Abstract
The authors hypothesized that preeclampsia may change the phenotype of umbilical cord vessels. Segments of umbilical cords were obtained from 29 pregnant women (20 healthy and 9 with preeclampsia), which were histomorphometrically assessed. Birth weight was 2928±613 g for the control group vs 1749±656 g for the preeclampsia group (P<.0001). A significantly shorter gestational period was noted in the preeclampsia group: 35 weeks vs 39 weeks in the healthy group. Measurements of the outer layer area (116.4±55 μm2 vs 56.5±25 μm2; P=.0038), the inner layer area (63.1±16 μm2 vs 28.6±8 μm2; P<.0001), the lumen area (8.4±1 μm2 vs 3.4±2 μm2; P=.0003), and the wall/lumen ratio (20.3±9 vs 3.1±0.6; P<.0001) of arteries were significantly larger in the preeclampsia umbilical cords. Concerning veins, the wall/lumen ratio was higher in the preeclampsia group. In this study, the umbilical cord in preeclampsia showed significant changes in the structure of umbilical arteries, with increases in wall areas and wall/lumen ratios. J Clin Hypertens (Greenwich). 2011;13:30–34. ©2010 Wiley Periodicals, Inc.
Preeclampsia is a common complication of pregnancy characterized by systemic endothelial dysfunction and is usually diagnosed by the appearance of hypertension and proteinuria. 1 , 2 The association between reduced fetal growth rate, small body size at birth, and a later risk of disease are the long‐term consequences of fetal adaptive responses, 3 , 4 following the developmental‐origins hypothesis. 5 Prematurity, independent of the size at gestational age, has been associated with insulin resistance and glucose intolerance in prepuberal children 4 , 6 , 7 that may track into young adulthood and may be linked with elevated blood pressure. 8 New studies now demonstrate that lower birth weight is associated with narrower retinal arterioles, 9 narrower bifurcation angle, 10 and higher retinal vessel tortuosity, 11 providing evidence that fetal origins of cardiovascular disease may partly be mediated by the microcirculation. It remains unclear whether the association is mediated through poor fetal growth or short gestational duration. However, given the strong correlation between duration of gestation and birth weight, it is possible that the association between low birth weight and disease may be linked in part to preterm birth. The umbilical cord is the nexus between the fetus and the placenta, thus the umbilical vessel configuration may be an indicator of fetal status. The objective of this study was to analyze the structure of umbilical cord vessels in pregnant women with preeclampsia compared with healthy controls.
Methods
Segments of umbilical cords obtained at 2 cm from the placental attachment were analyzed in 29 pregnancies (gestational age varied from 29 to 41 weeks). All mothers were free from Chagas’ disease and human immunodeficiency virus. From a clinical point of view, 20 cases were considered normal pregnancies and 9 were preeclamptic.
Preeclampsia was defined as an increase in blood pressure to at least 140/90 mm Hg after the 20th week of gestation, combined with proteinuria such that protein excretion was at least 0.3 g per 24 hours. 1 , 2 , 3
Transversal sections of the umbilical cords, 5 mm in thickness, were fixed in buffered formaldehyde (pH 7.0), embedded in paraffin, serially sectioned at 4 μm to 6 μm, and stained with hematoxylin and eosin, Masson trichromic, acetic orcein for elastic fibers, periodic acid‐Schiff, Alcian blue pH 2.5, and Mallory phosphotungstic acid hematoxylin.
Whole sections of the arteries and the vein were digitalized and used for histomorphometry. The following measurements were obtained in the veins (in pixels): (1) lumen area, and (2) area of the muscular layer. In the arteries, the (1) lumen area, (2) area of the internal muscular layer, and (3) area of the external muscular layer were also assessed, using a microscope WPI Professional H602 and the software IMAGE J version 1.37 (NIH, Bethesda, MD). All parameters were adjusted by the body weight of newborns. Values were expressed as mean ± standard deviation except for gestational age or gestational period, which were expressed as median (interquartile range). All statistical analyses were processed through GRAPH PAD PRISM version 4.0 for Windows (Graph Pad Software, San Diego, CA). The significance of differences between group parameters was evaluated by Fisher exact test or Student t unpaired test as appropriate. The selected level of significance was P<.05 (two‐tailed).
Results
Maternal and fetal demographic data are shown in Table I. Mean birth weight was 2928±613 g for the control group vs 1749±656 g for the preeclampsic group (P<.0001; unpaired t test). Gestation period was significantly shortened in the preeclampsic group (35 vs 39 weeks).
Table I.
Maternal and Fetal Demographic Data
| Birth Weight, g | Men | Women | Gestation Period, wk | Maternal Age, y | |
|---|---|---|---|---|---|
| Preeclampsia | 1749±656a | 5 | 4 | 35 (4) | 28 (16) |
| Normal | 2928±613 | 10 | 10 | 39 (1) | 31 (16.5) |
a P=.0002 vs normal (unpaired t test). Birth weight is expressed as mean ± standard deviation, while gestational period and maternal age are expressed as median (interquartile range).
Umbilical cords consisted of two arteries following a helical course around a unique vein, giving the cord a twisted appearance. No adventitia, external elastic lamina, or vasa vasorum were present. Both vessel lumens were lined by endothelium. The arterial lumen was constricted with a typical irregular branched shape. The media was particularly thick, showing an inner layer of longitudinal smooth muscle cells (SMCs) and an outer coat consisting of crossing spiraled SMCs (Figure 1). The lumen of veins demonstrated preservation of the circular shape, and the intima was thinner than in the arteries (Figure 2). Medial SMCs were arranged in circumferential branched lamina consisting of 2 or 3 cells separated by blebs positive for Alcian blue and containing cell debris. Scarce groups of longitudinally or oblique‐oriented SMCs were also observed. The values of histomorphometry of umbilical vessels are detailed in Table II.
Figure 1.
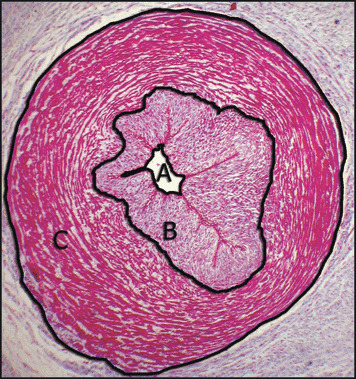
Umbilical artery: (A) lumen area, (B) inner layer area, (C) outer layer area. Masson’s trichrome ×40.
Figure 2.
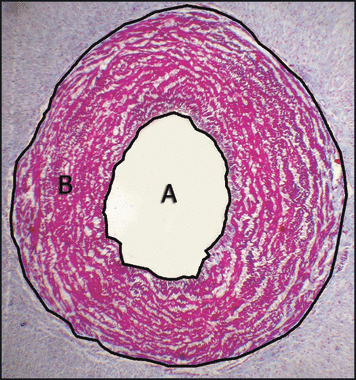
Umbilical vein: (A) lumen area and (B) wall area. Masson’s trichrome ×40.
Table II.
Histomorphometry of Umbilical Cord Vessels: Preeclampsia vs Normal Control Group
| Arteries | Veins | ||||||
|---|---|---|---|---|---|---|---|
| Outer Layer Area | Inner Layer Area | Lumen Area | Wall:Lumen Ratio | Wall Area | Lumen Area | Wall:Lumen Ratio | |
| Control | 56.6±25 | 28.6±8 | 3.4±2 | 3.1±0.6 | 109.8±35 | 36.6±18 | 2.9±1 |
| Preeclampsia | 116.4±55 | 63.1±16 | 8.4±1 | 20.3±9 | 155.9±86 | 35.9±29 | 4.8±2 |
| P Value | .0038 | <.0001 | .0003 | <.0001 | NS | NS | .0126 |
Abbreviation: NS, nonsignificant. Unpaired t test.
The outer layer area, inner layer area, lumen area, and wall/lumen ratio of arteries were significantly higher in the preeclamptic patients. In veins, wall area and the wall/lumen ratio were also higher in the preeclamptic patients, but only significantly so in the latter.
Discussion
Women with preeclampsia had a shorter gestational period and delivered newborns with lower birth weight and significant structural changes in umbilical cord vessels. Forced delivery of the preterm and low birth weight fetus is often necessitated by maternal and fetal indications, thereby shortening the gestational period. Lower birth weight may also be attributed to the same cause and, in part, to the disease itself.
Assessment of maternal disease during pregnancy as well as intrinsic fetal sickness is facilitated by umbilical cord evaluation by different methods because of its close relation to fetal evolution. 12 , 13 The imbalance between endogenous regulators of angiogenesis and compounds that modulate vascular tone in the placenta and umbilical cord can lead to pregnancy complications. Therefore, the finding of fetal growth restriction and low weight at birth in different diseases such as preeclampsia may be interpreted as a consequence of fetal adaptive responses. 14 , 15
Both epidemiologic and mechanistic studies have supported the notion that vascular and hemodynamic function are at least partially programmed in early life and that this background could play an important role in the process of vascular aging and arterial stiffening in later life. 16 , 17 Consequently, umbilical cord vessels may be useful in detecting differential phenotypes since vascular wall cells experience hormonal and hemodynamic changes during the fetal life period. These phenotypes can be studied through indirect assessment using noninvasive techniques such as pulse wave velocity and reflecting waves.
The changes in luminal areas observed in this study may be partially explained by the fact that throughout the last 2 weeks of pregnancy, the cord vessels show increasing responsiveness to mechanical irritation that is not present during the preceding periods of pregnancy. 18 The increase in thickness of the muscular layers of arteries and veins may be due to other mechanisms. Fetal responses to diseases during pregnancy or intrinsic fetal sickness may include hormone production, tissue sensitivity to these hormones, and alterations in metabolism that may affect fetal intrauterine development, leading to anatomic, physiologic, and metabolic changes. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26
Studies during the past decade have provided new insights into mechanisms that underlie the pathogenesis of preeclampsia. 19 , 20 , 21 , 22 , 23 , 24 It is accepted that placental ischemia/hypoxia induces the production of a variety of factors from the placenta that generate profound effects on the cardiovascular system. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 This dysfunction manifests itself as enhanced formation of factors such as endothelin, reactive oxygen species, and augmented vascular sensitivity to angiotensin II. Alternatively, the preeclampsia syndrome may also be evidenced by decreased formation of vasodilator agents such as nitric oxide and prostacyclin. 23 , 24 , 25 , 26 Taken together, these alterations may cause hypertension by impairing renal pressure and natriuresis, increasing total peripheral resistance, and inducing cardiac and vascular remodeling. However, the quantitative importance of the various endothelial and humoral factors that mediate vasoconstriction and elevation of arterial pressure during preeclampsia remains to be elucidated. Therefore, identifying the connection between placental ischemia/hypoxia and maternal cardiovascular abnormalities with the aim of developing therapeutic regimens remains an important area of investigation. 25 , 26
Diverse structural and functional changes occur within blood vessels during hypertension. Hypertension is generally associated with hypertrophy of the aorta and other large arteries as indicated by an increased cross‐sectional area of the vessel wall. Inward remodeling with or without increases in the cross‐sectional area is also a common finding in smaller resistance vessels. 27
Preeclampsia has been associated with changes in left ventricular structure and function 28 , 29 and, according to our results, with an increased outer layer area, the inner layer area, the lumen area, and the wall/lumen ratio of umbilical arteries. Similar results were reported by Junek and colleagues, 30 who found no changes in thickness of the umbilical veins but an increase in vessel wall thickness of umbilical arteries. The enlargement was caused by an increase of both the intima and the media. The thickening of the intima was attributed to a migration of SMCs toward the endothelium, accompanied by a splitting of the internal elastic lamina. 30 SMCs of vessels in preeclamptic pregnancies showed a metabolic activation demonstrated by highly dilated endoplasmic reticulum. These facts might represent part of the functional adaptation of umbilical cord arteries to altered hemodynamic conditions in preeclampsia.
Conclusions
Umbilical cord vessels in preeclampsia show significant structural changes, including increases in wall area and wall/lumen ratio. These vascular abnormalities may be expressions of “early vascular aging” susceptibility because the structural and mechanical properties of the large arteries can be permanently affected by altered hemodynamic stress early in life. Consequently, the analysis of umbilical cord vessels may be useful in detecting differential vascular phenotypes that may indicate predisposal to cardiovascular events later in life.
Acknowledgments and disclosures: The authors wish to acknowledge to Mrs Sarah Kantharia (SUNY‐Stony Brook) for her editorial assistance. This study received financial support from CONICET and the University of Buenos Aires, Argentina (UBACYT M047 – 2008–2010).
References
- 1. Walker JJ. Pre‐eclampsia. Lancet. 2000;356:1260–1265. [DOI] [PubMed] [Google Scholar]
- 2. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 3. Osmond C, Barker DJP, Winter PD, et al. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hales CN, Barker DJ. Type 2 (non‐insulin‐dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJP. In utero programming of chronic disease. Clin Sci (Lond). 1998;95:115–128. [PubMed] [Google Scholar]
- 6. Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–2186. [DOI] [PubMed] [Google Scholar]
- 7. Hovi P, Andersson S, Eriksson JG, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:2053–2063. [DOI] [PubMed] [Google Scholar]
- 8. Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–1315. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell P, Liew G, Rochtchina E, et al. Evidence of arteriolar narrowing in low‐birth‐weight children. Circulation. 2008;118:518–524. [DOI] [PubMed] [Google Scholar]
- 10. Chapman N, Mohamudally A, Cerutti A, et al. Retinal vascular network architecture in low‐birth‐weight men. J Hypertens. 1997;15:1449–1453. [DOI] [PubMed] [Google Scholar]
- 11. Tapp RJ, Williams C, Witt N, et al. Impact of size at birth on the microvasculature: the Avon Longitudinal Study of Parents and Children. Pediatrics. 2007;120:e1225–e1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raio L, Ghezzi F, Di Naro E, et al. Sonographic measurement of the umbilical cord and fetal anthropometric parameters. Eur J Obstet Gynecol Reprod Biol. 1999;83:131–135. [DOI] [PubMed] [Google Scholar]
- 13. De Vore GR, Mayden K, Tortora M, et al. Dilation of the fetal umbilical vein in rhesus hemolytic anemia: a predictor of severe disease. Am J Obstet Gynecol. 1881;141:464–466. [DOI] [PubMed] [Google Scholar]
- 14. Hales CN, Ozanne SE. The dangerous road of catch‐up growth. J Physiol. 2003;547:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. [DOI] [PubMed] [Google Scholar]
- 16. Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004;363:1642–1645. [DOI] [PubMed] [Google Scholar]
- 17. Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens. 2008;26:1049–1057. [DOI] [PubMed] [Google Scholar]
- 18. Shepherd JT. Bayliss response in the umbilical artery. Fed Proc. 1968;27:1408–1409. [PubMed] [Google Scholar]
- 19. Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. [DOI] [PubMed] [Google Scholar]
- 20. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 21. Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. [DOI] [PubMed] [Google Scholar]
- 22. Rana S, Karumanchi SA, Levine RJ, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–142. [DOI] [PubMed] [Google Scholar]
- 23. Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2004;70:223–232. [DOI] [PubMed] [Google Scholar]
- 24. Klockenbusch W, Goecke TW, Krüssel JS, et al. Prostacyclin deficiency and reduced fetoplacental blood flow in pregnancy‐induced hypertension and preeclampsia. Gynecol Obstet Invest. 2000;50:103–107. [DOI] [PubMed] [Google Scholar]
- 25. Taylor RN, Roberts JM. Endothelial cell dysfunction. In: Lindheimer M, Roberts JM, Cunningham FG, eds. Chelsey’s Hypertensive Disorders in Pregnancy. Norwalk, CT: Appleton & Lange; 2007: 395–429. [Google Scholar]
- 26. Gilbert JS, Ryan MJ, La Marca BB, et al. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. [DOI] [PubMed] [Google Scholar]
- 27. Schiffrin EL, Hayoz D. How to assess vascular remodelling in small and medium‐sized muscular arteries in humans. J Hypertens. 1997;15:571–584. [DOI] [PubMed] [Google Scholar]
- 28. Vazquez Blanco M, Grosso O, Bellido C, et al. Left ventricular geometry in pregnancy induced hypertension. Am J Hypertens. 2000;13:226–230. [DOI] [PubMed] [Google Scholar]
- 29. Vázquez Blanco M, Roisinblit J, Grosso O, et al. Left ventricular function impairment in pregnancy‐induced hypertension. Am J Hypertens. 2001;14:271–275. [DOI] [PubMed] [Google Scholar]
- 30. Junek T, Baum O, Läuter H, et al. Pre‐eclampsia associated alterations of the elastic fibre system in umbilical cord vessels. Anat Embryol (Berl). 2000;201:291–303. [DOI] [PubMed] [Google Scholar]


