Abstract
J Clin Hypertens (Greenwich).
There has been no report comparing the changes in home blood pressure (HBP) and target organ damage between depressive and nondepressive hypertensives receiving antihypertensive therapy based on HBP monitoring. This study was a multicenter prospective study conducted by 7 doctors at 2 institutions. The authors prospectively studied 42 hypertensive patients with home systolic blood pressure >135 mm Hg. Participants were divided into a depression group (Beck Depression Inventory score >10; n=21) and a nondepression group (Beck Depression Inventory score <9, matched for HBP level; n=21). The authors performed antihypertensive therapy to reduce home systolic blood pressure to below 135 mm Hg and, 6 months later, evaluated the urinary albumin/creatinine ratio (UACR). Although patients in the depression group tended to require the addition of a greater number of medications than those in the nondepression group (2.3±1.0 vs 1.7±1.0 drugs, P<.05), HBP was reduced similarly in both groups at 6 months (depression group: 150±17/78±11 mm Hg to 139±11/73±8 mm Hg, P<.001; nondepression group: 150±11/76±9 mm Hg to 135±9/70±8 mm Hg, P<.01). The reduction of UACR was smaller in the depression group than in the nondepression group (2.4 vs 10.1 mg/gCr, P<.05). Depressive hypertensive patients required a larger number of antihypertensive drugs to control HBP, and showed a smaller reduction in UACR than nondepressive hypertensives.
Depression is a risk factor for development of hypertension 1 and is also associated with poor prognosis of hypertensive patients. 2 Depression is associated with poor blood pressure (BP) control, which may be partly explained by poor adherence to drug regimens titrated by clinic BP. 3 The titration based on the home blood pressure (HBP) self‐measured by patients themselves may improve the adherence to antihypertensive medication. However, there has been no report comparing the changes in HBP and target organ damage between depressive and nondepressive hypertensives receiving antihypertensive therapy based on HBP monitoring.
In this study, we prospectively investigated whether there are significant differences in the changes in HBP and in measures of hypertensive target organ damage (urinary albumin/creatinine ratio (UACR); B‐type natriuretic peptide (BNP) 4 , 5 , 6 , 7 , 8 during antihypertensive therapy titrated by HBP monitoring between depressive hypertensives and nondepressive hypertensives.
Methods
Patients and Study Protocol
This study was a multicenter prospective study conducted by 7 doctors at 2 institutions. We enrolled 48 hypertensive patients with a home systolic BP ≥135 mm Hg. Patients were excluded if they had already been diagnosed with depression or were taking an antidepressant, or if they had been diagnosed with congestive heart failure. At baseline examination, a series of physical examinations was performed. Subjects were asked about their past history and lifestyle, and their depression status was determined using the Beck Depression Inventory (BDI). We prospectively studied those with a BDI score >10 (depression group: n=21) and those with a BDI score <9 (matched for home systolic BP level; nondepression group: n=21). 9 , 10 The physicians did not know the patient’s depression status, BNP level, or UACR during the study period. Physicians were asked to evaluate the patient’s HBP (average of morning and evening BP), and to attempt to reduce it to below 135 mm Hg within 6 months (the study period) using any antihypertensive drugs they considered appropriate.
Antihypertensive medications were classified as calcium channel blockers (this category included dihydropyridine calcium channel blockers as well as verapamil and diltiazem), angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, diuretics, and α‐blockers. We defined inhibitors of the renin‐angiotensin system as angiotensin‐converting enzyme inhibitors and/or angiotensin receptor blockers. UACR and BNP were measured at 6 months after the start of treatment (the end of the study). Written informed consent was obtained from all enrolled patients.
HBP Measurements
HBP was measured using a validated upper arm cuff‐oscillometric device (HEM‐5001; Omron Healthcare, Kyoto, Japan). 11 The HEM‐5001 device is equipped with BP memory for recalling measurements and produces a graph of the weekly‐averaged BP and pulse rate. 12
HBP was measured on the nondominant upper arm in the sitting position after 2 minutes of rest. In both groups, the HBP monitoring device automatically took 3 readings at 15 second intervals on each occasion, and stored the data in the monitor memory. Morning BP was measured within 1 hour after waking, after urination, and before breakfast and taking antihypertensive medication. 13 , 14 Evening BP was measured immediately before going to bed. Patients were instructed to avoid measuring BP just after taking a bath, drinking alcohol, or smoking. HBP was defined as an average of morning and evening BP over the 2 weeks immediately before visiting the physician’s office.
Clinic BP was taken in 3 readings per occasion after a rest of at least 5 minutes in a sitting position. Clinic BP was measured using the HBP monitoring device that patients brought with them to the clinic, by a physician pressing a casual BP measurement button. 12
Biochemical and Urine Examination
Blood samples and spot samples of urine were collected in the morning in a fasting state. Blood and urine examination were performed at the enrollment and after 6 months of treatment. The BNP level was measured using a radioimmunoassay (Shionogi Inc., Osaka, Japan). The urinary microalbumin level was measured using the immunoturbidimetric method (Mitsubishi Kagaku Iatron Inc., Tokyo, Japan). Urine creatinine was measured by Jaffe reaction without deproteinization and then quantified by a photometric method. The ratio of the urinary albumin level to the urinary creatinine level was calculated as the UACR. The estimated glomerular filtration rate was calculated by using the Japanese Society of Nephrology‐Chronic Kidney Disease Initiatives coefficient. 15
Statistical Analysis
All data were expressed as the mean ± standard deviation or a percentage. BNP and UACR were presented as the median value together with the 25th and 75th percentiles (25%, 75%), and log‐transformed before statistical analysis. An unpaired t‐test was used to compare HBP and the increase of medication between the depression group and nondepression group. A Mann‐Whitney U test was used to compare the change of UACR between the depression group and nondepression group. All statistical analyses were performed using the computer software package SPSS version 11.0J (SPSS Inc., Chicago, IL). A P value <.05 was considered statistically significant.
Results
Baseline characteristics are shown in the Table. The mean ± standard deviation age was 72±9 years and 38% of participants were men. The 2 groups were similar in terms of clinic/home BP, demographic characteristics, antihypertensive drug use before enrollment, and prevalence of coexisting cardiovascular conditions, but BNP in the depression group was higher than that in the nondepression group (37.7 vs 23.3 pg/mL, P <.05). HBP was reduced in both groups at 6 months (depression group: 150±17/78±11 mm Hg to 139±11/73 ±8mm Hg, P <.001; nondepression group: 150±11/76±9 mm Hg to 135±9/70±8 mm Hg, P <.01; Figure). The reduction of home/clinic BP was not significantly different between the 2 groups at the end of the study, although the depression group required the addition of a greater number of medications than the nondepression group (2.3±1.0 vs 1.7±1.0 drugs, P <.05; Figure). Significantly more inhibitors of the renin‐angiotensin system were added in the depression group than in the nondepression group (86% vs 38%, P <.01).
Table.
Baseline Characteristics
| Depression n=21 | Nondepression n=21 | P Value | |
|---|---|---|---|
| Age, y | 73.9±9.2 | 70.1±8.7 | NS |
| Male, % | 38 | 38 | NS |
| Body mass index, kg/m2 | 23.8±3.2 | 24.2±3.0 | NS |
| Current drinking, No. (%) | 6 (29) | 6 (29) | NS |
| Current smoking, No. (%) | 5 (24) | 2 (10) | NS |
| Duration of hypertension, y | 9.0 (7.0–17.5) | 10.0 (3.0–16.5) | NS |
| Duration of hypertensive therapy, y | 7.0 (3.0–14.5) | 7.0 (0.5–15.0) | NS |
| Diabetes, No. (%) | 5 (24) | 2 (10) | NS |
| Hyperlipidemia, No. (%) | 6 (29) | 6 (29) | NS |
| ARB, No. (%) | 6 (29) | 5 (24) | NS |
| ACE inhibitor, No. (%) | 8 (38) | 14 (67) | NS |
| Calcium channel blocker, No. (%) | 18 (86) | 17 (81) | NS |
| β‐Blocker, No. (%) | 4 (19) | 1 (5) | NS |
| Diuretics, No. (%) | 5 (24) | 2 (10) | NS |
| α‐Blocker, No. (%) | 4 (19) | 3 (14) | NS |
| Brain natriuretic peptide, pg/mL | 37.7 (22.1–60.1) | 23.3 (16.9–31.1) | <.05 |
| Serum creatinine, mg/dL | 0.7 ± 0.2 | 0.8 ± 0.2 | NS |
| Estimated GFR, mL/min/1.73 m2 | 91.5±21.9 | 79.7±14.6 | NS |
| Urinary albumin/creatinine ratio, mg/gCr | 30.9 (10.1–143.2) | 25.4 (11.5–83.7) | NS |
| Clinic systolic blood pressure, mm Hg | 164±27 | 162±14 | NS |
| Clinic diastolic blood pressure, mm Hg | 84±13 | 82±12 | NS |
| Clinic pulse rate, bpm | 76±11 | 77±15 | NS |
| Home systolic blood pressure, mm Hg | 150±17 | 150±11 | NS |
| Home diastolic blood pressure, mm Hg | 78±11 | 76±9 | NS |
| Home pulse rate, bpm | 70±8 | 70±10 | NS |
Data are shown as the No. (percentage) or mean ± standard deviation. Duration of hypertension, duration of hypertensive therapy, brain natriuretic peptide and urinary albumin ratio are the median values (25% value–75% value).
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; bpm, beats per minute; GFR, glomerular filtration rate; NS, not significant.
Figure.
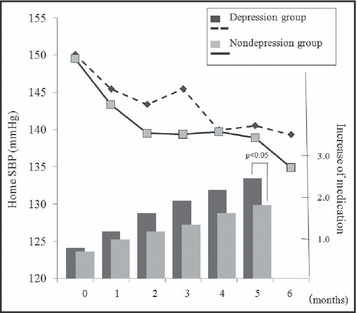
Home systolic blood pressure (BP) and increase of medication over 6 months.
The reduction of UACR in the nondepression group was greater than that in the depression group (10.1 vs 2.4 mg/gCr, P <.05). Estimated glomerular filtration rate was not changed in either group during this study (the depression group: 91.5 to 89.0 mg/gCr, P = not significant; nondepression group: 79.7 to 78.8 mg/gCr, P = not significant). BNP was higher in the depression group than in the nondepression group at baseline (37.7 vs 23.3 pg/mL, P <.05), and this difference between the depression group and nondepression group was similar at 6 months (40.5 vs 24.1 pg/mL, P <.05).
In the overall group (combined depression and nondepression groups, n=42), the BDI score was not associated with either log BNP at the baseline or UACR response.
Discussion
In this prospective study of antihypertensive therapy titrated by HBP monitoring, we demonstrated that depressive hypertensives required a greater number of additional antihypertensive drugs in order to achieve a similar level of HBP control. The reduction of UACR was smaller in the depression group than in the nondepression group.
In this study, depressive hypertensives required a greater number of antihypertensive drugs in order to control HBP to <135 mm Hg systolic. This result may partly be explained by poor adherence in depressive patients. Depression has been reported to be associated with poor adherence to treatment, 3 and poor adherence is an important cause of resistant hypertension. 14 , 16 , 17 In addition, it has been reported that physicians’ attitudes to antihypertensive therapy can contribute to inadequate BP control, 18 and thus physicians with a more proactive attitude to improving adherence might be needed to treat depressive hypertensives.
However, at 6 months after the start of medication, the reduction in HBP as well as that in clinic BP were comparable between the depression and nondepression groups. This suggests that poor adherence could not completely explain our findings. A previous study reported that increases in depression score (BDI) were significantly associated with greater 24‐hour urinary norepinephrine, 19 suggesting that increased neurohumoral activation and advanced target organ damage in depressive patients may partly contribute to the difficulty of BP control.
This possibility may be supported by the finding that the reduction of UACR was smaller in the depression group than in the nondepression group, even though renin‐angiotensin system inhibitors were more frequently used in the depression group than in the nondepression group. In addition to the increased neurohumoral activation, increased inflammation may contribute to poor UACR response. Depressive patients are reported to have increased levels of inflammatory markers. 20
BNP was higher in the depression group than in the nondepression group at baseline. It has been shown that depressive symptoms are associated with a higher BNP level in patients with heart failure. 21 Although the relationship between depression and BNP has never been demonstrated in hypertensive patients, the higher BNP level found in the depression group may have been due to the advanced hypertensive cardiac remodeling. The increased use of renin‐angiotensin inhibitors in the depression group might have been related to the advanced hypertensive cardiac remodeling.
There were several important limitations in this study. First, this study was not a randomized study and antihypertensive drugs were not added in a standardized fashion, while the doctors who titrated the antihypertensive medications were blinded to the depression score. Second, we did not objectively evaluate adherence by an objective method such as electronic medication monitoring. 22
Conclusions
In depressive hypertensive patients, a greater number of antihypertensive drugs was required to control HBP, and the reduction of urinary albumin excretion was smaller than that in nondepressive patients. Further studies will be needed to clarify the characteristics of the BP lowering and target organ protection conferred by antihypertensive therapy in depressive hypertensive patients.
Disclosure:
The authors declare no conflict of interest.
References
- 1. Davidson K, Jonas BS, Dixon KE, et al. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Arch Intern Med. 2000;160:1495–1500. [DOI] [PubMed] [Google Scholar]
- 2. Wassertheil‐Smoller S, Applegate WB, Berge K, et al. Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (Systolic Hypertension in the elderly). Arch Intern Med. 1996;156:553–561. [PubMed] [Google Scholar]
- 3. Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120:26–32. [DOI] [PubMed] [Google Scholar]
- 4. Jensen JS, Feldt‐Rasmussen B, Strandgaard S, et al. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35:898–903. [DOI] [PubMed] [Google Scholar]
- 5. De Leeuw PW, Ruilope LM, Palmer CR, et al. Clinical significance of renal function in hypertensive patients at high risk: results from the INSIGHT trial. Arch Intern Med. 2004;164:2459–2464. [DOI] [PubMed] [Google Scholar]
- 6. Olsen MH, Wachtell K, Tuxen C, et al. N‐terminal pro‐brain natriuretic peptide predicts cardiovascular events in patients with hypertension and left ventricular hypertrophy: a LIFE study. J Hypertens. 2004;22:1597–1604. [DOI] [PubMed] [Google Scholar]
- 7. Kario K, Matsui Y, Shibasaki S, et al. An alpha‐adrenergic blocker titrated by self‐measured blood pressure recordings lowered blood pressure and microalbuminuria in patients with morning hypertension: the Japan Morning Surge‐1 Study. J Hypertens. 2008;26:1257–1265. [DOI] [PubMed] [Google Scholar]
- 8. Fogari R, Derosa G, Zoppi A, et al. Effect of telmisartan‐amlodipine combination at different doses on urinary albumin excretion in hypertensive diabetic patients with microalbuminuria. Am J Hypertens. 2007;20:417–422. [DOI] [PubMed] [Google Scholar]
- 9. Furlanetto LM, Mendlowicz MV, Romildo Bueno J. The validity of the Beck Depression Inventory‐Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86:87–91. [DOI] [PubMed] [Google Scholar]
- 10. Thombs BD, De Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–2171. [DOI] [PubMed] [Google Scholar]
- 11. Anwar YA, Giacco S, McCabe EJ, et al. Evaluation of the efficacy of the Omron HEM‐737 IntelliSense device for use on adults according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1998;3:261–265. [PubMed] [Google Scholar]
- 12. Kabutoya T, Ishikawa J, Hoshide S, et al. A home blood pressure monitor equipped with a graphic function facilitates faster blood pressure control than the conventional home blood pressure monitor. J Clin Hypertens (Greenwich). 2009;11:422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imai Y, Otsuka K, Kawano Y, et al. Japanese society of hypertension (JSH) guidelines for self‐monitoring of blood pressure at home. Hypertens Res. 2003;26:771–782. [DOI] [PubMed] [Google Scholar]
- 14. Japanese Society of Hypertension . Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2004). Hypertens Res. 2006;29(suppl):S1–S105. [DOI] [PubMed] [Google Scholar]
- 15. Imai E, Horio M, Nitta K, et al. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50:927–937. [DOI] [PubMed] [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, De Backer G, Dominiczak A, et al; for The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 18. Ono A, Fujita T. Factors relating to inadequate control of blood pressure in hypertensive outpatients. Hypertens Res. 2003;26:219–224. [DOI] [PubMed] [Google Scholar]
- 19. Grewen KM, Girdler SS, Hinderliter A, et al. Depressive symptoms are related to higher ambulatory blood pressure in people with a family history of hypertension. Psychosom Med. 2004;66:9–16. [DOI] [PubMed] [Google Scholar]
- 20. Ranjit N, Diez‐Roux AV, Shea S, et al. Psychosocial factors and inflammation in the multi‐ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–181. [DOI] [PubMed] [Google Scholar]
- 21. Parissis JT, Nikolaou M, Farmakis D, et al. Clinical and prognostic implications of self‐rating depression scales and plasma B‐type natriuretic peptide in hospitalised patients with chronic heart failure. Heart. 2008;94:585–589. [DOI] [PubMed] [Google Scholar]
- 22. Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol. 2006;48:2218–2222. [DOI] [PubMed] [Google Scholar]


