Abstract
J Clin Hypertens (Greenwich). 2010;12:451–455. ©2010 Wiley Periodicals, Inc.
S‐allyl‐mercapto‐captopril (CPSSA) is a conjugate of captopril with allicin, an active principle in garlic with multiple beneficial actions on metabolic syndrome abnormalities, including weight preservation, observed by the authors in fructose‐induced hypertensive hyperinsulinemic rats and in Koletsky rats. The aim of the study was to examine blood pressure (BP) and glucose levels in the Cohen‐Rosenthal Diabetic Hypertensive (CRDH) model as well as to follow their weight preservation. CRDH rats (n=14) were fed the sugar‐rich copper‐free diet essential for the development of diabetes mellitus. Two months later BP and blood glucose levels were measured. CPSSA was diluted in drinking water and administered at a final dose of 53.5 mg/kg/d (n=8). Control rats (n=6) received no drug (vehicle group). In contrast to control group, CPSSA prevented progressive weight gain, without a detectable effect on food and water intake. CPSSA was effective in attenuating systolic and diastolic BP (P<0.01) as well as significantly reducing glucose levels (P<0.01). Control rats continued to gain weight, whereas the groups fed CPSSA did not. CPSSA was shown to have additional beneficial effects on improving BP and glucose level, as well as preserving weight gain. The authors conclude that the combined molecule CPSSA integrates the antihypertensive feature of both allicin and captopril, making it a potential antidiabetic and cardiovascular protective agent.
The garlic plant (Allium sativum) has been used for generations as a source of home remedies for a variety of human ailments. Numerous studies documenting garlic’s cardioprotective properties 1 , 2 , 3 , 4 , 5 and antimicrobial 6 ‐antiparasitic 7 effects have been reported. Allicin was first described over 65 years ago by Cavallito 8 as one of the major biologically active molecules of garlic. It is produced following the mechanical injury of a clove by the reaction of the enzyme alliinase (E.C.4.4.1.4), which is present in micro compartments in the cloves, and the inert, nonprotein amino acid, alliin [(+)S‐allyl‐cysteine sulfoxide], which is stored in a different microcompartment. 9 Crushing a clove breaks down the compartmentalization and brings the enzyme and its substrate into contact, leading to the production of allicin (diallyl‐dithiosulfinate). 10 The allicin that is released contributes to the defense of the garlic plant from soil microorganisms. 11 Using a novel method to produce pure allicin, 12 we have demonstrated in a number of animal models of human disease that allicin is the molecule responsible for several of the medicinal properties that were historically attributed to garlic. In hyperlipidemic rabbits there was a beneficial effect on serum lipid profile. 13 Investigations with pure allicin in the Reaven animal model of metabolic syndrome showed that daily oral intake of allicin reduced high blood pressure (BP), triglyceride and insulin levels, 14 and also prevented weight gain in the animals. 15 Daily oral administration of allicin was also shown to reduce formation of fatty streaks (atherosclerosis) in hyperlipidemic mice, a phenomenon that appeared not to occur due to an alteration in blood lipid profile. 16 , 17 Allicin was also found to upregulate cellular glutathione in vascular endothelial cells. 18 Allicin has been shown by others to attenuate tissue injury and to inhibit apoptosis. 19 , 20 Some of the problems of using pure allicin as a remedy are the following; (1) allicin is a molecule that is difficult to mass produce in high purity; (2) it is responsible for the typical pungent odor of garlic; and (3) it has a rather short shelf life and its activity decays at room temperature due to its chemical reactivity. In our quest to stabilize the allicin molecule, we found that it was possible to prepare a chemical derivative by reacting it with captopril, which was the first orally active angiotensin‐converting enzyme inhibitor and is still included in leading clinical trials. 21 Captopril is used not only as a hypotensive agent during emergency but as an ordinary drug in newborns and young infants having heart failure secondary to left‐to‐right shunt 22 and as a protector against ischemic stress. 23 , 24
The beneficial effects of captopril are due to its sulphydryl group which has demonstrated antioxidant properties improving endothelial dysfunction. 25 The product of the reaction between these two well known molecules yielded allyl‐mercapto‐captopril (CPSSA), a nonsymmetric disulfide compound that is chemically stable and relatively easy to purify. 26 The allylmercapto moiety of allicin and the reaction products of allicin with cysteine or glutathion (allyl‐mercapto‐cysteine and allyl‐mercapto‐glutathion) were shown to be biologically active. 27
We have demonstrated in a number of studies 26 , 28 , 29 that CPSSA combines the properties of both compounds: it acted as a thiol reactive reagent and had positive effects on BP, serum triglycerides, and insulin concentration in two well established animal models of metabolic syndrome, the Reaven Sprague Dawley fructose fed rats 26 , 28 and the spontaneously hypertensive, obese rat (SHROB) model. 29
Since one of the more negative aspects of metabolic syndrome is the diabetic state, we decided to confirm the effects of CPSSA in the Cohen‐Rosenthal Diabetic Hypertensive (CRDH) rat model. 30 , 31 The rats in this model reach high levels of BP and blood glucose. Our aim, therefore, was to see if daily oral intake of CPSSA would ameliorate the diabetic condition in these rats.
Materials and Methods
Preparation of Alliin, Allicin, and CPSSA
Alliin (S‐allyl‐cysteine‐sulfoxide) was synthesized from l‐cysteine and allyl‐bromide after oxidation with hydrogen peroxide by the procedure of Stoll and Seebeck. 32 Pure allicin was prepared by applying synthetic alliin onto a column containing immobilized garlic alliinase enzyme as previously described. 12
Captopril was purchased from Sigma (St. Louis, MO). CPSSA was synthesized by reacting captopril with pure allicin as described. 26 The chemical structure of CPSSA was confirmed by nuclear magnetic resonance and mass spectrometry.
Experimental Design
CRDH rats are the outcome of inbreeding the offspring of spontaneous hypertensive rats’ (SHR) and Cohen diabetic rats’ (CDR) siblings for many generations. Male CRDH rats at the age of 6–8 weeks were fed a sugar‐rich copper‐free diet containing 72% sucrose, 18% vitamin‐free casein (MP Biomedicals, LLC, Solon, OH), 5% salt mixture USP No. II (MP Biomedicals, LLC), 5% butter, 0.5% corn oil, and 0.23% water soluble vitamins, which is essential for the development of non–insulin dependent diabetes type II. 30 , 31 At 4–5 months of age CRDH rats (n=14) weighing 300–330 g were tested for BP prior to treatment and nearly every 2 weeks post treatment, using a noninvasive tail‐cuff instrument (BP‐2000 series II; Visitech Systems, Apex, NC). Blood glucose levels were determined by a glucometer device (Bayer Ltd., Leverkusen, Germany) every 10 days. The drug CPSSA was dissolved in the aqueous solution at a final dose of 53.5 mg/kg/d (equivalent to 40 mg/kg/d of captopril) and administered to 8 of the 14 rats for 60 days. The control CRDH rats received no drug (vehicle group). Procedures were conducted in accordance with the guidelines for animal studies and approved by the Institutional Animal Care and Use Committee. Rats were maintained on a 14 hour light/10 hour dark cycle in a room with a constant temperature of 23°C and humidity of approximately 50%.
Results
Both systolic and diastolic BP declined significantly (P<0.01) following treatment with CPSSA from starting levels 151/124±5/6 to 140/111±4/4 mm Hg on day 15 and further 121/96±4/5 mm Hg on day 30 of treatment (Figure 1). Control group systolic and diastolic BPs remained unchanged; 149/121±3/5 to 153/133±3/5 mm Hg, prior to and on day 30 of treatment, respectively. In contrast to the control group, the daily oral administration of CPSSA induced a decrease in glucose from an initial level of 317±37 to 225±17 mg/dL on day 30 post treatment. After nearly 2 months of treatment, glucose levels were reduced to 183±9 on day 55 compared to baseline as well as to the vehicle group (P<0.05, Figure 2). Treatment with CPSSA also prevented progressive weight gain with no detectable effect on food intake or other vital signs (Figure 3). The CPSSA‐treated group exhibited a decline (not significant) in weight, whereas the vehicle group showed a slight increase.
Figure 1.
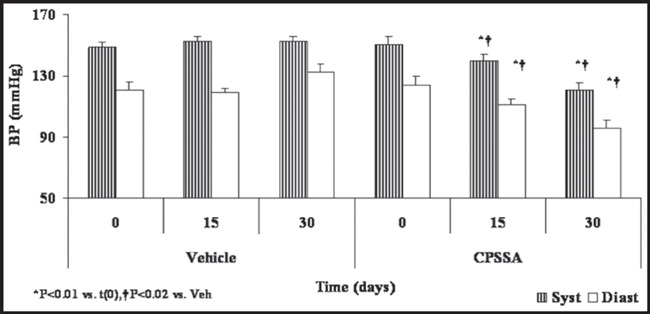
Blood pressure (BP) prior to and during treatment in both control (Veh) and S‐allyl‐mercapto‐captopril (CPSSA) treated groups. Data presented as mean ± standard error of the mean. Syst indicates systolic BP; Diast, diastolic BP.
Figure 2.
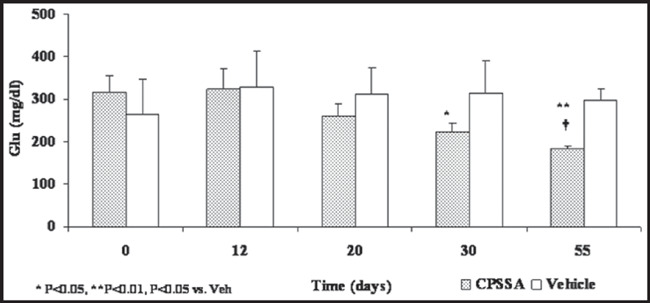
Blood glucose levels (Glu) prior to and during treatment in both control (Vehicle) and S‐allyl‐mercapto‐captopril (CPSSA) treated groups. Data presented as mean ± standard error of the mean.
Figure 3.
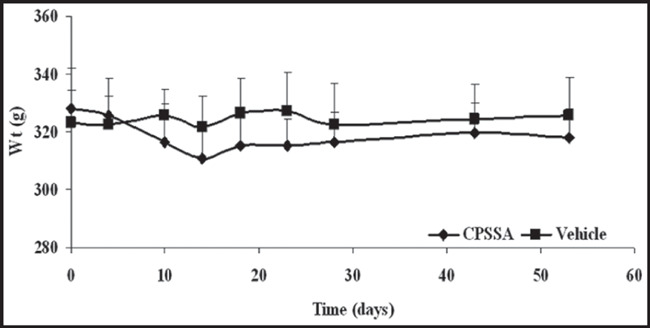
Animals’ weight (Wt) prior to and during treatment in both control (Vehicle) and S‐allyl‐mercapto‐captopril (CPSSA) treated groups. Data presented as mean ± standard error of the mean.
Discussion
Hypertension and type II diabetes frequently coexist 33 , 34 and this combination accelerates complications of each pathology which, by itself, is a major independent risk factor for cardiovascular disease. The CRDH rat model represents a unique model of combined genetic diabetes and hypertension. 30 , 31 This strain was developed after crossbreeding CDR‐sensitive substrain and SHR. Starting from the original CDR and SHR, CRDH rats were selected, and pairs displaying the highest spontaneous blood glucose and systolic BP levels were mated. At the 28th generation, noninsulin‐dependent diabetes mellitus and hypertension were evident. These rats have been extensively used in our studies of diabetes and hypertension.
Our present results in this animal model show that the long‐term administration of a daily oral dose of CPSSA induced an impressive decrease in glucose as well as in BP and prevented body weight gain compared to the vehicle group. These results are in agreement with our previous findings in the fructose‐induced hypertensive and hyperinsulinemic rats (Reaven’s model), 26 , 28 and in the Koletsky SHROB rat model of obesity. 29 In a comparative study between CPSSA and captopril, performed by Ernsberger and our group, 29 BP in CPSSA‐treated rats was found to be lower than in those treated with captopril. While glucose levels following an oral load, and insulin secretion in response to oral glucose resulted in low levels of glucose and insulin during CPSSA treatment, the above mentioned levels during captopril treatment were higher. Captopril produced a significant increase in area under the curve compared to both control and CPSSA groups, while rats treated with CPSSA showed a nearly two‐fold drop in area under the curve compared to control SHROB rats. 29
In all the experiments the prevention of weight gain was seen only in the CPSSA treated groups. This is an important observation in view of the health factors that go beyond esthetics. These include the well established relationship between excess body weight, hypertension, and type II diabetes 35 and the broad consensus that obesity is one of the biggest risk factors of the cardiometabolic syndrome. 36
Obesity is a common and serious disorder that requires appropriate management, with equal importance given to both weight reduction and weight maintenance. It is well known that weight loss is difficult to achieve, however, maintaining the weight loss is an even greater challenge. 37
Although there is a general perception that almost no one succeeds in long‐term maintenance of weight loss, research has shown that approximately 20% of overweight individuals succeed in maintaining the loss for at least 1 year. 38 Our results in experiments with 3 different models of metabolic syndrome have clearly demonstrated that the new molecule, CPSSA, integrates the antihypertensive and diabetic reducing effects as well as the induction of weight preservation. CPSSA in essence combines the pharmacologically beneficial properties of both allicin and captopril, and our results show that CPSSA was more potent than the equivalent dose of captopril that can be dissolved in water. The novel compound, CPSSA, thus combines the beneficial properties of allicin—the lowering of glucose and prevention of weight increase—with those of captopril—one of the first converting enzyme inhibitors that is still being used in patients to this day. 21 , 22 , 23 , 24 An additional advantage is that both compounds have been administered to humans for many years. Recently it was reported that when captopril was given to rats, this angiotensin‐converting enzyme inhibitor protected against the development of diet‐induced obesity and glucose intolerance. 39 The effects of CPSSA on several indicators of metabolic syndrome should encourage its testing in well controlled human clinical trials, where it may turn out to be a useful agent for reducing cardiovascular risk. CPSSA is a promising option that merits a place high on the list of antimetabolic drugs. The combination of pharmacological and nonpharmacological approach should be attractive to many patients, and increase compliance.
Conclusions
Hypertension and insulin resistance continue to be a fertile area of research that will undoubtedly lead to further treatment options for these detrimental comorbid conditions. 40 Our results show that administration of CPSSA improved BP and glucose levels and prevented weight gain in the CRDH rat model. CPSSA combines the beneficial properties of the two molecules allicin and captopril, making it a potential antidiabetic and cardiovascular protective agent for the treatment of metabolic syndrome. Preventing weight gain highlights another important advantage of this compound.
Disclosures: This work is supported by grants to the authors from Boxenbaum Neta Fund (0125‐1373)
References
- 1. Augusti KT. Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J Exp Biol. 1996;34(7):634–640. [PubMed] [Google Scholar]
- 2. Ashraf R, Aamir K, Shaikh AR, et al. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J Ayub Med Coll Abbottabad. 2005;17(3):60–64. [PubMed] [Google Scholar]
- 3. Rahman K. Effects of garlic on platelet biochemistry and physiology. Mol Nutr Food Res. 2007;51(11):1335–1344. [DOI] [PubMed] [Google Scholar]
- 4. Reinhart KM, Coleman CI, Teevan C, et al. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta‐analysis. Ann Pharmacother. 2008;42(12):1766–1771. [DOI] [PubMed] [Google Scholar]
- 5. Hiyasat B, Sabha D, Grotzinger K, et al. Antiplatelet activity of Allium ursinum and Allium sativum . Pharmacology. 2009;83(4):197–204. [DOI] [PubMed] [Google Scholar]
- 6. Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Review article: Research in Infectious Diseases. Microbes and Infection. 1999;1(2):125–129. [DOI] [PubMed] [Google Scholar]
- 7. Ankri S, Miron T, Rabinkov A, et al. Allicin from garlic strongly inhibits cysteine proteinases and cytopathic effects of Entamoeba histolytica . Antimicrob Agents Chemother. 1997;41:2286–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavallito CJ, Bailey JH. Allicin the antibacterial principle of Allium sativum. I. Isolation physical properties and antibacterial action. J Am Chem Soc. 1944;66:1950–1951. [DOI] [PubMed] [Google Scholar]
- 9. Miron T, Rabinkov A, Mirelman D, et al. A spectrophotometric assay for allicin and alliinase (Alliin lyase) activity: reaction of 2‐ nitro‐5‐thiobenzoate with thiosulfinates. Analytical Biochem. 1998;265:317–325. [DOI] [PubMed] [Google Scholar]
- 10. Rabinkov A, Miron T, Konstantinovski L, et al. The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochim Biophys Acta. 1998;1379:233–244. [DOI] [PubMed] [Google Scholar]
- 11. Schadkchan Y, Shemesh E, Mirelman D, et al. Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro and in a murine model of disseminated aspergillosis. J Antimicrob Chemother. 2004;53:832–836. [DOI] [PubMed] [Google Scholar]
- 12. Miron T, Sivaraman H, Rabinkov A, et al. A method for continuous production of allicin using immobilized alliinase. Anal Biochem. 2006;351:152–154. [DOI] [PubMed] [Google Scholar]
- 13. Eilat S, Oestraicher Y, Rabinkov D, et al. Alteration of lipid profile in hyperlipidemic rabbits by allicin, an active constituent of garlic. Coron Artery Dis. 1995;6:985–990. [PubMed] [Google Scholar]
- 14. Elkayam A, Mirelman D, Peleg E, et al. The effects of allicin and enalapril in fructose‐induced hyperinsulinemic hyperlipidemic hypertensive rats. Am J Hypertens. 2001;14:377–381. [DOI] [PubMed] [Google Scholar]
- 15. Elkayam A, Mirelman D, Peleg E, et al. The effects of allicin on weight in fructose‐induced hyperinsulinemic, hyperlipidemic, hypertensive rats. Am J Hypertens. 2003;16:1053–1056. [DOI] [PubMed] [Google Scholar]
- 16. Abramovitz D, Gavri S, Harats D, et al. Allicin‐induced decrease in formation of fatty streaks (atherosclerosis) in mice fed a cholesterol‐rich diet. Coron Artery Dis. 1999;10:515–519. [DOI] [PubMed] [Google Scholar]
- 17. Gonen A, Harats D, Rabinkov A, et al. The anti‐atherogenic effect of allicin: possible mode of action. Pathobiology. 2005;72:325–334. [DOI] [PubMed] [Google Scholar]
- 18. Horev‐Azaria L, Eliav S, Izigov N, et al. Allicin up‐regulates cellular glutathione level in vascular endothelial cells. Eur J Nutr. 2009;48(2):67–74. [DOI] [PubMed] [Google Scholar]
- 19. Cho SJ, Rhee DK, Pyo S. Allicin, a major component of garlic, inhibits apoptosis of macrophage in a depleted nutritional state. Nutrition. 2006;12:1177–1184. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Yao HP, Huang FF, et al. Allicin, a major component of garlic, inhibits apoptosis in vital organs in rats with trauma/hemorrhagic shock. Crit Care Med. 2008;36(12):3226–3232. [DOI] [PubMed] [Google Scholar]
- 21. Califf RM, Lokhnygina Y, Velazquez EJ, et al. Usefulness of beta blockers in high‐risk patients after myocardial infarction in conjunction with captopril and/or valsartan (from the VALsartan In Acute Myocardial Infarction [VALIANT] trial). Am J Cardiol. 2009;104:151–157. [DOI] [PubMed] [Google Scholar]
- 22. Shaw NJ, Wilson N, Dickinson DF. Captopril in heart failure secondary to a left to right shunt. Arch Dis Child. 1988;63:360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barbagallo M, Dominguez LJ, Resnick LM. Protective effects of captopril against ischemic stress: role of cellular Mg. Hypertension. 1999;34:958–963. [DOI] [PubMed] [Google Scholar]
- 24. Tokmakova MP, Skali H, Kenchaiah S, et al. Chronic kidney disease, cardiovascular risk, and response to angiotensin‐converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation. 2004;110:3667–3673. [DOI] [PubMed] [Google Scholar]
- 25. Liu YH, Liu LY, Wu JX, et al. Comparison of captopril and enalapril to study the role of the sulfhydryl‐group in improvement of endothelial dysfunction with ACE inhibitors in high dieted methionine mice. J Cardiovasc Pharmacol. 2006;47:82–88. [DOI] [PubMed] [Google Scholar]
- 26. Miron T, Rabinkov A, Peleg E, et al. Allylmercaptocaptopril: a new antihypertensive drug. Am J Hypertens. 2004;17:71–73. [DOI] [PubMed] [Google Scholar]
- 27. Rabinkov A, Miron T, Mirelman D, et al. S‐Allylmercaptoglutathione: the reaction product of allicin with glutathione possesses SH‐modifying and antioxidant properties. Biochim Biophys Acta. 2000;1499:144–153. [DOI] [PubMed] [Google Scholar]
- 28. Oron‐Herman M, Rosenthal T, Mirelman D, et al. The effects of S‐allylmercaptocaptopril, the synthetic product of allicin and captopril, on cardiovascular risk factors associated with the metabolic syndrome. Atherosclerosis. 2005;183(2):238–243. [DOI] [PubMed] [Google Scholar]
- 29. Ernsberger P, Johnson JL, Rosenthal T, et al. Therapeutic actions of allylmercaptocaptopril and captopril in a rat model of metabolic syndrome. Am J Hypertens. 2007;20:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen AM, Rosenmann E, Rosenthal T. The Cohen diabetic (non‐insulin‐dependent) hypertensive rat model: description of the model and pathologic findings. Am J Hypertens. 1993;6:989–995. [DOI] [PubMed] [Google Scholar]
- 31. Amenta F, Peleg E, Tomassoni D, et al. Effect of treatment with lercanidipine on heart of Cohen‐Rosenthal diabetic hypertensive rats. Hypertension. 2003;41(6):1330–1335. [DOI] [PubMed] [Google Scholar]
- 32. Stoll M, Seebeck E. Allium compounds V. The synthesis of natural alliin and its three optical isomers. Helv Chim Acta. 1951;34:481–487. [Google Scholar]
- 33. Ganne S, Arora SK, Dotsenko O, et al. Hypertension in people with diabetes and the metabolic syndrome: pathophysiologic insights and therapeutic update. Curr Diab Rep. 2007;7:208–217. [DOI] [PubMed] [Google Scholar]
- 34. Bakris G, Stockert J, Molitch M, et al. Risk factor assessment for new onset diabetes: literature review. Diabetes Obes Metab. 2009;11:177–187. [DOI] [PubMed] [Google Scholar]
- 35. El‐Atat F, Aneja A, Mcfarlane S, et al. Obesity and hypertension. Endocrinol Metab Clin North Am. 2003;32:823–854. [DOI] [PubMed] [Google Scholar]
- 36. Frohlich ED. Obesity: a central risk and cardiovascular target of the cardiometabolic syndrome. J Cardiometab Syndr. 2008;3:147–148. [DOI] [PubMed] [Google Scholar]
- 37. Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. [DOI] [PubMed] [Google Scholar]
- 38. Wing RR, Phelan S. Long‐term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. [DOI] [PubMed] [Google Scholar]
- 39. De Kloet AD, Krause EG, Kim DH, et al. The effect of angiotensin‐converting enzyme (ACE) inhibition using captopril on energy balance and glucose homeostasis. Endocrinology. 2009;150:4114–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Addison S, Stas S, Hayden MR, et al. Insulin resistance and blood pressure. Curr Hypertens Rep. 2008;10:319–325. [DOI] [PubMed] [Google Scholar]


