Abstract
Arterial hypertension and proteinuria are risk factors for chronic kidney disease. A mobile clinic was parked in a central plaza of 11 Italian cities to check blood pressure (BP), prescribe antihypertensive drugs, assess for proteinuria, and provide awareness about hypertension. Among 3757 patients, 56% were hypertensive, 37% were not diabetic nor proteinuric with BP ≥140/90 mm Hg, 17% were diabetic or proteinuric with BP ≥130/80 mm Hg, and 11% were on treatment with BP at target. Among 1204 treated patients, 400 (33%) had controlled BP. Among all 2114 hypertensive patients, only 1344 (64%) were aware of their hypertension. Awareness was greater among treated patients at target (99%). As many as 523 (14%) patients had proteinuria ≥30 mg/dL. The authors conclude that awareness of people walking in the street about their BP and proteinuria is insufficient. Mobile screening clinics may increase public awareness and detection of hypertension and proteinuria in the general community and detect patients at risk for chronic kidney disease.
Chronic kidney disease (CKD) is a major health problem that results in considerably increased cardiovascular morbidity and mortality. Surviving patients with CKD often develop end‐stage renal disease (ESRD) that requires replacement therapy (dialysis or renal transplant). The number of patients who enter chronic dialysis programs is increasing all over the world. In 2004 in Italy, 169 per million population (pmp) started chronic dialysis, compared with 90.4 pmp in 1993. 1 This number has reached 342 pmp in the United States. 2
Today we can slow the progression of CKD by controlling hypertension (HT) and using kidney‐protective drugs (eg, angiotensin‐converting enzyme inhibitors [ACEIs], angiotensin II receptor blockers [ARBs]). However, better results are achieved when prevention measures are taken early. Thus, it is important to detect patients at risk in the general population.
In 2006, the International Federation of Kidney Foundations and the International Society of Nephrology launched the first World Kidney Day to increase awareness of CKD and its associated cardiovascular morbidity and mortality. In 2004, the National Kidney Foundation of Italy (Fondazione Italiana del Rene Onlus [FIR Onlus]) had already planned an informative program (“Prevention of Renal Diseases”) to sensitize the general population about the major risk factors for CKD, including HT and proteinuria. 3 The present investigation was planned and conducted by the National Kidney Foundation of Italy in 2005, as its second informative program, with 2 major goals: (1) to inform the general Italian population about HT and proteinuria in a nontraditional medical scenario represented by the main squares of 11 Italian cities, and (2) to give a basic estimate of awareness and management of HT in the recruited population.
Material and Methods
Study Design
The aim of the present investigation was to provide a public informative program about CKD, HT, and proteinuria. The secondary goal was to illustrate the awareness and management of HT in the enrolled patients.
A mobile clinic with 2 examination rooms, a restroom for urine collection, and a waiting hall was used to evaluate participants. The truck had, on the external walls, big posters inviting people to a free evaluation of blood pressure (BP) and urine. The truck was parked for 4 consecutive days (from 10:00 am to 19:00 pm) in a central plaza of each 1 of 11 Italian cities (Avellino, Caserta, Foggia, Imperia, Marcianise, Messina, Milano, Milazzo, Napoli, Perugia, and Siracusa), moving from one city to another.
People walking in the squares had free access to the truck for measurement of BP and evaluation of urine. Trained personnel (nephrologists and nurses formally validated for their ability to accurately measure BP) assessed 3 measures of systolic BP (SBP) and diastolic BP (DBP) of passersby in the seated position after 5 minutes of rest for each recorded reading (the mean of 3 measures was recorded). Weight and height of dressed patients were assessed in order to estimate body mass index.
A urine sample was collected after BP measurement and proteinuria was tested by urine dipsticks (Aution Sticks 10EA; Arkray Inc, Kyoto, Japan) distributed by Menarini Diagnostics, Firenze, Italy, specifically measuring proteinuria and not albuminuria.
Enrolled patients verbally answered a questionnaire concerning personal data and presence of major cardiovascular risk factors such as HT, diabetes, hypercholesterolemia, hypertriglyceridemia, present or past smoking, coffee drinking, family history of HT, myocardial infarction or stroke, and antihypertensive medications. No data on socioeconomic or educational status were collected. Patients were considered aware of their HT when they answered “Yes” to the question “Do you suffer from HT?” No standardized questions were asked about knowledge of proteinuria or CKD. Nephrologists provided a brief and not standardized verbal message concerning the importance of controlling BP, proteinuria, and CKD.
The present investigation was not designed to assess the true prevalence of HT and related risk factors in the Italian population. Criteria for selection of cities and squares were only based on the willingness of nephrologists to participate in the project. Cities were not equally distributed across the country, and the population enrolled was not representative of the regional or national Italian community.
Statistical Analysis
Categoric and continuous variables are expressed as frequencies (percentages) and mean ± standard deviation, respectively. Data were stratified as a function of BP categories. Because it was not designed as a formal sample of the Italian population, inferential statistical analysis was not provided in the present study.
The prevalence of HT among patients screened was estimated according to the following criteria. Diabetic and proteinuric patients were considered hypertensive if they had BPs ≥130/80 mm Hg. Individuals who did not have diabetes or proteinuria were considered hypertensive if they had BPs ≥140/90 mm Hg. All patients taking antihypertensive drugs were considered hypertensive. Among individuals taking antihypertensive medications, BP was considered at target when SBP/DBP was <130/80 mm Hg for diabetic and proteinuric patients and when SBP/DBP was <140/90 mm Hg for nondiabetic and nonproteinuric patients.
Results
Patient Characteristics and Prevalence of HT
In our study, 3757 patients (aged 56±16 years; 60% male) were evaluated. All of the patients answered the questionnaire and received a BP measurement and urine analysis.
Patient characteristics are listed in the Table as a function of BP categories. A total of 1714 (46%) patients were hypertensive and 1087 (63%) were diabetic or proteinuric with SBP/DBP ≥130/80 mm Hg. Four hundred (11%) patients who were taking antihypertensive treatment and considered normotensive were also considered to be hypertensive. The prevalence of HT was 56% (2114 patients).
Table.
Characteristics of 3757 Patients Stratified as a Function of Hypertensive Status
| Total Population | Normotensive Population | Total Hypertensive Population | Patients With BP ≥140/90 Without Diabetes or Proteinuria | Patients With BP ≥130/80 With Diabetes or Proteinuria | Patients Taking Anti‐HT Treatment at Target | |
|---|---|---|---|---|---|---|
| All patients, No. (%) | 3757 | 1643 (44) | 2114 (56) | 1087 (37) | 627 (17) | 400 (11) |
| Referred anti‐HT treatment | 1204 (32) | – | 1204 (32) | 510 (47) | 294 (47) | – |
| Awareness of having HT | – | 60 (4) | 1344 (64) | 606 (56) | 344 (55) | 394 (99) |
| Diabetes | 397 (11) | 36 (2) | 361 (17) | – | 335 (53) | 26 (7) |
| Proteinuria ≥30 mg/dL | 523 (14) | 132 (8) | 391 (19) | – | 367 (56) | 24 (6) |
| Diabetes or proteinuria | 830 (22) | 161 (10) | 669 (32) | – | – | 42 (11) |
| SBP, mm Hg | 131±19 | 118±11 | 141±17 | 148±14 | 141±18 | 122±9 |
| DBP, mm Hg | 79±11 | 73±8 | 84±10 | 87±9 | 84±10 | 75±7 |
| Sex (male), No. (%) | 2270 (60) | 858 (52) | 1412 (67) | 707 (65) | 462 (74) | 243 (61) |
| Age, y | 56±16 | 48±16 | 62±13 | 61±13 | 62±13 | 64±11 |
| Body mass index, kg/m2 | 26±4 | 25±4 | 27±4 | 27±4 | 28±4 | 27±4 |
| Former smokers, No. (%) | 951 (25) | 307 (19) | 644 (31) | 324 (30) | 192 (31) | 128 (32) |
| Present smokers, No. (%) | 785 (21) | 399 (24) | 386 (18) | 201 (19) | 126 (20) | 59 (15) |
| Referred high triglycerides | 522 (14) | 126 (8) | 396 (19) | 171 (16) | 150 (24) | 75 (19) |
| Referred high cholesterol | 949 (25) | 280 (17) | 669 (32) | 334 (31) | 217 (35) | 118 (39) |
| Family history of hypertension, No. (%) | 1481 (39) | 598 (36) | 883 (42) | 455 (42) | 235 (38) | 193 (48) |
| Family history of stroke, No. (%) | 581 (16) | 210 (13) | 371 (18) | 193 (18) | 98 (16) | 80 (20) |
| Family history of myocardial infarction, No. (%) | 678 (18) | 248 (15) | 430 (20) | 208 (19) | 139 (22) | 83 (21) |
Abbreviations: BP, blood pressure; DBP, diastolic BP; HT, hypertension; SBP, systolic BP.
HT was descriptively associated with male sex, older age, greater body mass index, history of diabetes and smoking, dyslipidemia, family history of HT, and stroke or myocardial infarction (Table). HT was also associated with the presence of proteinuria ≥30 mg/dL (Table). As many as 200 (15%) individuals among the 1404 patients who were hypertensive, were not on therapy.
Awareness and Management of HT
A total of 64% of hypertensive patients were aware that their BP level was elevated (Table). Awareness was considerably greater (99%) among treated patients with BP at target levels (Table). Unaware and untreated persons were significantly younger than aware and treated patients (data not shown). Only 47% of the 627 diabetic or proteinuric individuals who were hypertensive and only 56% of the 1087 nondiabetic and nonproteinuric patients who were hypertensive were taking antihypertensive medications (Table). Among the 1204 treated patients, 400 (33%) were at target levels. The most commonly used medications were ACEIs (35%), diuretics (27%), ARBs (23%), and calcium channel blockers (CCBs) (23%).
A total of 397 individuals (11%) were diabetic (Table). Among them, only 210 (53%) were receiving antihypertensive therapy and only 26 achieved BP <130/80 mm Hg. The distribution of medication classes are noted in the Figure.
Figure.
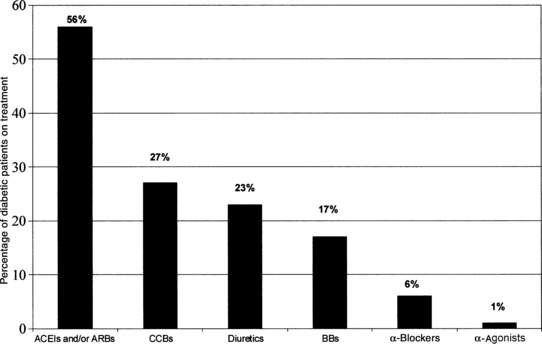
Classes of antihypertensive medications among 210 treated patients classified as diabetic. ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CCBs, calcium channel blockers; BBs, β‐blockers.
A total of 523 (14%) patients had proteinuria ≥30 mg/dL (Table). As expected, prevalence of proteinuria was greater in the hypertensive compared with the nonhypertensive population (19% vs 8%) (Table). Among the 523 patients with proteinuria ≥30 mg/dL, 88 (17%) had BP <120/80 mm Hg (81 of them without taking medications). One hundred seventy‐two individuals with proteinuria were on therapy: 53% ACEIs or ARBs, 2% combined therapy with both ACEIs and ARBs, 30% diuretics, 27% CCBs, 17%β‐blockers, 9%α‐blockers, and 2%α2‐agonists. Only 14% of 172 treated individuals with proteinuria had BP values <130/80 mm Hg.
Discussion
According to National Health and Nutrition Examination Survey (NHANES) reports, 11% of the US population is affected by CKD, 4 and its prevalence is expected to rapidly increase as a consequence of both longer life expectancy, the growing incidence of diabetic nephropathy, and the fact that hypertensive patients are not experiencing life‐threatening events in their 50s through 70s and are living longer. 5 Up to 64% of cases of renal insufficiency are diagnosed late. The frequent absence of symptoms and the insufficient awareness about kidney diseases contribute to late diagnosis of CKD and to the yearly increase of incident patients in dialysis therapy.
HT is a highly prevalent 6 and treatable risk factor for CKD 7 and represents a major target of prevention strategies against the onset and progression of CKD. 8 , 9 HT is often undiagnosed and not properly treated. One of the major limitations of adequate control of HT is the lack of awareness of HT in the general public, although awareness has improved in recent years. 10 Correction of HT greatly decreases progression of CKD both in diabetic and nondiabetic patients. 11 The use of specific antihypertensive drugs (ie, ACEIs and ARBs) usually given with a diuretic has resulted in a slowing down of ESRD in many patients. 12 , 13 , 14 , 15 , 16 , 17
In this study, the prevalence of HT was found to be 56%, consistent with the results of previous reports in Italy 3 , 18 and other European countries. 19 , 20 , 21 , 22 In hypertensive patients, 64% were aware of their high BP (Table). Despite awareness, however, as many as 217 hypertensive patients (104 of them diabetic or proteinuric) were not taking any antihypertensive drugs.
Reported public awareness of HT is disappointing. According to NHANES reports, 30% of the US population was unaware of their hypertensive status in the year 2000. 23 In recent studies, however, more than 75% to 80% were aware of their BPs. 10 , 24 In our study, patients who were treated and at target were aware that they had HT.
Proteinuria is considered an early marker of both cardiovascular and renal damage in apparently healthy individuals. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Proteinuria is a major independent risk factor of CKD progression 26 , 27 , 28 , 29 and cardiovascular disease. 30 , 31 Microalbuminuria is prevalent (40%) among diabetic 32 and hypertensive patients (8%–23%). 33 , 34 Individuals with microalbuminuria have been considered to be at a 4‐ and 2‐fold higher risk of new‐onset diabetes 35 and new‐onset HT, 36 respectively. It has been demonstrated that dipsticks can also detect microalbuminuria, 37 and the association of CKD progression with albuminuria, assessed by urine dipsticks, has been previously investigated. 28 In the present study, we assessed proteinuria by urine dipsticks. The overall prevalence of proteinuria ≥30 mg/dL was 14% (Table) and consistent with previous investigations. 3 , 32 In our analysis, ACEIs and ARBs were used in only 56% of patients with diabetes.
In our investigation, only 14% of 172 treated proteinuric individuals had BP values <130/80 mm Hg. Among the 397 diabetic individuals, 210 (53%) were on therapy, but only 26 of them had achieved BP <130/80 mm Hg.
Limitations
There were several limitations to the present study. (1) All data were collected only by questionnaires, thus a recall bias is expected; (2) all patients spontaneously entered the trucks, therefore we can expect a definite selection bias in favor of individuals more sensitive to health problems; (3) the use of antihypertensive medications was only suggested, not verified; (4) BP values might have been affected by the white‐coat HT syndrome, which could have been excluded only by further BP measurements; and (5) we assessed proteinuria by a urine dipstick on a single urine sample as the cheapest and most cost‐effective method for a public surveillance program in the general population; however, the colorimetric reaction on dipsticks may lead to a rate of intraoperator and interoperator error.
Conclusions
Despite these limitations, our results demonstrate that awareness concerning HT among Italians who volunteered to be screened for HT and proteinuria is less than optimal. Public screening and information programs using a mobile clinic such as in the present study may increase public awareness concerning CKD and its prevention.
Acknowledgments:
This paper has been prepared on the basis of what the National Kidney Foundation (NFK) of Italy (Fondazione Italiana del Rene Onlus) planned and carried out in 2005 in preparation of World Kidney Day. The president of the Italian Kidney Foundation, Vittorio Andreucci, directly planned and managed the study. None of the authors were on any of the contributing pharmaceutical companies’ speakers’ bureaus or consultant panels at the time of the study. NKF of Italy received funding from the listed sponsors to organize and manage the study. The authors were NKF volunteers and did not receive any grants by sponsors. We thank the following nephrologists and nurses who contributed to the success of our project: V. Bellizzi (Avellino); R. Alberico, C. Saviano, G. Carfora, M. Sorice, S. Mangiacapra, A. Barbato (Caserta); D. A. Procaccini, M. Querques, F. Aucella, A. De Min, C. Montemurno, G. Pompa, A. Latino (Foggia); A. Zollo, S. Galli, M. Piredda, M. Mji, F. Re (Imperia); V. Savica, G. Costantino, D. Santoro (Messina); L. Rocca Rey, A. Butti, G. Chiarelli (Milano); P. Esposito, M. L. Sirico, S. Manzi, M. De Cicco, M. Del Prete, G. Passaro, G. Palmiero, V. Serio (Napoli); and M. Gallo, M. Gianni, C. Caponetto, S. Salvatore (Siracusa). This study was financially supported by Amgen, Roche, Abbott, AstraZeneca, Baxter, Bellco, Boehringer Ingelheim Italia, B. Braun Carex, Chiesi Farmaceutici, Dompè Biotec, Estor, Ferrero, Fujisawa, Gambro, Hospal, Janssen Cilag, Merck, Sharp‐Dohme, Nephrocare, Novartis, Plasmon Dietetici Aliment, Schering‐Plough, Shire Italia, Sigma Tau, and Unicredit Banca.
References
- 1. Valderrabano F, Jones EH, Mallick NP. Report on management of renal failure in Europe, XXIV, 1993. Nephrol Dial Transplant. 1995;10(suppl 5):1–25. [DOI] [PubMed] [Google Scholar]
- 2. Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis. 2007;1(suppl 1):A6–A7, S1–S296. [DOI] [PubMed] [Google Scholar]
- 3. Russo D, Napolitano P, Sirico ML, et al. A project to prevent renal diseases in the general population. J Nephrol. 2007;20:36–42. [PubMed] [Google Scholar]
- 4. Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Xue JL, Ma JZ, Louis TA, et al. Forecast of the number of patients with end‐stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12: 2753–2758. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164:2126–2134. [DOI] [PubMed] [Google Scholar]
- 7. Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community‐based population: a 10‐year follow‐up study. Kidney Int. 2007;71:159–166. [DOI] [PubMed] [Google Scholar]
- 8. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 9. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 10. Moser M, Franklin SS. Hypertension management. Results of a new national survey for the Hypertension Education Foundation. Harris interactive. J Clin Hypertens (Greenwich). 2007;9:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. [DOI] [PubMed] [Google Scholar]
- 12. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. [DOI] [PubMed] [Google Scholar]
- 13. Jacobsen P, Andersen S, Jensen BR, et al. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J Am Soc Nephrol. 2003;14:992–999. [DOI] [PubMed] [Google Scholar]
- 14. Jacobsen P, Andersen S, Rossing K, et al. Dual blockade of the renin‐angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63:1874–1880. [DOI] [PubMed] [Google Scholar]
- 15. Nakao N, Yoshimura A, Morita H, et al. Combination treatment of angiotensin‐II receptor blocker and angiotensin‐converting‐enzyme inhibitor in non‐diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361:117–124. [DOI] [PubMed] [Google Scholar]
- 16. Bianchi S, Bigazzi R, Caiazza A, et al. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am J Kidney Dis. 2003;41:565–570. [DOI] [PubMed] [Google Scholar]
- 17. Sandhu S, Wiebe N, Fried LF, et al. Statins for improving renal outcomes: a meta‐analysis. J Am Soc Nephrol. 2006;17:2006–2016. [DOI] [PubMed] [Google Scholar]
- 18. Mancia G, Parati G, Borghi C, et al. Hypertension prevalence, awareness, control and association with metabolic abnormalities in the San Marino population: the SMOOTH study. J Hypertens. 2006;24:837–843. [DOI] [PubMed] [Google Scholar]
- 19. Macedo ME, Lima MJ, Silva AO, et al. Prevalence, awareness, treatment and control of hypertension in Portugal: the PAP study. J Hypertens. 2005;23:1661–1666. [DOI] [PubMed] [Google Scholar]
- 20. Colhoun HM, Dong W, Poulter NR. Blood pressure screening, management and control in England: results from the health survey for England 1994. J Hypertens. 1998;16:747–752. [DOI] [PubMed] [Google Scholar]
- 21. Banegas JR, Guallar‐Castillon P, Rodriguez‐Artalejo F, et al. Association between awareness, treatment, and control of hypertension, and quality of life among older adults in Spain. Am J Hypertens. 2006;19:686–693. [DOI] [PubMed] [Google Scholar]
- 22. Wolf‐Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in six European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. [DOI] [PubMed] [Google Scholar]
- 23. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 24. Viera AJ, Kshirsagar AV, Hinderliter AL. Lifestyle modifications to lower or control high blood pressure: is advice associated with action? The behavioral risk factor surveillance survey. J Clin Hypertens (Greenwich). 2008;10: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohmit SE, Flack JM, Peters RM, et al. Longitudinal Study of the National Kidney Foundation’s (NKF) Kidney Early Evaluation Program (KEEP). J Am Soc Nephrol. 2003;7(suppl 2):S117–S121. [DOI] [PubMed] [Google Scholar]
- 26. Arnlov J, Evans JC, Meigs JB, et al. Low‐grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. [DOI] [PubMed] [Google Scholar]
- 27. Verhave JC, Gansevoort RT, Hillege HL, et al. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl. 2004;66: S18–S21. [DOI] [PubMed] [Google Scholar]
- 28. Iseki K, Ikemiya Y, Iseki C, et al. Proteinuria and the risk of developing end‐stage renal disease. Kidney Int. 2003;63:1468–1474. [DOI] [PubMed] [Google Scholar]
- 29. Keane WF, Brenner BM, De Zeeuw D, et al. The risk of developing end‐stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–1507. [DOI] [PubMed] [Google Scholar]
- 30. Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. [DOI] [PubMed] [Google Scholar]
- 31. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 32. Parving HH, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057–2063. [DOI] [PubMed] [Google Scholar]
- 33. Wachtell K, Palmieri V, Olsen MH, et al. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Losartan intervention for endpoint reduction. Am Heart J. 2002;143:319–326. [DOI] [PubMed] [Google Scholar]
- 34. De Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17:2100–2105. [DOI] [PubMed] [Google Scholar]
- 35. Brantsma AH, Bakker SJ, De Zeeuw D, et al. Urinary albumin excretion as a predictor of the development of hypertension in the general population. J Am Soc Nephrol. 2006;17:331–335. [DOI] [PubMed] [Google Scholar]
- 36. Brantsma AH, Bakker SJ, Hillege HL, et al. Urinary albumin excretion and its relation with C‐reactive protein and the metabolic syndrome in the prediction of type 2 diabetes. Diabetes Care. 2005;28:2525–2530. [DOI] [PubMed] [Google Scholar]
- 37. De Jong PE, Van Der Velde M, Gansevoort RT, et al. Screening for chronic kidney disease: where does Europe go? Clin J Am Soc Nephrol. 2008;3:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]


