Abstract
J Clin Hypertens (Greenwich). 2010;12:117–124. ©2009 Wiley Periodicals, Inc.
The role of increased sympathetic nervous system (SNS) activity in the pathogenesis of obesity hypertension and insulin resistance is controversial. Eight dogs were instrumented and fed a high‐fat diet (HFD) for 6 weeks. Dogs were evaluated for changes in weight, blood pressure, insulin resistance, and norepinephrine (NE) kinetics using a two‐compartment model. The HFD resulted in weight gain, hypertension, and insulin resistance. During the 6 weeks of the HFD, although plasma NE concentration trended toward increasing (P=.09), SNS, assessed by NE kinetic studies, significantly increased (P=.009). Within 1 week of starting the HFD, NE release into the extravascular compartment (NE2) increased from 3.44±0.59 μg/mL to 4.87±0.80 μg/mL (P<.01) and this increase was maintained over the next 5 weeks of the HFD (NE2 at week 6 was 4.66±0.97 μg/mL). In addition to the increased NE2 there was also a significant increase in NE clearance (P=.04). There were significant correlations between the increase in NE2 and both the development of insulin resistance and hypertension. This study supports the hypothesis that activation of the SNS plays a pivotal role in the metabolic and hemodynamic changes that occur with weight gain induced by HFD.
The sympathetic nervous system (SNS) plays an important role in metabolic homeostasis. Landsberg and Young 1 reported that short‐term overfeeding of rats activated the SNS. These and other observations led Landsberg 2 to hypothesize that the increase in SNS activity with weight gain serves the homeostatic role of stimulating thermogenesis to prevent further weight gain. In an attempt to test this hypothesis there has been considerable controversy regarding the effects of obesity on SNS activation. Data from a review of more than 40 different studies suggests that whole body SNS activity, measured by plasma norepinephrine (NE) concentration, urinary NE excretion, and systemic NE spillover rate into the plasma, is either the same in lean and obese persons or increases with increasing obesity. 3 Masuo and colleagues 4 , 5 reported that plasma NE concentrations not only increased following weight gain in Japanese men but plasma NE also appeared to predict subsequent weight gain and blood pressure (BP) elevation. While Masuo’s findings from an observational, cross‐sectional study suggest that increases in plasma NE concentrations occur with modest weight gain, plasma NE concentrations are of limited value as a measure of sympathetic neural activity because they are influenced by the sampling site, as well as by NE appearance and clearance from the circulation. 6
Clinical measurements of NE release from sympathetic nerves to plasma and clinical measurements of rates of sympathetic nerve firing by clinical neurography provide the best methods for assessing sympathetic nervous function in humans. 7 Using isotope dilution methodology, regional heterogeneity in SNS activity has been demonstrated in obesity. Coppack and colleagues 8 measured whole body and regional (subcutaneous abdominal adipose tissue and forearm) NE kinetics in lean and obese women. Coppack found that although mean systemic NE spillover tended to be higher in obese than in lean persons, the differences were not statistically significant, whereas adipose tissue and forearm NE spillover rates were greater in lean than in obese persons. Similarly, Vaz and colleagues 9 demonstrated that whole body plasma NE spillover was unrelated to body mass index, whereas renal NE spillover was significantly correlated with body mass index and cardiac NE spillover was lower in obese compared with lean persons.
Microneurography has also been used to directly measure sympathetic neural activity in obese individuals. Most studies that measured muscle sympathetic nerve activity have demonstrated higher nerve activity in obese compared with lean individuals. 10 , 11 , 12 , 13 However, Huggett and colleagues 14 reported that muscle sympathetic nerve activity was nearly identical in normal weight and overweight men, whereas obese diabetic men had 50% higher muscle sympathetic nerve activity.
One of the major limitations of all of these studies relating whole body SNS activation to obesity is that they have been cross‐sectional. There have been few studies that have longitudinally evaluated SNS activity in the same individual prior to and after the development of obesity. Katzeff and colleagues 15 measured the thermogenic and hormonal responses to NE during overnutrition and undernutrition in 6 lean and 6 overweight male volunteers. Although these investigators demonstrated that overfeeding did not increase the thermogenetic responses to NE, they did demonstrate that both lean and obese individuals did increase their plasma NE concentration with overfeeding. Gentile and colleagues 16 demonstrated that overfeeding 12 nonobese male volunteers resulted in an increase in muscle sympathetic neural activity and that the change in muscle sympathetic nerve activity burst frequency was correlated with the percent increase in body weight, change in body fat, and percent change in body fat. Gentile did not measure the change in NE kinetics with overfeeding. In the current study, we serially measured NE kinetic parameters before and after 1 and 6 weeks of feeding dogs a high‐fat diet in order to test the hypothesis that weight gain results in altered whole body NE kinetics.
Methods
Eight adult mongrel dogs (5 males and 3 females) were trained to stand quietly in a padded sling. All dogs were then surgically instrumented with an ascending aortic catheter and 2 right atrial catheters. Surgical instrumentation of the animals was performed under methohexital induction (12 mg/kg) and isoflurane (0.5–1.5%). After surgery, the dogs were allowed to recover for 3 weeks before baseline measurements were made. Dogs received a regular diet (a diet that maintained weight constant), 1 to 2 cans of dog food (Ken‐L‐Ration; H. J. Heinz Company, Pittsburgh, PA), for 2 weeks followed by 6 weeks of a high‐fat diet consisting of approximately 0.8 kg of cooked beef fat in addition to their regular diet. 17
In addition, all dogs received vitamin supplements (VAL syrup; Fort Dodge Laboratories, Fort Dodge, IA) and antibiotics throughout the entire study. The dogs were housed in air‐conditioned cages and were fed between the hours of 1 pm and 3 pm each day. BP, heart rate, and body weight were measured daily. Plasma glucose and insulin were measured twice a week during the entire study. NE kinetics were measured before and after 1 and 6 weeks of the high‐fat diet in 7 of 8 dogs. In the other dog, the 1‐week post‐fat measurements could not be made due to catheter malfunction. All measurements were made between 8 am and 11 am and before the daily feeding (the dogs having not been fed since 5 pm the previous day). The animals were housed and cared for and all procedures in the study were in accordance with National Institutes of Health guidelines in addition to University of Michigan guidelines on animal experimentation.
Laboratory Measurements
Arterial pressure was measured daily with a pressure transducer mounted at the level of the heart. BP signals were recorded and the analog signals were sent to a computer to be analyzed. The computer calculated the average systolic, diastolic, and mean BP and heart rate (over a 15‐ to 30‐minute period while the dog was resting quietly).
Insulin resistance was assessed by a single insulin dose of 2 mU/(kg·min) by euglycemic hyperinsulinemic clamp. The euglycemic hyperinsulinemic clamp was performed in all dogs before starting the high‐fat diet and at 1 (day 8 or 9) and 6 weeks (day 43 or 44) of the high‐fat diet. 18
NE kinetics was measured on the day before the euglycemic clamp using 3H‐NE. On the days that NE kinetics was measured, the dogs were placed in the padded sling for 30 minutes before starting the measurements. 3H‐NE (specific activity 40 to 60 Ci/mmol; Perkin‐Elmer, Norwalk, CT) was given into the right atrial catheter as a continuous infusion for 60 minutes at a rate of 0.013 μCi/(min·kg). Arterial plasma samples were collected at 40, 50, and 60 minutes during 3H‐NE infusion for measurement of steady‐state 3H‐NE and plasma NE concentrations. The 3H‐NE infusion was then stopped and blood samples were obtained at 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 minutes after discontinuing the infusion. The zero time value for analysis of the disappearance curves was taken as the mean of the 40‐, 50‐, and 60‐minute samples.
Analytic Methods
Blood for serum glucose determination was drawn, put in untreated polypropylene tubes, and centrifuged using an Eppendorf microcentrifuge (Brinkman Instruments, Westbury, NY). The glucose concentration of the supernatant was then measured in duplicate by the glucose oxidase method using a glucose analyzer (model A23; Yellow Springs Instruments, Yellow Spring, OH). Serum insulin was measured by double‐antibody radioimmunoassay (ICN Biomedicals, Inc, Costa Mesa, CA). Plasma electrolytes were measured by flame photometry.
Blood samples for NE kinetics were collected into chilled plastic tubes containing ethyleneglycotetraacetic acid (EGTA) and reduced glutathione and immediately placed on ice. They were promptly centrifuged at 4°C and the plasma was stored at −70°C. Plasma NE and epinephrine levels were measured using single isotope assay, with all samples from a given dog analyzed in the same assay. 19 3H‐NE concentrations were determined by liquid scintillation counting of radiolabeled catecholamines after alumina extraction. 20
Compartmental analysis of NE kinetics was performed using the two‐compartment model as described by Linares and colleagues. 21 Mathematic modeling was performed using the simulation, analysis, and modeling (SAAM29) and conversational simulation, analysis, and modeling (CONSAM) programs. 22 , 23 The modeling involves determining a simultaneous fit of all the plasma NE and 3H‐NE data by solving differential equations for both the endogenous tracee (plasma NE) and tracer (3H‐NE) system by the method of weighted nonlinear least squares. Parameters that are estimated by SAAM29 are the fractional transfer coefficients (L ij ), the volume of distribution of NE in compartment 1 (VD1, L), and the steady‐state input rate into compartment 2 (NE2, μg/min). Additional measures include the mass of NE in each compartment, the flux rates of NE between compartments, and the metabolic clearance rate of NE from plasma‐containing compartment. Calculation of the rate of NE appearance into plasma and the rate of NE clearance from plasma was by the isotope dilution technique. 24
Statistical Analysis
All values are mean ± standard error. Weekly BPs, heart rates, and body weights were determined by averaging the daily values for each week. Plasma glucose and insulin were determined by averaging the two values that were obtained each week. Since we have previously demonstrated in dogs fed a high‐fat diet that hepatic glucose output was completely suppressed at an insulin infusion rate of >1 mU/(kg/min), the amount of glucose required to maintain euglycemia during the last 30 minutes of each insulin infusion was used as the index of whole‐body glucose uptake. 18 In an attempt to measure whole‐body glucose uptake independent of the degree of obesity, some investigators have indexed whole‐body glucose uptake to lean body mass as opposed to total body weight. 25 In the current study, since we did not measure lean body mass in our dogs because we have previously demonstrated that within 3 weeks of stopping the fat diet the dogs returned to their pre‐fat diet weight, 26 and because the dogs were fully mature and did not change their height or length during the 6 weeks of the high‐fat diet, we felt that the pre‐fat diet weight was a reasonable surrogate for lean body mass. Therefore, we expressed whole‐body glucose as glucose per kilogram of pre‐fat diet weight per minute (MPRF).
A mixed model repeated‐measures analysis of variance was performed for each variable to determine whether a significant change in the variable occurred over time. A paired t test was used to determine whether significant differences were present between the 3 time periods (before and after 1 and 6 weeks of the high‐fat diet). Due to the multiple comparisons made in these paired t tests, a Bonferroni correction was made such that a P<.025 rather than a P<.05 was used to document a significant difference between the time periods.
Linear regression analysis was used to evaluate whether a relation existed between sympathetic activation and the development of insulin resistance and hypertension.
All of the statistical analyses were performed using SAS software programs (SAS Institute, Cary, NC).
Results
Hemodynamic and Hormonal Results
During the 6 weeks of the high‐fat diet, the dogs significantly increased their body weight from 22.4±1.5 to 29.8±0.7 kg (P<.001) (Table I). The gain in weight was associated with a significant increase in mean arterial BP (P<.001) and heart rate (P<.001). Plasma glucose concentration did not significantly increase with the high‐fat diet. However, there was a significant increase in fasting insulin concentration (78.1±3.1 pmol/L to 219±7.1 pmol/L; P<.001). Plasma epinephrine concentration significantly decreased from 230.1±24.8 pg/mL to 14.1±28.4 pg/mL (P<.02) during the 6 weeks of the fat diet.
Table I.
Hemodynamic, Hormonal, and Euglycemic Clamp Changes Associated With 6 Weeks of a High‐Fat Diet
| Week 0 | Week 1 | Week 6 | ||
|---|---|---|---|---|
| Dogs | 8 | 7 | 8 | P Value |
| Weight, kg | 22.4±0.5 (range 5.1) | 25.8±.6b (range 4.8) | 29.8±0.7c,e (range 7) | <.0001 |
| Mean arterial blood pressure, mm Hg | 89.6±2.4 (range 15.8) | 94.6±2.8a (range 20.4) | 106.4±4.1c,e (range 4.1) | .02 |
| Heart rate, beats per min | 94.2±1.9 (range 10) | 107.1±3.5b (range 14) | 114.5±3.5c,e (range 16) | <.0001 |
| Fasting insulin, pmol/L | 78.1±3.1 (range 27.7) | 112.8±2.2b (range 20.8) | 219.6±7.1c,e (range 55.6) | <.0001 |
| Glucose, mmol/L | 5.3±0.4 (range 0.6) | 5.2±0.4 (range 1.1) | 5.4±0.5 (range 1.1) | .652 |
| MPRF, mg glucose, kg of pre‐fat per wt/min | 11.4±0.2 (range 2.3) | 7.16±0.42b (range 3.21) | 4.02±0.29c,e (range 2.37) | <.0001 |
| Epinephrine, pg/mL | 230.1±24.8 (range 182) | 185.3±40 (range 276) | 147.1±28.4d (range 245) | .02 |
Values are expressed as mean ± standard error. Abbreviations: MPRF,whole body glucose uptake during a euglycemic clamp (indexed to pre‐fat diet weight); Week 0, before starting the high‐fat diet; Week 1, after 1 week of high‐fat diet; Week 6, after 6 weeks of the high‐fat diet. aSignificant difference P<.05 week 1 vs week 0. bSignificant difference P<.01 week 1 vs week 0. cSignificant difference P<.01 week 6 vs week 0. dSignificant difference P<.05 week 6 vs week 0. eSignificant difference P<.01 week 6 vs week 1.
Euglycemic Clamp Data
During the euglycemic clamp studies, the steady state blood glucose concentration averaged approximately5.3 mm and did not differ from the fasting concentration prior to the insulin infusion. The coefficient of variation of glucose level at the insulin plateau was <5%. Feeding the dogs the high‐fat diet was associated with a significant reduction in insulin‐mediated glucose uptake (P<.001) (Table I). The reduction in insulin‐mediated glucose uptake (MPRF) was apparent within the first week of starting the high‐fat diet.
NE Kinetics
During the 6 weeks of the high‐fat diet, the dogs tended to increase their plasma NE concentration from 241±16 pg/mL to 285±27 pg/mL (P=.094). We used a two‐compartment model to evaluate the kinetics of distribution and metabolism of NE before and during the development of weight gain. 21 , Table II summarizes the changes in NE kinetics that occurred when the dogs were fed a high‐fat diet. Within 1 week of starting the fat diet, NE release into the extravascular compartment (NE2) increased from 3.44±0.59 μg/mL to 4.87±0.80 μg/mL and this increase was maintained over the next 5 weeks of the high‐fat diet (NE2 at week 6 was 4.66±0.97 μg/mL). We also observed that within 1 week of starting the fat diet that NE metabolic clearance rate (MRC1) also increased from 1.58±0.11 mL/min to 2.49±0.51 mL/min and this increase was also maintained during the next 5 weeks of the fat diet. The increase in MRC1 was associated with a significant increase in the volume of distribution of NE in the intravascular compartment (VD1) (VD1 increased from 1.37±0.27 L to 2.41±0.57 L [P=.02] after 6 weeks of the high‐fat diet). With weight gain, there was a small increase in the mass of NE in compartment 1 (Q1) and no significant increase in the mass of NE in compartment 2 (Q2). There was over 80% more NE mass exchanged between Q1 and Q2 after 6 weeks of the high‐fat diet (rate of NE appearance into the intravascular compartment [R12] 0.426±0.047 μg/min to 0.785±0.112 μg/min; P=.036). There were no significant differences in NE spillover faction (SF%) or in the fraction transport rate coefficients (L ij ).
Table II.
Changes in Norepinephrine Kinetic Variables Associated With 6 Weeks of a High‐Fat Diet
| Week 0 | Week 1 | Week 6 | ||
|---|---|---|---|---|
| Dogs | 8 | 7 | 8 | P Value |
| NE, pg/mL | 241±16 (range 154) | 254±33 (range 224) | 285±27 (range 225) | .09 |
| NECl, L/min | 1.36±0.12 (range 1.01) | 2.39±0.56 (range 4.31) | 2.52±.39a (range 2.93) | .039 |
| NEAP, μg/min | 0.396±.033 (range .307) | 0.704±.167 (range 1.279) | 0.682±.080b (range .693) | .021 |
| NE2, μg/min | 3.44±0.59 (range 4.97) | 4.87±0.80c (range 6.74) | 4.66±0.97a (range 7.73) | .009 |
| Q1, μg | 0.378±.07 (range 0.60) | 0.492±0.066 (range 0.537) | 0.539±0.07a (range 0.496) | .031 |
| Q2, μg | 103.3±20.8 (range 165.8) | 147±32.6 (range 224.9) | 107.2±17.9 (range 146.3) | .138 |
| R12, μg/min | 0.426±.047 (range .389) | 0.840±.253 (range 1.909) | 0.785±.112b (range 1.038) | .036 |
| MRC1, mL/min | 1.580±.106 (range 0.925) | 2.49±.506 (range 3.929) | 2.351±.294a (range 2.130) | .04 |
| VD1, L | 1.37±.27 (range 1.95) | 2.26±.57 (range 4.12) | 2.41±.57a (range 4.05) | .02 |
Values are expressed as mean ± standard error. Abbreviations: MRC1, norepinephrine clearance rate from the intravascular compartment; NE, plasma norepinephrine; NE2, norepinephrine release into the extravascular compartment; NEAP, noncompartmental norepinephrine appearance rate in plasma; NECl, noncompartmental norepinephrine clearance form plasma; Q1, norepinephrine mass in the intravascular compartment; Q2, norepinephrine mass in the extravascular compartment; R12, rate of norepinephrine appearance into the intravascular compartment; VD1, volume of distribution of norepinephrine in the intravascular compartment; Week 0, before starting the high‐fat diet; week 1, after 1 week of high‐fat diet; Week 6, after 6 weeks of the high‐fat diet. aSignificant difference P<.05 week 6 vs week 0. bSignificant difference P<.01 week 6 vs week 0. cSignificant difference P<.01 week 1 vs week 0.
Calculations based on the isotope dilution method demonstrated that both noncompartmental NE appearance rate into plasma (NEAP) (P=.021) and noncompartmental NE clearance from the plasma (NECL) (P=.039) increased with the high‐fat diet (Table II). R12 is the parameter of the two‐compartment model that is most analogous to NEAP, and tended to be similar in magnitude to NEAP both before and after the high‐fat diet. NEAP was much less than NE2 both before and after the high‐fat diet. NECL and MRC1 were similar in magnitude both before and after the high‐fat diet.
We also observed a significant correlation between the increase in NE2 (ΔNE2) and both the increase in whole‐body insulin‐mediated glucose uptake (ΔMPRF) and the increase in mean arterial pressure (ΔBP) (1, 2). However, unlike the relationship between ΔNE2 and ΔMPRF, which was present after both 1 and 6 weeks of receiving the high‐fat diet, the ΔBP was significantly related to the increase in NE2 only after the dogs had been on the high‐fat diet for 6 weeks.
Figure 1.
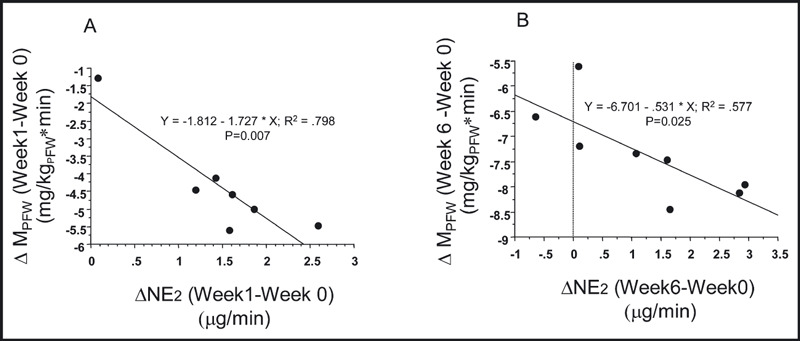
(A) The regression relationship between the change in NE release into the extravascular compartment (ΔNE2) after 1 week of the high fat diet and the change in insulin mediated glucose uptake (Δ MPRF) in mg glucose per kg of pre‐fat body weight per min. The data from the seven animals that had week 1 measurements made are shown. (B) The regression relationship between the change in NE release into the extravascular compartment (ΔNE2) after 6 weeks of the high fat diet and the change in insulin mediated [2 mU/(kg min)] glucose uptake (Δ MPRF) in mg glucose per kg of pre‐fat body weight per min. The data from all eight animals that had week six measurements made are shown.
Figure 2.
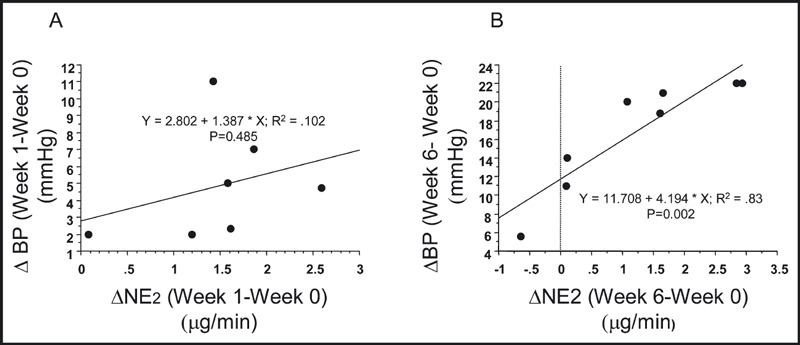
(A) The regression relationship between the change in NE release into the extravascular compartment (ΔNE2) after 1 week of the high fat diet and the change in mean arterial pressure (ΔBP). The data from the seven animals that had week 1 measurements made are shown. (B) The regression relationship between the change in NE release into the extravascular compartment (ΔNE2) after 6 weeks of the high fat diet and the change in mean arterial pressure (ΔBP). The data from all eight animals that had week six measurements made are shown.
Discussion
The results from the current study provide evidence to support the hypothesis that weight gain induced by feeding dogs a high‐fat diet is associated with altered whole‐body NE kinetics. In addition, these results also suggest that the rate of NE release into the extravascular compartment correlates with both the development of insulin resistance and hypertension in the high fat–fed dog model of obesity.
The role of enhanced sympathetic activity in the pathogenesis of obesity hypertension and insulin resistance remains controversial. A significant component of this controversy is related both to the fact that few studies have serially followed changes in sympathetic activity in the same animal or individual as they gain weight and to limitations in the methods used to assess sympathetic activity.
There have been few studies that have longitudinally evaluated SNS activity prior to and after the development of obesity. Katzeff and colleagues 15 measured the thermogenic and hormonal responses to NE during overnutrition and undernutrition in 6 lean and 6 overweight male volunteers. These investigators demonstrated that both lean and obese individuals increased their plasma NE concentration with overfeeding. Gentile and colleagues 16 demonstrated that overfeeding 12 nonobese male volunteers resulted in an increase in muscle sympathetic neural activity and that the change in muscle sympathetic nerve activity burst frequency correlated with the percent increase in body weight, change in body fat, and percent change in body fat. Gentile did not measure the change in NE kinetics with overfeeding.
Plasma NE levels provide an indirect assessment of systemic sympathetic activity, since they reflect the net balance between NE appearance and removal mechanisms and provide no information concerning what happens to NE after it is released from presynaptic sympathetic nerve terminals. NE kinetic studies using isotope dilution methods were developed to estimate systemic rates of NE appearance into and NE clearance from the plasma. Using this methodology, Coppack and colleagues 8 and Eikelis and colleagues 27 found that compared with lean individuals, obese individuals had slightly higher spillover rates but the differences were not significant. In addition, Bazelmans and colleagues 28 found marginally lower systemic NE spillover rates in obese compared with lean subjects, whereas one other study 29 found a direct positive correlation between adiposity and systemic NE spillover. In the current study we demonstrated during 6 weeks of feeding dogs a high‐fat diet that, although plasma NE concentration trended towards increasing (P=.09), systemic sympathetic activity assessed by NE kinetic studies significantly increased (P=.009). Using both a single compartment model and a minimal two‐compartment model 21 we demonstrated that within 1 week of starting the high‐fat diet, there was a significant increase in both NEAP (single‐compartment model) and NE2 (two‐compartment model) and that this increase was maintained during the next 5 weeks of the fat diet. The most likely reason that we did not demonstrate a significant increase in plasma NE levels was because, in addition to the increased rate of NE release from the sympathetic nerve terminals, we also observed a significant increase in NE clearance (NECL [single‐compartment model] and MCR1 [two‐compartment model]). Compartmental analysis demonstrates that the increase in MCR1 could be explained in large part by the increase in VD1. It is unlikely that the increase in NE clearance was primarily due to a change in uptake1 or uptake2, since we observed only small changes in the fractional transport rate coefficients (L ij ) of the two‐compartment model. 21 Since the VD1 for NE is known to be somewhat larger than plasma volume but much less than total extracellular fluid volume, 21 it is not unexpected that VD1 would increase in the high fat–fed dogs since we and others have demonstrated that this model is associated with significant fluid retention. 26 , 30 , 31 The increase in VD1 and the associated increase in clearance of epinephrine is also the most likely explanation for the decrease in plasma epinephrine concentration that was observed during the 6 weeks of the high‐fat diet (Table 1). In addition, the decrease in plasma epinephrine concentration with the fat diet makes it unlikely that increased adrenal release of catecholamines was the cause of the increase in both NEAP and NE2 that occurred with feeding the dogs the high‐fat diet.
The results from this present study also help to explain the conflicting results from the numerous cross‐sectional studies evaluating the differences in whole‐body SNS activity in lean and obese patients. As can be seen in Table II, we observed marked variability in NE kinetics between the 8 animals that were studied. It was only with our longitudinal design that we were able to document a significant increase in whole animal NE release.
The other important finding from the current study was the association between sympathetic activation and the development of insulin resistance and hypertension. In the high fat–fed dog, we previously reported that insulin resistance occurs within 1 week of starting the high‐fat diet. 18 In the current study, we observed both at 1 and 6 weeks of the high‐fat diet a significant correlation between the increase in NE2 and the decrease in MPRF (Figure 1). This current finding, along with our previous findings that clonidine prevents the insulin resistance associated with dogs fed a high‐fat diet 32 , 33 and the recent report of Chazova and colleagues 34 demonstrating that moxonidine improves glycemic control in overweight individuals, are consistent with but do not prove our hypothesis that increased sympathetic activity is potentially important in the pathogenesis of insulin resistance in the high fat–fed model of obesity.
Unlike insulin resistance, the increase in BP that occurs with the high‐fat diet did not correlate to the increase in sympathetic activity until the animals had received 6 weeks of the diet (Figure 2). This observation is also consistent with our knowledge of BP regulation in this animal model of obesity. We and others have demonstrated that the hypertension associated with feeding dogs a high‐fat diet is due to chronic sodium retention. 26 , 30 We also know that the SNS is involved in the hypertension based on both pharmacologic studies 32 , 33 as well as the studies of Kassab and colleagues, 31 which demonstrated that obesity‐induced hypertension could be prevented by renal sympathetic denervation. In addition, Vaz and colleagues, 9 using regional analysis of NE kinetics, demonstrated increased renal NE spillover in obese patients. Since the renal sympathetic–mediated fluid retention takes weeks to fully develop, it is not surprising that the significant relation between the increase in NE2 and increase in mean arterial pressure did not become evident until week 6 of the high‐fat diet.
Conclusions
We observed that when dogs are fed a high‐fat diet there is both a significant increase in NE release from sympathetic nerve terminals and an increase in the metabolic clearance of NE from the plasma. In addition, this increase in sympathetic activity correlates with both the development of insulin resistance and hypertension.
Disclosures: Research was supported in part by grant P2P01HL18575‐24, K07 AG028403 and K24 AG00924 from the National Institutes of Health and the VA Ann Arbor and Salt Lake City GRECC programs.
References
- 1. Landsberg L, Young JB. Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. 1978;298:1295–1301. [DOI] [PubMed] [Google Scholar]
- 2. Landsberg L. Diet, obesity and hypertension: a hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. QJM. 1986;61:1081–1090. [PubMed] [Google Scholar]
- 3. Young JB, MacDonald IA. Sympathoadrenal activity in human obesity: heterogeneity of findings since 1980. Int J Obes. 1992;16:959–967. [PubMed] [Google Scholar]
- 4. Masuo K, Kawaguchi H, Mikami H, et al. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. [DOI] [PubMed] [Google Scholar]
- 5. Masuo K, Mikami H, Ogihara T, et al. Weight gain –induced blood pressure elevation. Hypertension. 2000;35:1135–1140. [DOI] [PubMed] [Google Scholar]
- 6. Esler M, Jennings B, Lambert G, et al. Overflow of catecholamine neurotransmitters to the circulation: source, fate and functions. Physiol Rev. 1990;70:936–985. [DOI] [PubMed] [Google Scholar]
- 7. Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. [DOI] [PubMed] [Google Scholar]
- 8. Coppack SW, Horowitz JF, Paramore DS, et al. Whole body, adipose tissue and forearm norepinephrine kinetics in lean and obese women. Am J Physiol Endocrinol Metab. 1998;275:E830–E834. [DOI] [PubMed] [Google Scholar]
- 9. Vaz M, Jennings G, Turner A, et al. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. [DOI] [PubMed] [Google Scholar]
- 10. Alvarez GE, Beske SD, Ballard TP, et al. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. [DOI] [PubMed] [Google Scholar]
- 11. Grassi G, Seravalle G, Dell’Oro R, et al. Adrenergic and reflex abnormalities in obesity‐related hypertension. Hypertension. 2000;36:538–542. [DOI] [PubMed] [Google Scholar]
- 12. Gudbjornsdottir S, Lonnroth P, Sverrisdottir Y, et al. Sympathetic nerve activity and insulin in obese normotensive and hypertensive men. Hypertension. 1996;27:276–280. [DOI] [PubMed] [Google Scholar]
- 13. Spraul M, Anderson EA, Bogardus C, et al. Muscle sympathetic nerve activity in response to glucose ingestion. Impact of plasma insulin and body fat. Diabetes. 1994;43:191–196. [DOI] [PubMed] [Google Scholar]
- 14. Huggett RJ, Scott EM, Gilbey SG, et al. Disparity of autonomic control in type 2 diabetes mellitus. Diabetolgia. 2005;48:172–179. [DOI] [PubMed] [Google Scholar]
- 15. Katzeff HL, O’Connell M, Horton ES, et al. Metabolic studies in human obesity during overnutrition and undernutrition: thermogenic and hormonal responses to norepinephrine. Metabolism. 1986;35:166–175. [DOI] [PubMed] [Google Scholar]
- 16. Gentile CL, Orr JS, Davy BM, et al. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007; 292: R1834–R1838. [DOI] [PubMed] [Google Scholar]
- 17. Rocchini AP, Moorehead CS, DeRemer S, et al. Pathogenesis of weight related changes in pressure in the dog. Hypertension. 1989;13:922–928. [DOI] [PubMed] [Google Scholar]
- 18. Rocchini AP, Marker P, Cervenka T. Time course of insulin resistance associated with feeding dogs a high fat diet. Am J Physiol Endocrinol Metab. 1997;272:E147–E154. [DOI] [PubMed] [Google Scholar]
- 19. Evans MI, Halter JB, Porte D. Comparison of double‐ and single‐ isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem. 1978;24:567–570. [PubMed] [Google Scholar]
- 20. Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide‐trihydroxyindole procedure for analysis of catecholamines. J Pharmacol Exp Ther. 1962;138:360–375. [PubMed] [Google Scholar]
- 21. Linares OA, Jacquez JA, Zech LA, et al. Norepinephrine metabolism in humans: kinetic analysis and model. J Clin Invest. 1987;80:1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berman MW, Beltz F, Grief PC, et al. CONSAAM User’s Guide. No. 1983‐421:3279. Washington, DC: United States Government Printing Office; 1984. [Google Scholar]
- 23. Berman MW, Weiss MF. SAAM Manual. National institutes of Health Publication No. 78‐180. Washington, DC: United States Department of Health, Education, and Welfare; 1978. [Google Scholar]
- 24. Esler M. Assessment of sympathetic nervous system function in humans from noradrenaline plasma kinetics. Clin Sci (Lond). 1982;62:247–254. [DOI] [PubMed] [Google Scholar]
- 25. Sinaiko AR, Jacobs DR, Steinberger J, et al. Insulin resistance syndrome in childhood: association of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139:700–707. [DOI] [PubMed] [Google Scholar]
- 26. Rocchini AP, Moorehead C, Wentz E, et al. Obesity‐induced hypertension in the dog. Hypertension. 1987;9 (suppl 3):III 64–68. [DOI] [PubMed] [Google Scholar]
- 27. Eikelis N, Lambert G, Wiesner G, et al. Extra‐adipocyte Leptin release in human obesity and its relation to sympathoadrenal function. Am J Physiol Endocrinol Metab. 2004;286:E744–E752. [DOI] [PubMed] [Google Scholar]
- 28. Bazelmans JP, Nestel K, O’Dea K, et al. Blunted norepinephrine responsiveness to changing energy states in obese subjects. Metabolism. 1985;34:154–160. [DOI] [PubMed] [Google Scholar]
- 29. Schwartz R, Jaeger L, Veith R. The importance of body composition to the increase in palama norepinephrine appearance rate in elderly men. J Gerontol. 1987;42:546–551. [DOI] [PubMed] [Google Scholar]
- 30. Hall JE, Brands MW, Dixon WN, et al. Obesity‐induced hypertension: renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. [DOI] [PubMed] [Google Scholar]
- 31. Kassab S, Kato T, Wilkins C, et al. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. [DOI] [PubMed] [Google Scholar]
- 32. Rocchini AP, Mao HZ, Babu K, et al. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension. 1999;33:548–553. [DOI] [PubMed] [Google Scholar]
- 33. Rocchini AP, Yang Q, Gokee A. Hypertension and insulin resistance are not directly related in obese dogs. Hypertension. 2004;43:1011–1016. [DOI] [PubMed] [Google Scholar]
- 34. Chazova I, Almazov VA, Shlyakhto E. Moxonidine improves glycaemic control in mildly hypertensive, overweight patients: a comparison with metformin. Diabetes Obes Metab. 2006;8:456–465. [DOI] [PubMed] [Google Scholar]


