Abstract
J Clin Hypertens (Greenwich). 2010;12:422–430. ©2010 Wiley Periodicals, Inc.
Cardiovascular and chronic kidney disease are epidemic throughout industrialized societies. Diabetes leads to premature cardiovascular disease and is regarded by many as the most common etiological factor for chronic kidney disease. Because most studies of blood‐pressure lowering agents in people with diabetes and hypertension have been conducted in individuals who already have some target organ damage, it is unclear whether earlier intervention could prevent or delay the onset of renal or systemic vascular disease. In early disease there is only a low possibility of observing cardiovascular or renal events; thus intervention trials in this population must rely on disease markers such as microalbuminuria. Accordingly, the authors review the evidence to support the use of microalbuminuria as a disease marker in diabetic patients based on its strong association with renal and cardiovascular events, and discuss recent trials that examine the impact of preventing or delaying the onset of microalbuminuria.
Diseases of the cardiovascular system can be seen as a continuum that frequently includes chronic kidney disease. Damage to the renal and systemic vasculature is generally accompanied by a rise in blood pressure and a progression through asymptomatic and symptomatic target organ damage to end‐stage cardiovascular and renal disease and premature death (Figure). 1 , 2 Most clinical trials involving blood pressure‐lowering agents have been conducted in individuals with several associated cardiovascular risk factors, asymptomatic or symptomatic target organ damage, or diabetes 3 , 4 , 5 because fewer subjects are necessary to achieve an adequate number of cardiovascular events in high‐risk populations. The questions whether populations at lower risk for cardiovascular or renal disease also achieve meaningful benefits from lowering of blood pressure or microalbuminuria are not fully answered because such studies require very large sample sizes and very long follow‐up periods.
Figure.
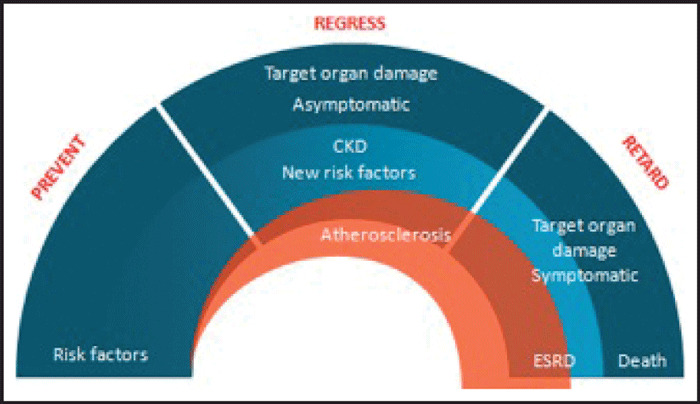
The renal and cardiovascular continuum (modified from reference 1 ). Three stages can be identified. In the first, only cardiovascular risk factors are present. In the second, the disease is asymptomatic, but clinical work‐up demonstrates signs of organ damage. In the third stage, renal or cardiovascular events develop, leading to organ failure and death. Efforts during the first phase should be directed to preventive interventions. During the second phase, attempts should be made to slow the progression of existing arterial or target organ damage. During the third phase, the priority is to delay events. CKD indicates chronic renal disease; ESRD, end‐stage renal disease.
Although regulatory agencies accept only blood pressure, cholesterol, and glucose as cardiovascular disease surrogates, there is a strong argument that microalbuminuria and increased serum creatinine are equally important indicators of future cardiovascular and renal diseases. 6 , 7 The discussion of serum creatinine as a disease surrogate is beyond the scope of this review but utility of albuminuria as a predictor of cardiovascular and renal events is of particular interest. 8 The aim of this paper is to review available basic science and clinical evidence to determine the likelihood that early treatment can prevent microalbuminuria and cardiovascular disease in people with type 2 diabetes.
Pathogenesis of Microalbuminuria
There are several processes acting on the kidney that can initiate or exacerbate albuminuria. In the absence of glomerular or tubular diseases with specific pathogenetic mechanisms (immunological, metabolic, toxic, etc.), it is believed that hemodynamic forces, neurohormones, oxidative stresses, and inflammatory processes interact to promote micro‐ and macroalbuminuria and eventually may lead to chronic kidney disease. Microalbuminuria reflects an abnormality in glomerular capillary permeability to proteins and is also dependent on the tubular capacity to reabsorb filtered albumin. Microalbuminuria is defined clinically as values between 20 and 199 mg albumin/g creatinine in males and 30 to 299 mg albumin/g creatinine in females in at least 2 out of 3 positive spot urine samples on different days. 9 Values above the upper limit for males and females are considered as macroalbuminuria or proteinuria.
Endothelial Injury and Permeability Changes
Based on animal and human experimentation, critical hemodynamic driving forces underlying albuminuria and progressive kidney disease are systemic arterial and glomerular capillary blood pressure. 10 Accompanying the hemodynamic abnormalities of “prediabetes” is increased glomerular permeability. Glomerular structural changes typical of diabetic nephropathy can be observed by the time microalbuminuria becomes apparent. 11 Similar lesions have been demonstrated in conjunctival capillaries in individuals with borderline hypertension and high cardiac output. 12 It is believed that increased oxidative stress and inflammatory cytokines directly injure the endothelium, accelerating the glomerular albumin leakage rate. The dysfunctional endothelium may further exacerbate glomerular hemodynamic changes, further increasing intraglomerular pressure. Concomitant injury to podocytes (epithelial cells on the outside of the glomerulus) further enhances the vicious cycle of hemodynamic changes and endothelial injury and promotes albumin leakage into the urine. Maintenance of hyperglycemia continues to increase oxidative stress through overproduction of superoxide and other reactive oxygen species (ROS) produced by podocytes in response to high glucose levels. 13 Evidence also suggests a direct role of angiotensin II in ROS generation as well as in the inflammatory processes leading to albuminuria. 14 , 15
Microalbuminuria is a sign of systemic and renal microvascular disease as well as progressive diabetic nephropathy. Factors similar to those promoting albuminuria facilitate increased vascular permeability to albumin, a change that also affects other organs such as large arteries, heart, and brain. 16 Indeed, the endothelium lining glomerular capillaries shares many properties with endothelial cells of other organs. The endothelium is damaged early in the pathogenesis of atherosclerotic vascular disease in patients with diabetes, as demonstrated by evidence of endothelial dysfunction with increased levels of von Willebrand factor, C‐reactive protein, and tumor necrosis factor‐alpha. 17 , 18 , 19 These findings coexist with the increased systemic albumin leakage that appears in diabetes even before microalbuminuria is detected and which worsens with disease duration. 17 , 18 Vascular endothelial growth factor, a key regulator of vascular permeability and angiogenesis produced in large amounts by glomerular podocytes, is also implicated in the pathogenesis of diabetic retinal neovascularization and in the development of albuminuria. 20 , 21 Thus, inflammation and oxidative stress play a central role in the pathogenesis of both microvascular and macrovascular disease, with the evidence implicating the vascular endothelium as a site of the initial damage.
Microalbuminuria Is an Important Tool for the Early Diagnosis of Diabetic Nephropathy and Is a Risk Factor for Cardiovascular Disease
Microalbuminuria is most often used in the assessment of diabetic patients based on 2 lines of thought: (1) it is an important marker of diabetic nephropathy; and (2) it is a validated risk factor for cardiovascular disease, including microvascular and macrovascular damage. 22 Microalbuminuria is a frequent finding in patients with diabetes, with or without hypertension. As many as 1 in 5 of people with newly diagnosed type 2 diabetes has microalbuminuria, 23 which eventually can progress to levels of macroalbuminuria or overt proteinuria that define the presence of diabetic nephropathy. Microalbuminuria predicts the development of nephropathy; the greater the urinary albumin excretion, the higher the risk of developing end‐stage renal disease. 23 , 24 On the other hand, several studies summarized in a recent review 25 have highlighted the role of microalbuminuria as an independent marker of cardiovascular morbidity and mortality in patients with type 2 diabetes and hypertension. Because of the association between albuminuria and both renal and cardiovascular disease, international hypertension guidelines advise regular screening for albuminuria in diabetic patients. 26 , 27
Role of Renin‐Angiotensin Blocking Agents
A number of studies using renin‐angiotensin system (RAS) suppression have demonstrated that treatment with RAS blockers is associated with reduction of preexisting micro‐ or macroalbuminuria and with lower risk of cardiovascular events. 28 , 29 , 30 Similarly, suppression of the RAS has been shown to prevent the development of macroalbuminuria in patients with type 2 diabetes. 28 , 31 , 32 The findings of these studies underlie the current recommendations to use a drug that suppresses the RAS (either an angiotensin‐converting enzyme inhibitor or an angiotensin II receptor blocker) as first‐line therapy in diabetic patients. 26 , 27 One “prevention‐oriented” trial with angiotensin II type 1 receptor blockade is the Trial of Preventing Hypertension (TROPHY), 33 which demonstrated a slowing of the age‐related rise of blood pressure with active therapy compared to placebo. The benefit continued for an additional 2 years when subjects were switched to placebo after discontinuation of treatment at year 2. In order to achieve optimal cardiovascular and renal protection, however, strict blood pressure control must accompany the positive effects of RAS suppression. Recent guidelines 26 , 27 raise the possibility of early pharmacological intervention in patients with diabetes or elevated cardiovascular disease risk when blood pressure is at the stage of prehypertension (120–139/80–89 mm Hg). This approach is consistent with current international blood pressure guidelines that suggest that individuals with diabetes or chronic kidney disease (stage 3 or beyond) require blood pressure control to levels below 130/80 mm Hg. 26 , 27 This recommendation was based initially on the close correlation between chronic blood pressure level and rate of decline in glomerular filtration rate 34 but more recent clinical trials have corroborated the additive positive effect of a simultaneous RAS suppression and strict blood pressure control in slowing the progression of chronic kidney disease and in preventing cardiovascular events. 28 , 35 In fact, the maintenance of blood pressure control and RAS suppression for up to 5 years has been shown to impede progressive increases in albuminuria. 36
Is It Possible to Prevent Microalbuminuria and Cardiovascular Disease in Patients With Diabetes?
Most older studies have been conducted in patients with varying degrees of albuminuria, many of whom had advanced kidney or cardiovascular disease. More recently, attention has turned to the possibility that early intervention may prevent not only microalbuminuria but also the onset of subsequent cardiovascular disease.
The Table summarizes the characteristics, outcomes and caveats of these studies, 28 , 37 , 38 , 39 , 40 , 41 , 42 , 43 which, in short, show that RAS suppression tends to blunt or prevent the early increase in albuminuria in individuals with type 2 diabetes if the blood pressure is above optimal levels. In the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT), Action in Diabetes and Vascular Disease: Preterax and Diamicron‐MR Controlled Evaluation (ADVANCE), and the Microalbuminuria, Cardiovascular, and Renal Outcomes in the Heart Outcomes Prevention Evaluation (MICRO‐HOPE) substudy, blood pressure levels were above 130/80 mm Hg. In the Diabetic Retinopathy Candesartan Trials (DIRECT), which showed no benefit of angiotensin receptor blocker on microalbuminuria in patients with type 1 diabetes, the initial blood pressures were 123/75 mm Hg and microalbuminuria was defined on the basis of 3 of 4 rather than 2 of 3 positive urine samples. In the Renin Angiotensin System Study (RASS), in individuals with type 1 diabetes, blood pressures were truly in the optimal range (<115 mm Hg).
Table.
Studies of Prevention of Microalbuminuria in Patients With Type 2 Diabetes and Hypertension
| Study | Population | Design | Endpoint(s) | Mean Baseline BP (mm Hg) | Between‐Group Difference in Mean BP Change (mm Hg) | Outcome(s) | Investigators’ Interpretation | Comments |
|---|---|---|---|---|---|---|---|---|
| ADVANCE 28 | 11,140 patients with type 2 diabetes plus a history of CVD or ≥1 other cardiovascular risk factor. 26% had microalbuminuria at baseline. | RCT of fixed combination of perindopril/indapamide or matching placebo in addition to current therapy. Follow‐up: mean of 4.3 y. | Primary: composites of major macrovascular and microvascular events (death from CVD, nonfatal stroke or nonfatal MI, and new or worsening renal or diabetic eye disease). New or worsening nephropathy and development of microalbuminuria were secondary outcomes. | 145/81 | Mean reduction of 5.6/2.2 vs placebo. | Relative risk of major macrovascular or microvascular event reduced by 9% vs placebo (P=.04). Significant 21% reduction in all renal events (95% CI, 15%–27%; P<.0001), driven by reduced risks for developing microalbuminuria and macroalbuminuria (both P<.003). | BP‐lowering treatment with perindopril‐indapamide administered routinely to individuals with type 2 diabetes provides important renoprotection, even among those with initial BP <120/70 mm Hg. Unable to identify a BP threshold below which renal benefit is lost. | Selection bias: patients intolerant of perindopril/indapamide withdrawn before randomization. Difference in outcome may be attributable to indapamide rather than the combination. Unknown whether ”normotensive” patients and those developing microalbuminuria were already on RAS blockers, although likely (recommended by current management guidelines). Between‐group differences in glycemic control may have influenced results. |
| HOPE and MICROHOPE substudy 38 | 3654 high‐risk patients, aged ≥55 y with type 2 diabetes but no clinical proteinuria. However >30% of patients in each treatment arm had baseline microalbuminuria. | Double‐blind RCT of ramipril vs placebo. ITT analysis. | Primary: composite of MI, stroke, or death from cardiovascular causes. Overt nephropathy was the main outcome in the substudy. | 139/79 | −1.92/−3.3 in the ramipril group vs +0.55/−2.3 in the placebo group. | Study stopped 6 months early because of consistent benefit of ramipril vs placebo. Ramipril lowered risk of the primary outcome by 25% (95% CI, 12–36, P=.0004) (even after adjustment for BP changes), MI by 22% (95% CI, 6–36), stroke by 33% (95% CI, 10–50), cardiovascular death by 37% (95% CI, 21–51), total mortality by 24% (95% CI, 8–37), revascularization by 17% (95% CI, 2–30), and overt nephropathy by 24% (95% CI, 3–40, P=.027). In patients without baseline microalbuminuria, the risk of new‐onset microalbuminuria was reduced, but not significantly, by 9% [–4 to 20], P=.17. | Ramipril significantly lowered the risk of major cardiovascular outcomes and overt nephropathy in a broad range of high‐risk patients with diabetes. | No target BP. No significant reduction of new‐onset microalbuminuria. Ambulatory BP lower in the ramipril group than placebo group. |
| BENEDICT 37 | 1204 patients with type 2 diabetes and normal urinary albumin excretion. | ≥3 y randomized treatment with trandolapril plus verapamil SR, trandolapril alone or verapamil alone. Target HbA1c: <7.0%. Target BP: ≤120/80 mm Hg or ≤130/80 mm Hg in separate publications. | Primary: development of persistent microalbuminuria. | ∼151/87 | 12/7, 12/6, 10/5, and 9/4, respectively in trandolapril + verapamil, trandolapril, verapamil, and placebo groups. | Reached in 5.7% of patients receiving trandolapril plus verapamil, 6% receiving trandolapril, 11.9% receiving verapamil, and 10% receiving placebo. | Optimal BP was achieved in these normoalbuminuric patients with type 2 diabetes. Onset of microalbuminuria was significantly delayed with verapamil SR plus trandolapril compared with placebo (P=.01), and with trandolapril alone vs placebo. The delay in onset of microalbuminuria with verapamil SR plus trandolapril and trandolapril alone exceeded expectations based on changes in BP alone. | Atypical diabetic population: mean HbA1c 5.8%. Mean on‐study BP was 140/80+ mm Hg. Half the patients received SNS blockers, which are known to reduce albuminuria. Thus BENEDICT did NOT show that controlling BP to a low level can prevent microalbuminuria in typical diabetic patients. |
| DIRECT 39 | 1905 patients, aged 37–75 y, with type 2 diabetes who were normoalbuminuric and either normotensive or receiving antihypertensive therapy. | 3 related RCTs to examine effect of candesartan vs placebo on retinopathy in type 1 and type 2 diabetes. Median follow‐up of 4.7 y. Patients who developed microalbuminuria were prescribed open‐label ACE inhibitor therapy. | Primary: incidence of new microalbuminuria in the pooled study population. Secondary: rate of change of albuminuria in the pooled study population. | 123/74.5 (139/79.5 for those on antihypertensive therapy) | −4.3/−2.5 and −2.9/−1.3 vs placebo in patients receiving or not receiving antihypertensive therapy at baseline, respectively (P<.005). | Primary and secondary outcomes did not differ importantly between candesartan and placebo groups. The adjusted rate of change of UAER was lower with candesartan: (by 10% and 6.8%, respectively, in the normotensive patients and those receiving antihypertensive therapy at baseline. | Candesartan had no effect on incidence of microalbuminuria over 4.7 y in a study sample of normoalbuminuric and normotensive patients with type 1 diabetes and normoalbuminuric patients with type 2 diabetes with or without treated hypertension. The adjusted rate of change in UAER, although lower with candesartan, was modest, and its clinical significance is uncertain. | Patients at low risk for microalbuminuria. Studies powered for retinopathy not renal endpoints. Nonsignificant difference between groups might have become significant had study continued for a further 2 y. No information about effect on BP or renal and cardiovascular events in subgroup of patients on dual RAS blockade with candesartan and open‐label ACE inhibitor therapy. |
| ROADMAP (ongoing) 40 , 41 | 4449 patients with type 2 diabetes and confirmed normoalbuminuria and ≥1 additional cardiovascular risk factor. | Double‐blind, randomized RCT of olmesartan vs placebo in addition to standard antihypertensive therapy (excluding RAS inhibitors) to achieve a BP target ≤130/80 mm Hg. Expected duration: median of 5 y. | Primary: occurrence of microalbuminuria. Secondary: include cumulative incidence of cardiovascular and renal disease mortality and morbidity. | 141/84 | Not available; study continues. At 1 year, 61% of total study population had achieved BP goal. | Not available; study continues. | Not applicable. | Avoids many of the shortcomings of previous studies, eg: almost all patients are RAS blocker‐naive; defined target BP; excellent BP control: target achieved by majority of patients at 1 year; ABPM substudy to assess effects on 24‐hour blood pressure profile. |
| TRANSCEND 42 | 5926 subjects with CVD or diabetes with end organ damage intolerant to ACE inhibitors. At baseline 36% and 76%, respectively, had diabetes or hypertension, and 10% had microalbuminuria. | RCT vs placebo. Median follow‐up of 56 months. | Primary renal outcome was first occurrence of dialysis, renal transplantation, doubling of serum creatinine, or death. Secondary outcome was composite of dialysis of doubling of serum creatinine. Other renal outcomes included changes in eGFR and UAER, progression of proteinuria, development of new microalbuminuria. | 141/82 | −4.0/−2.2 for telmisartan vs placebo. | No important difference in the composite renal outcome with telmisartan (58 patients [1.96%]) vs placebo (46 patients [1.55%]) (hazard ratio, 1.29 [95% CI, 0.87 –1.89]; P=0.20). Among the telmisartan and placebo groups, 7 and 10 patients had dialysis and 56 and 36 patients had doubling of serum creatinine, respectively (hazard ratio, 1.59 [CI, 1.04–2.41]; P=.031). Albuminuria increased less with telmisartan than with placebo (32% [CI, 23%–41%] vs 63% [CI, 52%–76%]; P<.001). Declines in eGFR were greater with telmisartan than placebo (mean change in eGFR, −3.2 mL/min per 1.73 m2 (SD, 18.3) vs –0.26 mL/min per 1.73 m2 (SD, 18.0); P<.001). | ARBs offer no renal benefit in ACE‐intolerant subjects at high vascular risk but without macroalbuminuria. Although telmisartan reduced proteinuria, the implications of this reduction as a surrogate marker for progression of renal disease are uncertain in patients with relatively stable eGFR. | At baseline, all patients had CVD and a minority (10%) had microalbuminuria, despite previous treatment with RAS blocker. Results therefore difficult to interpret. |
| RASS 43 | 285 normotensive individuals with type 1 diabetes and normoalbuminuria. | RCT of losartan, enalapril, or placebo. 5‐year follow‐up. | Primary: change in fraction of glomerular volume occupied by mesangium in kidney‐biopsy specimens. Retinopathy: progression on retinopathy severity scale of ≥2 steps. | 120/71 | −4.0/0.0 for losartan vs placebo; −2.0/−2.0 for enalapril vs placebo. | No significant difference in changes in mesangial fractional volume per glomerulus between enalapril, losartan, and placebo groups. 5‐year cumulative incidence of microalbuminuria was 6% in placebo group, 4% with enalapril (P=.96, log‐rank test), and 17% with losartan (P=.01, log‐rank test). Odds of retinopathy progression reduced by 65% with enalapril vs placebo and by 70% with losartan vs placebo, independently of BP changes. | Early blockade of the RAS in patients with type 1 diabetes slowed progression of retinopathy but NOT of nephropathy. Blockade of RAS for primary prevention of diabetic nephropathy in type 1 diabetes is not supported by the present evidence. | Carefully conducted study in patients with BP truly in the normal range. |
Abbreviations: ABPM, ambulatory blood pressure monitoring; ACE, angiotensin‐converting enzyme; ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron‐MR Controlled Evaluation; ARB, angiotensin receptor blocker; BP, blood pressure; BENEDICT, Bergamo Nephrologic Diabetes Complications Trial; CI, confidence interval; CVD, cardiovascular disease; DIRECT, Diabetic Retinopathy Candesartan Trials; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HOPE, Heart Outcomes Prevention Evaluation study; ITT, intention‐to‐treat; MI, myocardial infarction; MICRO‐HOPE, Microalbuminuria, Cardiovascular, and Renal Outcomes in the HOPE substudy; RAS, renin‐angiotensin system; RASS, Renin Angiotensin System Study; RCT, randomized controlled trial; ROADMAP, Randomized Olmesartan and Diabetes Microalbuminuria Prevention study; SD, standard deviation; TRANSCEND, Telmisartan Randomised Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease; UAER, urinary albumin excretion rate.
The Randomized Olmesartan and Diabetes Microalbuminuria Prevention Study
Against this background, what new insight does the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study offer? Early RAS suppression and strict blood pressure control are still believed to be pivotal to the early protection against cardiovascular and renal disease of patients with type 2 diabetes. However, the capacity of blood pressure control by itself, independent of intervention on the RAS, to prevent microalbuminuria remains unproven. ROADMAP is a placebo‐controlled, multicenter, double‐blind, randomized trial to examine the effect of olmesartan medoxomil compared with placebo. 40 This trial incorporates a number of important design features (Table) intended to avoid the shortcomings of previous studies. Of particular interest is the fact that 1‐year interim results from ROADMAP show excellent blood pressure control in the overall study population with 61% of patients achieving the target of ≤130/80 mm Hg. 41 Thus ROADMAP should be able to answer the question of whether blockade of the RAS, in patients achieving target blood pressure, provides primary prevention against microalbuminuria and subsequent cardiovascular disease.
Acknowledgments
Acknowledgment and disclosures: This article originated from the proceedings of a meeting of most of the authors (LR, HH, WM, SO, JI, and BW). Editorial assistance on the transcription of the remarks of the proceedings was funded by Daiichi Sankyo and the authors wish to thank Liz McNeil Grist, PhD for her initial editing of the proceedings. No honoraria for this article were paid to any of the authors, and all were involved in the final manuscript. Dr Bakris: Grant/Research Support—Juvenile Diabetes Research Foundation (JDRF), GSK, Forest Labs, CVRx; Consultant/Advisor—GSK, Merck, Novartis, Boehringer‐Ingelheim, Takeda, Abbott, Walgreen’s; Formulary Committee—BMS/Sanofi, Gilead, Forest, National Kidney Foundation, American Heart Association; Speakers Bureau—Novartis, Forest, GSK. Dr Oparil: Recipient of grants‐in‐aid—Daiichi Sankyo Inc, Forest Laboratories, Gilead, Novartis, and Takeda; Consultant—Boehringer Ingelheim, Bristol Myers‐Squibb, Daiichi Sankyo Inc, Forest Laboratories, NicOx, Novartis, Sanofi Aventis, The Salt Institute.
References
- 1. Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991;121:1244–1263. [DOI] [PubMed] [Google Scholar]
- 2. Ruilope L, Kjeldsen SE, De La Sierra A, et al. The kidney and cardiovascular risk – implications for management: a consensus statement from the European Society of Hypertension. Blood Press. 2007;16:72–79. [DOI] [PubMed] [Google Scholar]
- 3. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 4. Turnbull F, Neal B, Algert C, et al. Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410–1419. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 6. Gerstein HC, Mann JF, Pogue J, et al. Prevalence and determinants of microalbuminuria in high‐risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study. The HOPE Study Investigators. Diabetes Care. 2000;23:B35–B39. [PubMed] [Google Scholar]
- 7. Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity‐onset diabetes. N Engl J Med. 1984;310:356–360. [DOI] [PubMed] [Google Scholar]
- 8. Gansevoort RT, De Jong PE. The case for using albuminuria in staging chronic kidney disease. J Am Soc Nephrol. 2009;20:465–468. [DOI] [PubMed] [Google Scholar]
- 9. De Jong P, Curhan GC. Screening, monitoring, and treatment of albuminuria: public health perspectives. J Am Soc Nephrol. 2006;17:2120–2126. [DOI] [PubMed] [Google Scholar]
- 10. Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70:1694–1705. [DOI] [PubMed] [Google Scholar]
- 11. Dalla Vestra M, Masiero A, Roiter AM, et al. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes Diabetes. 2003;52:1031–1035. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan JM, Prewitt RL, Josephs JA. Attenuation of the microcirculation in young patients with high‐output borderline hypertension. Hypertension. 1983;5:844–851. [DOI] [PubMed] [Google Scholar]
- 13. Susztak K, Raff AC, Schiffer M, et al. Glucose‐induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 14. Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol. 2007;22:311–315. [DOI] [PubMed] [Google Scholar]
- 15. Nickenig G, Harrison DG. The AT(1)‐type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–396. [DOI] [PubMed] [Google Scholar]
- 16. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. [DOI] [PubMed] [Google Scholar]
- 17. Schalkwijk CG, Poland DC, Van Dijk W, et al. Plasma concentration of C‐reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42:351–357. [DOI] [PubMed] [Google Scholar]
- 18. Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond). 2005;109:143–159. [DOI] [PubMed] [Google Scholar]
- 19. Willerson J, Ridker P. Inflammation as a cardiovascular risk factor. Circulation. 2004;21(suppl 1):II2–II10. [DOI] [PubMed] [Google Scholar]
- 20. Chiarelli F, Spagnoli A, Basciani F, et al. Vascular endothelial growth factor (VEGF) in children, adolescents and young adults with Type 1 diabetes mellitus: relation to glycaemic control and microvascular complications. Diabet Med. 2000;17:650–656. [DOI] [PubMed] [Google Scholar]
- 21. Kim NH, Oh JH, Seo JA, et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT‐1 in diabetic nephropathy. Kidney Int. 2005;67:167–177. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. [DOI] [PubMed] [Google Scholar]
- 23. Tobe SW, McFarlane PA, Naimark DM. Microalbuminuria in diabetes mellitus. CMAJ. 2002;167:499–503. [PMC free article] [PubMed] [Google Scholar]
- 24. Collins AJ, Li S, Gilbertson DT, et al. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;Nov:S24–S31. [DOI] [PubMed] [Google Scholar]
- 25. Basi S, Fesler P, Mimran A, et al. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabetes Care. 2008;31(suppl 2):S194–S201. [DOI] [PubMed] [Google Scholar]
- 26. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 27. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 28. De Galan BE, Perkovic V, Ninomiya T, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol. 2009;20:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–927. [DOI] [PubMed] [Google Scholar]
- 30. Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. [DOI] [PubMed] [Google Scholar]
- 31. Makino H, Haneda M, Babazono T, et al. Microalbuminuria reduction with telmisartan in normotensive and hypertensive Japanese patients with type 2 diabetes: a post‐hoc analysis of The Incipient to Overt: Angiotensin II Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) study. Hypertens Res. 2008;31:657–664. [DOI] [PubMed] [Google Scholar]
- 32. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. [DOI] [PubMed] [Google Scholar]
- 33. Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin‐receptor blocker. N Engl J Med. 2006;354:1685–1697. [DOI] [PubMed] [Google Scholar]
- 34. Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end‐stage renal disease in men. N Engl J Med. 1996;334:13–18. [DOI] [PubMed] [Google Scholar]
- 35. Berl T, Hunsicker LG, Lewis JB, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16:2170–2179. [DOI] [PubMed] [Google Scholar]
- 36. Barnett AH, Bain SC, Bouter P, et al. Angiotensin‐receptor blockade versus converting‐enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. [DOI] [PubMed] [Google Scholar]
- 37. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–1951. [DOI] [PubMed] [Google Scholar]
- 38. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 39. Bilous R, Chaturvedi N, Sjolie AK, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med. 2009;151:11–20. [DOI] [PubMed] [Google Scholar]
- 40. Haller H, Viberti GC, Mimran A, et al. Preventing microalbuminuria in patients with diabetes: rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. J Hypertens. 2006;24:403–408. [DOI] [PubMed] [Google Scholar]
- 41. Izzo JL Jr, Pecan L, Ito S, et al. Influence of JNC7 blood pressure (BP) stage on antihypertensive drug benefits in diabetics: the ROADMAP experience at one year. J Clin Hypertens (Greenwich). 2009;11:A121–A122. [Google Scholar]
- 42. Mann JF, Schmieder RE, Dyal L, et al. Effect of telmisartan on renal outcomes: a randomized trial. Ann Intern Med. 2009;151:1–10. [DOI] [PubMed] [Google Scholar]
- 43. Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


