Abstract
The purpose of the present study was to determine the effect of an interval exercise training program on C‐reactive protein and erectile dysfunction (ED) management in older male hypertensive patients. Twenty‐two male hypertensive patients with ED (n=22; 62.10±5.23 years) were involved in interval exercise training (60%–79% heart rate max reserve) for 8 weeks for a duration of between 45 to 60 min/d, while an age‐matched control hypertensive (n=21; 64.00±4.77 years) group remained sedentary during this period. The study revealed a significant effect of an exercise training program on erectile function of hypertensive patients with ED (P<.05). It was concluded that an exercise program is a possible effective noninvasive and nonpharmacologic management of ED in male hypertensive patients.
Erectile dysfunction (ED) is a common problem, affecting more than half of all men between the ages of 40 and 70 years. 1 , 2 , 3 ED has been defined by the National Institutes of Health Consensus Panel on Impotence as the persistent inability to attain and maintain an erection sufficient to permit satisfactory sexual performance. 4
Numerous studies have shown that ED shares several modifiable risk factors with cardiovascular disease including atherosclerosis, 5 hypertension, hyperlipidemia, diabetes mellitus, 6 smoking, 7 obesity, and sedentary lifestyle. 8 Data from several studies involving patients with cardiac disease have shown a high prevalence (42% to 75%) of ED in this patient population. 9 , 10 , 11 According to Feldman and colleagues, 12 the most common etiology for organic ED is vasculogenic. Men with hypertension have a 15% probability of developing complete ED (increasing to 20% if they smoke).
The influence and significance of lifestyle factors in ED have been demonstrated in cross‐sectional and prospective, randomized, controlled trials. Recent epidemiologic studies in several countries have shown that modifiable lifestyle or risk factors, including physical activity in particular, are directly related to the occurrence of ED. In a review of several recent studies, it was concluded the levels of physical activity predicted ED prevalence and incidence. Furthermore, the role of lifestyle changes (weight loss, physical activity) was demonstrated to be effective in modifying ED in moderately obese sedentary men. 13
It is an established fact that sedentary lifestyle contributes to increased risk of cardiovascular disease, especially hypertension. Studies 14 , 15 , 16 , 17 have shown that exercise training lowers blood pressure in many hypertensive patients. Studies have also supported the value of risk factor modification and lifestyle change in the clinical management of men with ED and concomitant cardiovascular illness. 13 However, few studies have actually investigated the effect of physical training in the management of ED. The purpose of the present study was to access the therapeutic efficacy of interval exercise in the management of ED in older male hypertensive patients.
Methodology
Research Design
In the present study, an age‐matched randomized double‐blind independent group design was used to determine the influence of an exercise interval training program on a biomarker of inflammation, C‐reactive protein (CRP), and ED. Patients were arranged in ascending order of age (50–70 years) and then assigned to exercise and control groups in an alternating pattern (age‐matched). Pretest was administered to all patients. The exercise group was involved in a training program for 8 weeks, while the control group remained sedentary during this period. At the end of the training and sedentary period, a post‐test was administered to all patients.
Patients
The patient population of the study was 357 essential hypertensive men attending the hypertensive clinic of Murtala Muhammed Specialist Hospital (MMSH), Kano, Nigeria. Those complaining of ED were referred to a specialized clinic. Levels of ED were established using the international index of erectile function (IIEF), 13 and all men were questioned about previous ED treatment. Those 50 patients who met the inclusion criteria were recruited for the study.
Inclusion Criteria
Only patients diagnosed with ED and who volunteered to participate in the study were recruited. Patients between the age range of 50 and 70 years with chronic and stable (>1‐year duration) hypertension (systolic blood pressure [SBP] between 140 and 180 mm Hg and diastolic blood pressure [DBP] between 90 and 109 mm Hg) and body mass index (BMI) between 20 and 30 kg/m2 were selected. Patients whose blood pressures (BPs) had been responding favorably to methyldopa and with no apparent psychological/psychiatric disorders were recruited. 14 All patients in both the exercise and control groups were taking methyldopa. At the end of the trial, the drug was stopped in all cases. Post‐tests were conducted 7 days after discontinuation of therapy.
Exclusion Criteria
Exclusion criteria included obese (BMI >30 kg/m2) and underweight (BMI <20 kg/m2) patients, smokers, alcoholics, diabetics, and patients with other cardiac or renal conditions, respiratory disease, benign prostatic hypertrophy, liver failure, low testosterone levels, and multiple sclerosis. Patients involved in vigorous physical activities and above average physical fitness (maximal oxygen consumption [VO2max] >27 and >33 mL/kg/min for >60 and 50 years of age, respectively) were also excluded.
Fifty hypertensive patients with ED were assigned to 2 groups: exercise (n=25) and control (n=25) groups.
Ethical Permission
Ethical approval was granted by the ethical committee of Kano State Hospitals management board.
Pretest Procedure
Wash‐Out Period
All patients taking antihypertensive drugs were asked to stop all forms of medication and were given placebo tablets (consisting of mainly lactose and inert substance) in a double‐blind method. 18 All patients including those not taking any antihypertensive medications were placed on placebo tablets for 1 week (wash‐out period). During the wash‐out period, all patients were instructed to report to the hypertensive clinic for daily BP monitoring and general observation. The pretest procedure was conducted on the last day of the wash‐out period.
Blood Sample Collection (Venipuncture Method)
Both pretreatment venous blood samples were obtained between 8 am and 10 am after about a 12‐hour overnight fast (fasting blood sample). 19 All samples were stored in a refrigerator at −80°C until analysis. 20
C‐Reactive Protein
The high‐sensitivity CRP was determined qualitatively and semiquantitatively using a commercial latex agglutination method. A titer (highest dilution showing a positive result or lowest detection point) was then established at 0.2 mg/L (values <0.2 were regarded as 0.1 mg/L).
Test Procedure
All the tests and procedures were conducted in the Department of Physiotherapy at MMSH, Kano, between 8 am and 10 am.
Stress Test
The Young Men Christian Association (YMCA) submaximal cycle ergometry test protocol was used to assess patients’ aerobic power as described by the American College of Sports Medicine (ACSM) 21 and Golding and colleagues. 22
Training Program
The Interval Group (Group 1).
Patients in the treatment group exercised on a bicycle ergometer at a low intensity of between 60% and 79% of their heart rate maximum (HR max) reserve (estimated from 220 minus the patient age as recommended by ACSM). 17 The starting workload was 100 kgm (17 watts), which was increased at a pedal speed of 50 revolutions per minute to obtain an HR max reserve 60% was increased in the first 2 weeks to a level up at 79% HR max reserve at a work/rest ratio of 1:1. 17 This was maintained throughout the remaining part of the training period. The initial exercise session was increased from 45 minutes in the first 2 weeks of training to up to 60 minutes throughout the remaining part of the training. During the training period (8 weeks) all patients were placed on methyldopa according to their pre‐recruitment doses (250 mg and 500 mg daily).
Methyldopa was preferred because it does not alter normal hemodynamic responses to exercise. 23 It is a well‐tolerated and widely prescribed antihypertensive drug in Nigeria, 24 particularly in Northern Nigeria, where the study was conducted. It is also useful in the treatment of mild to moderately severe hypertension. 25 Three exercise sessions per week were maintained throughout the 8‐week period of training for the exercise (interval) group.
The Control Group (Group 2).
Patients in the control group remained sedentary and were instructed not to undertake any vigorous physical activity during the period of study. Patients in the control group were also placed on methyldopa according to their pre‐recruitment doses and responses (doses of 250 mg and 500 mg daily for the same period [8 weeks] of time). At the end of the 8‐week training period, a post‐test was conducted for all patients.
Post‐Test Procedure
-
•
Wash‐out period: At the end of the 8 weeks training period, all patients were asked to stop methyldopa and were prescribed placebo tablets in a single‐blinded method for 1 week.
-
•
Blood sample collection: On the last day of the post‐training wash‐out period, fasting blood samples were collected.
-
•
Post‐test stress test: Stress test was also conducted on the last day of the post‐test wash‐out period.
Forty‐three patients (22 from the interval and 21 from the control group) completed the 8‐week training program. Seven patients (3 from the exercise and 4 from the control group) had dropped out because of noncompliance, unfavorable responses to methyldopa and exercise training, or had incomplete data. The data of 43 patients were used in the statistical analysis.
Data Analysis
Following data collection, the variables were statistically analyzed. Descriptive statistics (means, standard deviations) of the patients’ physical characteristics were included. Data were analyzed using SPSS for Windows (version 15.0, Chicago, IL). Independent Student t test (SBP, DBP, white blood cell count, and VO2max) and Mann–Whitney U test (CRP and IIEF) was used to find the significant difference in all variables of interest (in the SPSS t test, data were entered as ordinal data for both IIEF and CRP and interval for SBP, DBP, and VO2max). In the t test and Mann–Whitney test, the difference between the pretest and post‐test values (changed score) was used as the dependent variables. Spearman correlation was also performed. A value of P<.05 was considered statistically significant.
Results
The patients’ age ranged between 50 and 70 years. Mean ± standard deviation values for age, fasting blood sugar (FBS), BMI, and total cholesterol (TC) for the exercise group were 62.10±5.23 years, 3.93±0.55 mmol/L, 24.19±3.07 kg/m2, and 100.54±45.04 mg/dL, and for the control group were 64.00±4.77 years, 3.99±0.85 mmol/L, 25.21±3.75 kg/m2, and 100.57±38.65 mg/dL, respectively. Results indicated no significant differences in the age (t=−1.249, P=.219), BMI (t=−.978, P=.334), FBS (t=−.271, P=.787), and TC (t=−.230, P=.819) values between groups.
Patients’ pre‐treatment compared with post‐treatment values of BP (mm Hg) and VO2max (mL/kg/min) for the exercise group were SBP, 164.02±15.20; DBP, 96.14±4.41; and VO2max, 24.80±9.62 vs SBP, 149.00±16.99; DBP, 90.80±5.40; and VO2max, 36.65±7.88 and for the control group were SBP, 159.64±13.23; DBP, 97.19±2.94; and VO2max, 23.12±8.40 vs SBP, 161.98±15.60; DBP, 96.05±2.94; and VO2max, 29.83±10.5. Results indicated significant reduction in the exercise groups over controls in SBP (t=−4.914, P=.000), DBP (t=−3.645, P=.001), and VO2max (t=6.053, P=.000) at P<.05.
Patients’ pre‐treatment compared with post‐treatment CRP and IIEF values for the exercise group were CRP, 0.15±0.05 and IIEF, 11.50±5.30 vs 0.12±0.04 and 15.14±4.92 and for the control group CRP, 0.13±0.05 and IIEF, 8.10±4.02 vs 0.14±0.05 and 8.95±3.90, respectively. In the Table, Mann–Whitney U test analysis for changes in CRP and IIEF, results indicated that groups differed significantly in CRP (t=142.500, P=.002) and IIRF (t=85.000, P=.000) values.
Table.
CRP and IIEF Response to Exercise (Mann–Whitney U) (N=43)
| Variables | t Value | P Value |
|---|---|---|
| IIEF | 85.000 | .000a |
| CRP | 142.000 | .002a |
Abbreviations: CRP, C‐reactive protein; IIEF, international index of erectile function. aSignificant: P<.05.
The Figure shows a significant negative correlation between changes in CRP and IIEF score (r=−.588).
Figure.
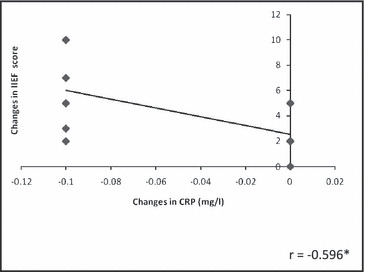
Correlation between training changes in C‐reactive protein (CRP) and international index of erectile function (IIEF) score.
Discussion
The purpose of the present study was to determine the role and effect of exercise on the association of CRP and ED in older male hypertensive patients. Results suggested a significant effect of exercise on ED. Results also showed a significant negative correlation between CRP and ED following aerobic exercise.
Results of the present study are in agreement with findings from several studies 26 , 27 that reported a significant reduction in CRP following aerobic exercise and an inverse correlation between CRP and VO2max in healthy, mild to moderate hypertensive, and coronary heart disease patients. Several other studies of large population cohorts, including the British Regional Heart Study (BRHS), 28 the Third National Health and Nutrition Examination Survey (NHANES), 29 , 30 the Cardiovascular Health Study (CHS), 31 the men’s Health Professionals Follow‐Up Study (HPFS), the Nurses’ Health Study II (NHS II), 32 and the Health, Aging and Body Composition Study (Health ABC), 33 provide evidence for an inverse, independent dose–response relationship between plasma CRP concentration and the level of physical activity in both men and women.
The physiologic basis for the therapeutic role of interval exercise in the management of ED in hypertension as reported in the present study could be related to the biochemical, neural, and hormonal changes in the blood vessel walls that induce an acute and long‐term blood vessel relaxation. The blood vessels may relax after each exercise session because of body warming effects; local production of certain chemicals, such as lactic acid and nitric oxide; decreases in nerve activity; and changes in hormones and their receptors. 34 , 35 Over time, as exercise is repeated, there is growing evidence of a longer‐lasting effect. Chronic (regular, long‐term) physical training may reduce basal concentrations of inflammatory markers. Data from cross‐sectional observational studies have shown an inverse association between markers of systemic inflammation and physical activity and fitness status. 28 , 29 , 30 , 31 , 32 , 33 , 36
Our data demonstrate that exercise of a relatively short period improves ED and reduces BP in older hypertensive men with ED. This improvement was associated with a reduction in the marker (CRP) of systemic vascular inflammation, which may likely be related to the amelioration of endothelial function.
Although the present study indicated a significant effect of exercise in the management of ED in hypertension, there are limitations of the study. They included failure to objectively assess the endothelial physiology and few participants. These limiting factors warrant more attention in future studies. Future studies are also needed to investigate the effects of different antihypertensive drugs coupled with exercise on ED of hypertensive patients with different racial and sociocultural backgrounds.
References
- 1. TEDES . An epidemiological study of erectile dysfunction in Thailand (part 1: prevalence). Thai Erectile Dysfunction Epidemiologic Study Group (TEDES). J Med Assoc Thai. 2000;83:872–879. [PubMed] [Google Scholar]
- 2. Braun M, Wassmer G, Klotz T. Epidemiology of erectile dysfunction: results of the “Cologne Male Survey”. Int J Impot Res. 2000;12:305–311. [DOI] [PubMed] [Google Scholar]
- 3. Martin‐Morales A, Sanchez‐Cruz JJ, Saenz TI. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569–574. [DOI] [PubMed] [Google Scholar]
- 4. NIH Consensus Conference . Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 5. Kaiser FE, Viosca SP, Morley JE, et al. Impotence and aging: clinical and hormonal factors. J Am Geriatr Soc. 1988;36:511–519. [DOI] [PubMed] [Google Scholar]
- 6. Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol. 2004;171:2341–2345. [DOI] [PubMed] [Google Scholar]
- 7. Shabsigh R, Fishman IJ, Schum C, et al. Cigarette smoking and other vascular risk factors in vasculogenic impotence. Urology. 1991;38:227–232. [DOI] [PubMed] [Google Scholar]
- 8. Derby CA, Mohr BA, Goldstein I, et al. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56:302–306. [DOI] [PubMed] [Google Scholar]
- 9. Solomon H, et al. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003;89:251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montorsi F, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–365. [DOI] [PubMed] [Google Scholar]
- 11. Kloner R. Erectile dysfunction in the cardiac patient: how common and how should we treat? J Urol. 2003;170:S46–S50. [DOI] [PubMed] [Google Scholar]
- 12. Feldman HA, Goldstein I, Hatzichristou DG. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151:54–61. [DOI] [PubMed] [Google Scholar]
- 13. Rosen RC, Kostis JB. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003;92:9M–18M. [DOI] [PubMed] [Google Scholar]
- 14. Stewart KJ, Bacher AC, Turner KL, et al. Effects of exercise on blood pressure in older person. Arch Intern Med. 2005;165:756–762. [DOI] [PubMed] [Google Scholar]
- 15. Ishikawa‐Takata K, Ohta T, Tanaka H. How much exercise is required to reduce blood pressure in essential hypertension. A dose response study. Am J Hypertens. 2003;16:629–633. [DOI] [PubMed] [Google Scholar]
- 16. Engstrom G, Hedblad B, Janzon L. Hypertensive men who exercise regularly have lower rate of cardiovascular mortality. J Hypertens. 1999;17:737–742. [DOI] [PubMed] [Google Scholar]
- 17. American College of Sport Medicine . Physical activity, physical Fitness and hypertension. Med Sci Sports Exerc. 1993;25:i–x. [PubMed] [Google Scholar]
- 18. Townsend RR, Mcfadden TC, Ford V, et al. A randomized double blind, placebo‐controlled trial of casein protein hydrolysnte (C12 peptide) in human essential hypertension. Am J Hypertens. 2004;17:1056–1058. [DOI] [PubMed] [Google Scholar]
- 19. Bachorik PS. Collection of blood sample for lipoprotein analysis. Clin Chem. 1982;28:1375–1378. [PubMed] [Google Scholar]
- 20. Barbieri M, Ferrucci L, Corsi AM, et al. Is chronic inflammation a determinant of blood pressure in the elderly? AJH. 2003;16:537–543. [DOI] [PubMed] [Google Scholar]
- 21. American College of Sports Medicine . ASCM’s Guidelines for Exercise Testing and Prescription 5th ed. Baltimore, MD: Williams & Wilkins; 1995. [Google Scholar]
- 22. Golding LA, Meyers CR, Sinniny WE. Way to Physical Fitness. The Complete Carnote to Fitness Testing and Instruction, 3rd ed. Champaign IL: Human Kinetics Publishers; 1989. [Google Scholar]
- 23. Mancia G, Parati G, Pomidossi G, et al. Alerting reaction and rise in blood pressure during measurement by physician and nurse. Hypertension. 1987;9:209–215. [DOI] [PubMed] [Google Scholar]
- 24. Salako LA. Treatment of Hypertension: Cardiovascular Disease in Africa. Ibadan: Ciba Geigy Ltd.; 1976. [Google Scholar]
- 25. Katzung BG. Basic and Clinical Pharmacology, 7th ed. New York: Lange Medical Books/McGraw (Craw) Hill; 1998. [Google Scholar]
- 26. Kullo DI, Khaleghi M, Hensrud DD. Markers of inflammation Are inversely associated with VO2max in symptomatic men. J Appl Physiol. 2007;102:1374–1379. [DOI] [PubMed] [Google Scholar]
- 27. Hjestuen A, Anderssen SA, Holme I, et al. Makers of inflammatory are inversely related to physical activity and fitness in sedentary men with treated hypertension. Am J Hypertens. 2006;19:669–675. [DOI] [PubMed] [Google Scholar]
- 28. Wannamethee SG, Lowe GD, Whincup PH, et al. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–1790. [DOI] [PubMed] [Google Scholar]
- 29. Abramson JL, Vaccarino V. Relationship between physical activity and ninflammation among apparently healthy middle‐aged and older US adults. Arch Intern Med. 2002;162:1286–1292. [DOI] [PubMed] [Google Scholar]
- 30. King DE, Carek P, Mainous AG III, et al. Inflammatory markers and exercise: differences related to exercise type. Med Sci Sports Exerc. 2003;35:575–581. [DOI] [PubMed] [Google Scholar]
- 31. Geffken D, Cushman M, Burke G, et al. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. [DOI] [PubMed] [Google Scholar]
- 32. Pischon T, Hankinson SE, Hotamisligil GS, et al. Leisure‐time physical activity and reduced plasma levels of obesity‐related inflammatory markers. Obes Res. 2003;11:1055–1064. [DOI] [PubMed] [Google Scholar]
- 33. Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. [DOI] [PubMed] [Google Scholar]
- 34. MacDonald JR, Hogben CD, Tarnopolski MA, et al. Post exercise hypertension is sustained during subsequent bouts of mild exercise and simulated activities of daily living. J Hum Hypertens. 2001;15:567–571. [DOI] [PubMed] [Google Scholar]
- 35. Halliwill JR. Mechanisms and clinical implications of post exercise hypertension in human exercise and sports. Sci Rev. 2001;29:65. [DOI] [PubMed] [Google Scholar]
- 36. Albert MA, Glynn RJ, Ridker PM. Effect of physical activity on serum C‐reactive protein. Am J Cardiol. 2004;93:221–225. [DOI] [PubMed] [Google Scholar]


