Abstract
This report updates concepts on hypertension management in patients with diabetes. It focuses on clinical outcomes literature published within the last 3 years and incorporates these observations into modifications of established guidelines. While the fundamentals of treatment and goal blood pressures remain unchanged, approaches to specific patient‐related issues has changed. This update focuses on questions such as what to do when a patient has an elevated potassium level when therapy is initiated and whether combinations of agents that block the renin‐angiotensin system still be used. In addition, there are updates from trials, just published and in press, that focus on related management issues influencing cardiovascular outcomes in persons with diabetes. Last, an updated algorithm is provided that incorporates many of the new findings and is suggested as a starting point to achieve blood pressure goals.
This review provides the reader with an update on treatment of hypertension in patients with diabetes as reviewed by the American Society of Hypertension. Hypertension, which affects more than 70 million Americans, is the most prevalent risk factor for development of cardiovascular and kidney disease. 1 , 2 The prevalence of hypertension is estimated at about 30% of the adult population in developed countries and is predicted to increase by almost 60% in the next 2 decades. 3 , 4 Diabetes is a major risk factor for cardiovascular disease and the most common cause of kidney failure in the Western world. 1 , 5 Moreover, cardiovascular mortality and morbidity is increased substantially in the presence of diabetes. 6
More than 75% of adults with diabetes have blood pressure (BP) levels ≥130/80 mm Hg or are using antihypertensive medication. 1 In the natural history of type 1 diabetes, development of an elevated BP (ie, >130/80 mm Hg) is a major predictor of nephropathy and future declines in kidney function. 1 , 7 In contrast, hypertension is already evident in most patients with type 2 diabetes at the time of diagnosis. The implications of hypertension on cardiovascular risk, however, are similar in both types of diabetes. 1 , 8 Mortality is increased 7.2‐fold when hypertension is present in patients with diabetes. 1
Since the publication of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), several important observations regarding BP management and glycemic control in patients with diabetes are now apparent. First, post hoc analyses of 2 different cardiovascular outcome trials note that even though diuretics worsen glycemic control, cardiovascular event rates were not higher. 9 , 10 Specifically, a post hoc analysis of the Systolic Hypertension in the Elderly Program (SHEP) notes that worsening of glycemic control with diuretics did not result in a reduced long‐term benefit of thiazide‐type diuretic (chlorthalidone)–induced lowering of systolic pressures on cardiovascular risk. 10 In addition, an analysis of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) subgroup with diabetes failed to show a higher cardiovascular event rate in the diuretic group even though they had the greatest worsening of glycemic control. 9 Note, however, that these trials do not answer the question fully, as these were post hoc analyses and patients were followed only over a limited period of time. Thus, the true implications of new‐onset diabetes on mortality are not known. Further, the impact of drug‐induced increases in diabetes incidence on microvascular diseases such as retinopathy and nephropathy, although not systemically studied, are likely substantial.
Many post hoc analyses, however, uniformly demonstrate that diuretics and β‐blockers not only worsen glycemic status among those with diabetes but also increase development of new‐onset diabetes in those with impaired fasting glucose. 11 , 12 , 13 Hence, they increase number of medications taken and need for more frequent physician visits. Both thiazide diuretics, through hypokalemia and other mechanisms related to increased visceral adiposity, 14 and vasoconstricting β‐blockers worsen insulin sensitivity 15 ; exceptions to this statement include the newer vasodilating β‐blockers, such as carvedilol and nebivolol. These vasodilating agents have neutral effects on glycemic control and increase insulin sensitivity. 16 , 17 , 18 Angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and calcium channel blockers (CCBs) have beneficial or neutral effects on insulin sensitivity and glycemic control. 11 , 15 , 19 Note also that renin‐angiotensin system (RAS) blockers administered concomitantly with thiazide diuretics do not prevent worsening of glycemic control in obese persons with impaired fasting glucose. 20 These data, taken together with the findings of the most recent meta‐analysis by the blood pressure trialists 21 indicate that since it is BP lowering and not the class of antihypertensive agent used that reduces cardiovascular events, one should use antihypertensive agents that do not worsen preexisting metabolic conditions.
Second, a substantial amount of epidemiologic and post hoc analyses’ clinical trial data supports the notion that presence of proteinuria (ie, >300 mg/d in patients with diabetes) is associated with higher cardiovascular event rates. 22 , 23 Moreover, all studies among patients with diabetes indicate that proteinuria reduction of >30% within the first 6 to 12 months of BP‐lowering therapy reduces cardiovascular events and development of heart failure as well as slows kidney disease progression. 24 , 25 Taken together, these data support the notion that treatment of BP in persons with diabetes must focus not only on achievement of BP goal but also on reducing proteinuria if present. Thus, as suggested by the most recent diabetes guidelines, all patients with diabetes should be evaluated for albuminuria at least once annually. 1 Antihypertensive agents found to maximally reduce proteinuria when BP is reduced include blockers of the RAS either alone or combined along with nondihydropyridine CCBs. 1 , 26
Last, there has been an improvement in achievement of BP goals over the past decade. All current guidelines recommend a BP goal of <130/80 mm Hg in patients with diabetes to maximally reduce cardiovascular events and progression of nephropathy. 27 , 28 , 29 An analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2003 data demonstrates that the recommended BP goal of <140/90 mm Hg is achieved in only about one‐third of persons with diabetes; 25% are at a goal of <130/80 mm Hg. 30 More recent analysis of NHANES 2003–2004 notes that 84% of those with hypertension and diabetes were treated, and the number in whom the BP goal of <130/80 mm Hg was achieved increased to 35%. 31
In cardiovascular outcome trials among patients with hypertension, the proportion of participants in whom BP goals are achieved is roughly double that in clinical practice. An assessment of the subgroup with diabetes in these outcome trials over the past decade indicates that an average of 2.9 appropriately dosed antihypertensive medications are required to achieve BP goals. Among persons with diabetes and preexisting kidney disease, stage 3 or higher, this average increases to about 3.5 medications. 32 Thus, a key tenet in the approach to achieve BP goal in patients with diabetes is to select agents for maximal efficacy and tolerability to achieve BP goal that have the fewest adverse effects and, if possible, the lowest cost.
Strategies for Controlling BP
The basic paradigm to achieve BP goals in persons with diabetes has not changed appreciably from that suggested in JNC 7, but there are some important considerations that have emerged. Specifically, blockers of the RAS are still recommended as initial agents for BP management along with a second agent, usually a CCB or thiazide‐like diuretic, if BP is >20/10 mm Hg above the goal pressure of <130/80 mm Hg. Since no difference in cardiovascular outcomes has been noted between antihypertensive agents if BP is appropriately lowered, this approach mitigates against worsening of metabolic control and is in concert with both JNC 7 and recent European guidelines. 27 , 28 It places RAS‐blocking agents as appropriate agents for those with the compelling indication of diabetes.
Lifestyle changes should have a central role in helping to manage hypertension in all patients with BP values >130/80 mm Hg (Figure). These include weight loss, increase in physical exercise, reduction of alcohol intake, smoking cessation and, perhaps most important, low sodium intake to levels <2.4 g/d. Low salt intake should be encouraged through appropriate dietary counseling and encouragement by the physician and staff (Table I). In addition, the American Diabetes Association guidelines should also be followed to optimize glycemic control. 33 This is important especially for morbidity reduction (ie, reduction of neuropathy and blindness). While mortality reduction is associated with good glycemic control, the level to which glucose needs reduction appears to be higher than previously thought. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial tested whether a lower level of glucose, defined as a hemoglobin A1c value <6.5%, would result in a lower cardiovascular event rate was stopped early by the data safety monitoring board secondary to a higher cardiovascular event rate in the lower glucose control group. 34 Similarly, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation (ADVANCE) trial did not show any improvement in cardiovascular outcome with aggressive treatment of glycated hemoglobin to <6.5%. 35 This study did show a 20% reduction in new‐onset nephropathy with aggressive glycemic treatment, however. Thus, the guideline put forth by the American Diabetes Association of a hemoglobin A1c value of <7% appears to be the one that would provide the greatest cardiovascular risk reduction along with BP reduction.
Figure.
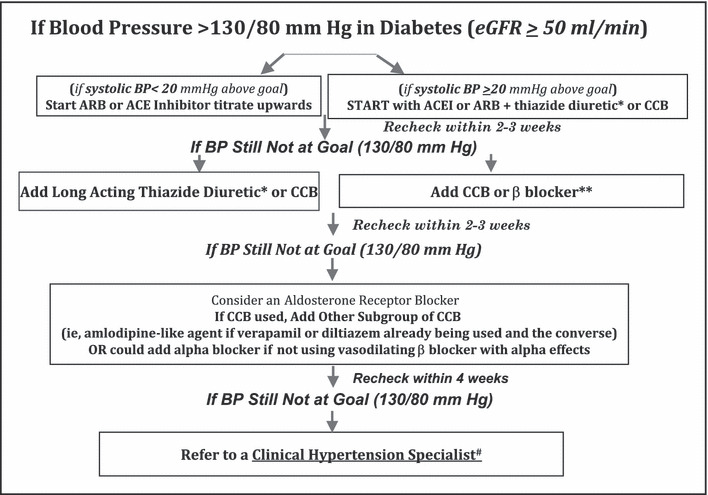
A Suggested Approach to Achieve BP Goal in Patients with Diabetes. ^Represents kidney function (estimated glomerular filtration rate‐eGFR) that generally responds well to thiazide diuretics. *Chlorthalidone is the suggested thiazide like diuretic since this is the diuretic used in clinical trials and forms the bases for the cardiovascular outcome data. **Vasodilating beta blockers have a better tolerability profile and less metabolic consequences as compared to older agents such as atenolol. #Specialists can be found at http://www.ash‐us.org/specialist_program/directory.htm# Adapted from Ruilope et al.50
Table I.
Lifestyle Modifications to Prevent and Manage Hypertensiona
| Weight reduction | Maintain normal body weight (body mass index 18.5–24.9 kg/m2). |
| Adopt DASH eating plan | Consume a diet rich in fruits, vegetables, and low‐fat dairy products with a reduced content of saturated and total fat. |
| Dietary sodium reduction | Reduce dietary sodium intake to no more than 100 mmol per day (2.4 g sodium or 6 g sodium chloride). |
| Physical activity | Engage in regular aerobic physical activity such as brisk walking (at least 30 minutes per day, most days of the week). |
| Moderate alcohol use | Limit consumption to no more than 2 drinks (e.g., 24 oz beer, 10 oz wine, or 3 oz 80‐proof whiskey) per day in most men and to no more than 1 drink per day in women and lighter weight persons. |
aAdopted from JNC 727.
In addition to the lifestyle measures, all patients with diabetes and a BP >130/80 mm Hg should be started on a once‐daily RAS blocker and dose‐maximized within the first month of treatment if BP is not <130/80 mm Hg. If BP is >20/10 mm Hg above goal, then combination therapy with an RAS blocker and either a thiazide‐like diuretic, if kidney function is appropriate, or a CCB should be initiated. Whether choosing an ACE inhibitor or an ARB, dosage should be titrated to the highest tolerated level necessary for BP to reach goal. If an ACE inhibitor is started and the adverse effect of cough appears, treatment should be changed to an appropriate dose of an ARB. If within a month after monotherapy titration the BP goal is not achieved, then either a low‐dose thiazide diuretic (12.5 mg of chlorthalidone or hydrochlorothiazide) or a CCB should be added. In the case of a patient with an estimated glomerular filtration rate (eGFR) <50 mL/min, the thiazide diuretic should be replaced by a loop diuretic in adequate doses (once‐daily torsemide or twice‐daily furosemide or bumetinide). Note that chlorthalidone can be used in such patients down to an eGFR of 40 mL/min.
It should be noted that this algorithm (Figure) serves as a general guide, as there is no substitute or guide for good clinical judgment for any given patient. Therefore, if potassium levels are elevated (>5 mEq/L), either due to long‐standing diabetes and consequent type IV renal tubular acidosis or chronic kidney disease (usually an eGFR <40 mL/min), before initiating RAS‐blocking therapy, a review of all high potassium–containing foods and substances as well as over‐the‐counter agents that cause hyperkalemia, such as NSAIDs, must be discussed with the patient. Observational data support that reductions of up to 0.6 mEq/L in serum potassium can be achieved just by following these lifestyle interventions. Under circumstances when potassium levels are elevated, use of loop diuretics twice or thrice daily may be appropriate to enable the use of RAS‐blocking agents. While there are no cardiovascular outcome data from clinical trials in patients with relatively high potassium levels, post hoc analyses of heart failure and kidney disease progression studies report cardiovascular risk reduction in those with eGFR values of <50 mL/min with serum potassium levels up to 5.6 mEq/L on RAS‐blocking therapy. 24 , 36
Minimization of the number of antihypertensive pills improves patient adherence and the effectiveness of lowering BP. 37 , 38 Thus, conversion of the full combination treatment to a fixed‐dose combination of an RAS blocker/diuretic or an RAS blocker/CCB should be given strong consideration. It should also be noted that based on the data from the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET), use of an ACE inhibitor/ARB combination is not supported either for BP reduction or reduction in cardiovascular outcomes. 39 This combination does have advantages for further proteinuria reduction in persons with advanced diabetic nephropathy 40 but this has not been shown to translate into a cardiovascular risk reduction in those with diabetic nephropathy.
If after 2 to 4 weeks of adding a diuretic or CCB, BP is still not at goal, titration of the thiazide to 25 mg/d and of the CCB to the maximum tolerated dose is recommended. This combination of medications will ensure that target BP is achieved in the majority of cases (Figure). However, in at least of 20% of the remaining cases, a fourth and possibly a fifth agent will be needed. Under these circumstances, a β‐blocker is useful. Moreover, a vasodilating β‐blocker is generally better tolerated and metabolically neutral compared with vasoconstricting agents. 41 β‐Blockers are especially useful in patients with elevated pulse rates and should be considered for BP control if the pulse rate is elevated on at least 2 separate antihypertensive medications. 42 Alternatively, combination of a nondihydropyridine CCB (verapamil or diltiazem) in moderate doses with a dihydropyridine CCB has additive effects on BP reduction 43 and will help achieve goal BP.
There is potentially a role for α‐blockers for BP control as a fourth‐ or fifth‐line agents; however, these agents are major culprits of orthostatic hypotension, especially in patients with diabetes, and should be avoided if an α/β‐blocker is already being used or if the patient has diabetic neuropathy with a substantial decrease in BP or symptoms on standing.
Last, the role of aldosterone blockade as a fourth‐line strategy is very important in patients with diabetes and obesity. Individuals with obstructive sleep apnea and central obesity have demonstrated major benefits of BP reduction with the use of aldosterone antagonism. 44 , 45 In a study of 76 patients with uncontrolled BP on an average of 4 medications, including an ACE inhibitor or ARB and a thiazide diuretic, addition of spironolactone (12.5–25 mg/d) resulted in an average 25‐mm Hg reduction in systolic BP and an average 12‐mm Hg reduction in diastolic BP after 6 months of follow‐up. 46 Reductions in BP were similar in African American and Caucasian individuals. Moreover, the BP‐lowering response was not predicted by baseline plasma aldosterone, 24‐hour urinary aldosterone, plasma renin activity, or plasma aldosterone/renin ratio. These BP‐lowering effects of aldosterone receptor blockade were confirmed in a report of 1411 participants in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA) unselected for plasma aldosterone and plasma renin activity. They received spironolactone mainly as a fourth‐line antihypertensive agent for uncontrolled BP and were receiving an average of 3 drugs. 47 Use of spironolactone was again associated with a BP drop of 21.9/9.5 mm Hg that was largely unaffected by factors like age, sex, smoking, and diabetic status. Recent data in obese patients demonstrates that the adipocyte releases substances that increase aldosterone, and this may be the reason for this observation. 48 Given the benefits aldosterone blockade in these individuals and those with sleep apnea, one is reminded of hyperkalemia as a limiting factor in their use. 44 , 47 The reader is referred to the earlier discussion on this topic.
Conclusions
The high cardiovascular risk in these patients requires an integrated therapeutic intervention that apart from effective antihypertensive therapy should include optimal achievement of goals for glycemic and lipid control, as well as inhibition of platelet aggregation (Table II). The treatment goals for glycemic control are set to a hemoglobin A1c level of <7% and plasma preprandial glucose concentrations (average of several measurements) of 70 to 130 mg/dL. 33 All patients with diabetes should be treated with a statin and, if needed, complimentary lipid‐lowering drugs to reduce low‐density lipoprotein cholesterol to <70 mg/dL, triglycerides to <150 mg/dL, and to raise high‐density lipoprotein cholesterol to >40 mg/dL in men and >45 mg/dL in women. 49 Further, in patients with diabetes and hypertension, antiplatelet therapy should generally consist of aspirin in dosages of 75 to 162 mg/d. 1
Table II.
Approach Needed to Maximally Reduce Cardio‐Renal Riska
| ? Lifestyle modifications‐as per Table 1 |
| ? Achieve BP <130/80 mmHg |
| ? Achieve LDL <70 mg/dl |
| ? Achieve glycemic control (<7% HbA1c)a |
| ? Antiplatelet therapy‐low dose aspirin 75–162 mg/day |
| ? In those with albuminuria or proteinuria‐ reduce by >30% after starting treatment within 6 months. |
aBased on ADA guidelines, AACE guidelines indicates <6.5% HbA1c.
Acknowledgments
Acknowledgments and disclosure: This article was reviewed by Joshua Barzilay, MD; Vivian A. Fonseca, MD and Nathan D. Wong, PhD. The American Society of Hypertension Writing Group Steering Committee: Thomas D. Giles, MD, Chair; Henry R. Black, MD; Joseph L. Izzo, Jr, MD; Barry J. Materson, MD, MBA; Suzanne Oparil, MD; and Michael A. Weber, MD. Dr Bakris is a consultant for Abbott, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi‐Sankyo, Forest Laboratories, Gilead, GlaxoSmithKline, Merck, Novartis, Sanofi Aventis, and Walgreens (formulary committee). He has received grant/research support from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases/National Heart, Lung, and Blood Institute), GlaxoSmithKline, and Forest Laboratories. Dr Sowers has received grant/research support from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases/National Heart, Lung, and Blood Institute), VA, and Forest Laboratories. He is a consultant for Forest Laboratories, Merck, and Novartis.
References
The American Society of Hypertension will publish a series of Position Papers in their official journals throughout the coming months; this article is the second in the series. The first in the series addressed the topic of Home and Ambulatory Blood Pressure Monitoring and appeared in The Journal of the American Society of Hypertension; it will be reprinted for the readership of The Journal of Clinical Hypertension in an upcoming issue. The citation for the first Position Paper follows:
Pickering TG and White WB, on behalf of the American Society of Hypertension Writing Group. When and how to use self (home) and ambulatory blood pressure monitoring. JASH. 2008;2(3):119–124.
- 1. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12–S154. [DOI] [PubMed] [Google Scholar]
- 2. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153(5):598–615. [DOI] [PubMed] [Google Scholar]
- 3. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. [DOI] [PubMed] [Google Scholar]
- 4. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. [DOI] [PubMed] [Google Scholar]
- 5. Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115(1):114–126. [DOI] [PubMed] [Google Scholar]
- 6. Nag S, Bilous R, Kelly W, et al. All‐cause and cardiovascular mortality in diabetic subjects increases significantly with reduced estimated glomerular filtration rate (eGFR): 10 years’ data from the South Tees Diabetes Mortality study. Diabet Med. 2007;24(1):10–17. [DOI] [PubMed] [Google Scholar]
- 7. Bakris GL. The importance of blood pressure control in the patient with diabetes. Am J Med. 2004;116(suppl 5A):30S–38S. [DOI] [PubMed] [Google Scholar]
- 8. Sarafidis PA, Bakris GL. State of hypertension management in the united states: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich). 2008;10:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whelton PK, Barzilay J, Cushman WC, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med. 2005;165(12):1401–1409. [DOI] [PubMed] [Google Scholar]
- 10. Kostis JB, Wilson AC, Freudenberger RS, et al. Long‐term effect of diuretic‐based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005;95(1):29–35. [DOI] [PubMed] [Google Scholar]
- 11. Sarafidis PA, Bakris GL. Antihypertensive therapy and the risk of new‐onset diabetes. Diabetes Care. 2006;29(5):1167–1169. [DOI] [PubMed] [Google Scholar]
- 12. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet. 2007;369(9557):201–207. [DOI] [PubMed] [Google Scholar]
- 13. Mancia G, Grassi G, Zanchetti A. New‐onset diabetes and antihypertensive drugs. J Hypertens. 2006;24(1):3–10. [DOI] [PubMed] [Google Scholar]
- 14. Carter BL, Einhorn PT, Brands M, et al. Thiazide‐induced dysglycemia: call for research from a working group from the National Heart, Lung, and Blood Institute. Hypertension. 2008;52:30–36. [DOI] [PubMed] [Google Scholar]
- 15. Sarafidis PA, McFarlane SI, Bakris GL. Antihypertensive agents, insulin sensitivity and new‐onset diabetes. Curr Diab Rep. 2007;7:191–199. [DOI] [PubMed] [Google Scholar]
- 16. Sarafidis PA, Bakris GL. Antihypertensive treatment with beta‐blockers and the spectrum of glycaemic control. QJM. 2006;99(7):431–436. [DOI] [PubMed] [Google Scholar]
- 17. Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292(18):2227–2236. [DOI] [PubMed] [Google Scholar]
- 18. Kaiser T, Heise T, Nosek L, et al. Influence of nebivolol and enalapril on metabolic parameters and arterial stiffness in hypertensive type 2 diabetic patients. J Hypertens. 2006;24(7):1397–1403. [DOI] [PubMed] [Google Scholar]
- 19. Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551–1562. [DOI] [PubMed] [Google Scholar]
- 20. Bakris G, Molitch M, Hewkin A, et al. Differences in glucose tolerance between fixed‐dose antihypertensive drug combinations in people with metabolic syndrome. Diabetes Care. 2006;29(12):2592–2597. [DOI] [PubMed] [Google Scholar]
- 21. Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta‐analysis of randomised trials. BMJ. 2008;336(7653):1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. So WY, Kong AP, Ma RC, et al. Glomerular filtration rate, cardiorenal end points, and all‐cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29(9):2046–2052. [DOI] [PubMed] [Google Scholar]
- 23. Ibsen H, Wachtell K, Olsen MH, et al. Does albuminuria predict cardiovascular outcome on treatment with losartan versus atenolol in hypertension with left ventricular hypertrophy? A LIFE substudy. J Hypertens. 2004;22(9):1805–1811. [DOI] [PubMed] [Google Scholar]
- 24. Berl T, Hunsicker LG, Lewis JB, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16(7):2170–2179. [DOI] [PubMed] [Google Scholar]
- 25. De Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110(8):921–927. [DOI] [PubMed] [Google Scholar]
- 26. Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis. 2007;49(1):12–26. [DOI] [PubMed] [Google Scholar]
- 27. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 28. Mancia G, De BG, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–1187. [DOI] [PubMed] [Google Scholar]
- 29. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 2):S1–S290. [PubMed] [Google Scholar]
- 30. Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–342. [DOI] [PubMed] [Google Scholar]
- 31. Wong ND, Lopez VA, L’Italien G, et al. Inadequate control of hypertension in US adults with cardiovascular disease comorbidities in 2003–2004. Arch Intern Med. 2007;167(22):2431–2436. [DOI] [PubMed] [Google Scholar]
- 32. Chua DC, Bakris GL. Is proteinuria a plausible target of therapy? Curr Hypertens Rep. 2004;6(3):177–181. [DOI] [PubMed] [Google Scholar]
- 33. Pitt B, Bakris GL, Ruilope L, et al. EPHESUS investigators. Serum potassium and clinical outcomes in the eplerenone post‐acute myocardial infarction heart failure efficacy and survival study. Circulation. In Press. [DOI] [PubMed] [Google Scholar]
- 34. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. [DOI] [PubMed] [Google Scholar]
- 36. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. [DOI] [PubMed] [Google Scholar]
- 37. Gerbino PP, Shoheiber O. Adherence patterns among patients treated with fixed‐dose combination versus separate antihypertensive agents. Am J Health Syst Pharm. 2007;64(12):1279–1283. [DOI] [PubMed] [Google Scholar]
- 38. Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed‐dose combinations improve medication compliance: a meta‐analysis. Am J Med. 2007;120(8):713–719. [DOI] [PubMed] [Google Scholar]
- 39. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. [DOI] [PubMed] [Google Scholar]
- 40. Kunz R, Friedrich C, Wolbers M, et al. Meta‐analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148(1):30–48. [DOI] [PubMed] [Google Scholar]
- 41. Hart PD, Bakris GL. Should beta‐blockers be used to control hypertension in people with chronic kidney disease? Semin Nephrol. 2007;27(5):555–564. [DOI] [PubMed] [Google Scholar]
- 42. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36(3):646–661. [DOI] [PubMed] [Google Scholar]
- 43. Saseen JJ, Carter BL, Brown TE, et al. Comparison of nifedipine alone and with diltiazem or verapamil in hypertension. Hypertension. 1996;28(1):109–114. [DOI] [PubMed] [Google Scholar]
- 44. Pratt‐Ubunama MN, Nishizaka MK, Boedefeld RL, et al. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131(2):453–459. [DOI] [PubMed] [Google Scholar]
- 45. Calhoun DA, Jones D, Textor S, et al.; American Heart Association Professional Education Committee . Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–e526. [DOI] [PubMed] [Google Scholar]
- 46. Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low‐dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16(11, pt 1):925–930. [DOI] [PubMed] [Google Scholar]
- 47. Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49(4):839–845. [DOI] [PubMed] [Google Scholar]
- 48. Krug AW, Ehrhart‐Bornstein M. Aldosterone and metabolic syndrome: is increased aldosterone in metabolic syndrome patients an additional risk factor? Hypertension. 2008;51(5):1252–1258. [DOI] [PubMed] [Google Scholar]
- 49. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113(19):2363–2372. [DOI] [PubMed] [Google Scholar]
- 50. Ruilope L, Kjeldsen SE, De La Sierra A, Mancia G, Ruggenenti P, Stergiou GS, Bakris GL, Giles TD. The kidney and cardiovascular risk–implications for management: a consensus statement from the European Society of Hypertension. Blood Press. 2007;16(2):72–79. [DOI] [PubMed] [Google Scholar]


