Unger T. Inhibiting angiotensin type 1 receptors as a target for diabetes. Expert Opin Ther Targets. 2008;12:1257–1263. [DOI] [PubMed] [Google Scholar]
- 28. Larochelle P. Circadian variation in blood pressure: dipper or nondipper. J Clin Hypertens (Greenwich). 2002; 4(suppl 1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


