Abstract
J Clin Hypertens (Greenwich). 2010;12:64–72. © 2009 Wiley Periodicals, Inc.
To determine whether resistance training effectively maintains improvements in cardiometabolic syndrome risk factors during weight regain, 9 individuals lost 4% to 6% of their body weight during an 8‐ to 12‐week diet‐ and aerobic exercise–induced weight loss phase followed by a controlled weight regain phase (8–12 weeks), during which they regained approximately 50% of the lost weight while participating in a supervised resistance training program. Following weight loss (6.0%±0.3%), body mass index, body fat percentage, waist circumference, all abdominal adipose tissue depots, total cholesterol, low‐density lipoprotein cholesterol, insulin, and homeostasis model assessment (HOMA) were significantly reduced, while quantitative insulin‐sensitivity check index (QUICKI) and cardiorespiratory fitness (maximal oxygen consumption) significantly increased. During weight regain (48.3%±3.3% of lost weight), body fat percentage, waist circumference, and maximal oxygen consumption were maintained and muscular strength and lean body mass significantly increased. Abdominal adipose tissue depots, insulin, HOMA, and QUICKI did not significantly change after weight regain. Resistance training was effective in maintaining improvements in metabolic health during weight regain.
The incidence of overweight and obesity among Americans is climbing at an alarming rate. 1 Accompanying this rise is an increase in the development of chronic diseases such as the cardiometabolic syndrome, type II diabetes mellitus, and coronary heart disease. 2 Lifestyle modifications such as weight loss (WL), diet, and exercise have proven effective at beneficially altering many markers of metabolic health, 3 , 4 including abdominal adiposity, insulin sensitivity, and blood lipid profile. WL is associated with reductions in visceral adiposity 5 and improvements in plasma triglycerides (TGs), 6 high‐density lipoprotein cholesterol (HDL‐C), 4 low‐density lipoprotein cholesterol (LDL‐C), 4 and measures of insulin sensitivity. 4 , 6 While the effect of WL is clear, there is still great value in determining the impact of exercise training during a period of weight gain on metabolic health. Although many adults are able to successfully lose weight, only a small percentage are able to maintain long‐term WL. 7
Resistance training (RT), independent of WL, was shown to improve cardiometabolic syndrome risk factors including abdominal obesity 8 , 9 and insulin resistance. 8 , 10 Therefore, it is plausible that RT can protect against the detrimental metabolic consequences of weight regain (WR). Also, RT has been shown to increase muscle mass and strength. 11 As skeletal muscle is the major consumer of blood glucose, 12 it is plausible that an exercise training program with the potential to increase skeletal muscle mass might also improve insulin sensitivity in addition to body composition. Additionally, muscular strength was shown to be inversely associated with the incidence of the metabolic syndrome in men, and this relationship was found to be independent of cardiorespiratory fitness. 13 Moreover, RT is associated with increased resting energy expenditure, 11 , 14 fat oxidation, 14 and adipose tissue (AT) lipolysis, 14 and therefore could provide mechanisms by which RT could beneficially affect body composition as well as intramuscular and plasma lipid concentrations.
Maintaining significant long‐term WL is difficult and remains an elusive goal for most individuals. In at‐risk individuals, the possible retention of major health benefits may provide the needed incentive to maintain an active lifestyle even when WL or weight maintenance goals are not achieved. Therefore, the purpose of this study was to determine whether RT effectively maintains diet‐ and exercise‐induced improvements in cardiometabolic syndrome risk factors during a period of controlled WR. Given the documented positive effects of RT on cardiometabolic syndrome risk factors that can be achieved in the absence of WL, it was hypothesized that RT would maintain metabolic health during a period of WR.
Methods
Participants
Nine participants (1 man and 8 premenopausal women; aged 37±3 years with body mass indexes [BMIs] of 33.8±1.5 kg/m2) completed the study. The study originally began with 14 participants (5 men and 9 women) but 5 (4 men and 1 woman) discontinued participation due to the weekly time commitment. The primary criteria were overweight to class II obese (BMI, 25–39.9 kg/m2) and sedentary, defined as no more than 60 min/wk or energy expenditure of 500 kcal/wk from planned exercise. All patients were nonsmokers and not taking any antihypertensive or lipid‐lowering medication. Any potential patient having known diabetes or cardiovascular disease was excluded from the study. Each participant completed health history, physical activity, and dietary questionnaires before beginning the study. Prior to participation, each patient was informed of the risks and benefits associated with the study and signed a written consent form approved by the University of Missouri Health Sciences institutional review board.
Experimental Design
The study consisted of 2 phases: short‐term WL followed by partial WR. During the WL phase, patients lost 4% to 6% of their initial body weight during an 8‐ to 12‐week diet‐ and aerobic exercise–induced WL intervention. The duration of the WL phase was dependent on each participant’s rate of WL, and the average duration of the WL phase was 11 weeks. After WL, all patients participated in a controlled WR phase, during which they regained approximately 50% of the lost weight while participating in a supervised RT program. The targeted duration of the WR phase was 8 to 12 weeks, and the length of time spent in this phase was dependent on each participant’s rate of WR (mean duration, 10 weeks). Outcome measures were assessed at baseline, post‐WL, and post‐WR.
Diet. The diet component of the WL phase consisted of caloric restriction (goal of ∼500 kcal/d), and partial WR during the WR phase was achieved by increasing caloric intake above that which was consumed during the WL phase. Seven‐day dietary records were collected from all participants prior to the start of the study, and dietary intake was recorded daily throughout the study. The total energy and macronutrient and micronutrient composition of the dietary records were analyzed using Food Processor SQL software (ESHA Research, Salem, OR) and were reviewed during an initial dietary consultation with each participant. Each patient received individual nutritional counseling prior to the start of the study and weekly follow‐up counseling sessions throughout the study. Dietary consultations served to provide nutritional information and suggestions based on the participants’ current diet to produce a modest WL of approximately 1.5 to 2 lb/wk during the WL phase. Dietary consultations during WR served to provide nutritional guidance aimed at maintaining healthy macronutrient and micronutrient diet composition while promoting a modest increase in caloric intake (compared with the WL phase) resulting in partial WR.
WL and Aerobic Exercise Training. A progressive protocol was used to attain the desired level of aerobic training during the initial WL phase. The study began with an exercise intensity equal to approximately 50% of maximal oxygen consumption (VO2max) for 30 min/d, 5 d/wk and progressed to the desired intensity of 60% of VO2max (75% heart rate maximum) for 45 min/d, 5 d/wk where they remained for the duration of the WL phase (energy expenditure from exercise ∼1750 kcal/wk). Aerobic exercise training consisted of mainly treadmill exercise with the occasional use of stationary cycling or an elliptical machine. Intensity was monitored with the use of heart rate monitors. After the first 6 weeks of training, participants were required to have at least 3 supervised sessions per week and were allowed 2 unsupervised sessions per week. The intensity of all unsupervised exercise sessions was self‐monitored by the patient with the use of a heart rate monitor. All exercise sessions were recorded in a training log.
WR and RT. During the WR phase, all patients ceased aerobic exercise training and participated in a supervised whole‐body RT program on 3 nonconsecutive days per week. Patients ultimately performed 3 sets of 6 to 12 repetitions at 70% to 80% of one‐repetition maximum (1RM). A progressive protocol was followed to safely reach the desired intensity and is described in Table I. Patients were allowed a 2‐ to 3‐minute rest period between sets. Each RT session began with a 5‐minute warmup on the treadmill and ended with a 5‐ to 10‐minute cooldown period that included stretching. Energy expenditure from RT was estimated to be approximately 1350 kcal/wk. 15 The exercises performed included bench, incline, and overhead presses; T‐bar and bent‐over rows; bicep curls; tricep extensions; squats; leg press; leg extensions; leg curls; and abdominal crunches. Exercises were performed in the Exercise Physiology Fitness Center using free weights and weight stack resistance machines (Body Masters, Rayne, LA).
Table I.
Resistance Training Progression
| Week | Sessions per Week | Sets | Repetitions | Intensity,% 1RM |
|---|---|---|---|---|
| 1 | 3 | 1 | 10–12 | 55 |
| 2 | 3 | 2 | 10–12 | 60 |
| 3 | 3 | 3 | 10–12 | 65 |
| 4 | 3 | 3 | 6–8 | 70 |
| 5–7 | 2 | 3 | 6–8 | 75 |
| 1 | 3 | 10–12 | 65 | |
| 8–12 | 2 | 3 | 6–8 | 80 |
| 1 | 3 | 10–12 | 70 |
Abbreviation: 1RM, one repetition maximum. During weeks 5–12, higher‐intensity sessions were performed on Monday and Friday with the lower intensity session performed on Wednesday.
Outcome Measures
VO2max and Muscular Strength. VO2max was determined by indirect calorimetry during a treadmill stress test using the standard Bruce protocol. 16 Peak torque for knee extension and flexion was measured using an isokinetic dynamometer. Patients performed 3 sets of 5 repetitions at 60 degrees per second and the peak torque achieved was recorded. Additionally, 1RM was determined for 4 exercises (bench press, bicep curl, leg ext, leg curl). Patients were familiarized to the RT equipment and the 4 exercises to be tested approximately 1 week prior to 1RM testing. All 1RM tests began with a 5‐minute warmup on the treadmill. During 1RM testing, participants attempted to lift a weight estimated to be 50% of their maximal ability. Weight was increased following each successful attempt until the maximum amount of weight that could be lifted at one time was determined. A 3‐minute rest period was incorporated between each attempt, and all patients reached their 1RM within 3 to 5 attempts for each exercise. Isokinetic strength tests and all 1RM tests were performed at baseline, post‐WL, and post‐WR, and the post‐WL 1RM values were used to determine RT intensity during the WR phase.
Resting Blood Pressure. Resting blood pressure (BP) was measured following 15 minutes of rest in the seated position using an aneroid sphygmomanometer at the brachial artery with the arm supported at the level of the heart.
Body Composition and Abdominal and Thigh AT Distribution. Body composition was assessed using BMI, waist circumference (WC), waist‐to‐hip ratio (WHR), and dual‐energy x‐ray absorptiometry (DXA). Weight was measured to the nearest 0.05 kg and height was measured to the nearest 0.1 cm. Waist and hip circumferences were measured to the nearest 0.1 cm using a spring‐mounted tape measure. BMI and WHR were calculated. Body fat percentage and lean body mass were determined by whole‐body DXA scanning. The reliability of DXA scans was determined using a paired sample t test on data taken from the same individual on two separate occasions (n=6). There was no statistically significant difference between the two measurements (paired samples correlation, r=0.99 [P<.01]; and mean absolute difference, 0.90%±0.39%). Computed tomography (CT) was used for the determination of total (TATabd), subcutaneous (SATabd), and visceral (VAT) abdominal AT as previously described. 17 Briefly, a single cross‐sectional scan of 10‐mm thickness centered at the L4 to L5 vertebral disc space was made with the patient in a supine position. A second scan (10‐mm thickness) was obtained at the midpoint between the anterior superior iliac spine and the center of the patella in order to determine total AT at mid‐thigh (TATthigh). Subcutaneous (SATthigh) and intermuscular AT or fat residing within the fascia surrounding the muscle and within the muscle bed itself was also determined at mid‐thigh. Area (cm2) of AT in the density range corresponding to fat (−190 to −30 HU) was determined for each depot using software integral to the CT scanner. Additionally, skeletal muscle attenuation was assessed by determining the area (cm2) of muscle within the total (0–100 HU), low (0–30 HU), and high (31–100 HU) density ranges. All CT scans were analyzed by the same investigator.
Blood Collection and Analysis. All blood samples were obtained in the fasted state (12‐hour overnight fast) and under dietary control. Patients recorded a self‐selected control diet for 48 hours prior to baseline testing and this diet was repeated 48 hours prior to subsequent blood draws. Patients were also asked to abstain from alcohol for the 48 hours prior to blood collections. Additionally, all blood sampling was performed following 48 hours of no exercise. All blood samples were collected from the antecubital vein with the patient in a semi‐supine position. Samples were collected in 10‐mL tubes containing anticoagulant and chelating agent or serum separator, separated by centrifugation at 4°C for 15 minutes at 2000 g and stored at −80°C until analyzed. Plasma TGs, total cholesterol (TC), HDL‐C, and glucose were measured enzymatically using diagnostic kits and a spectrophotometer using known standards (intra‐assay coefficients of variation [cv] of 2.0%, 1.8%, 0.8%, and 1.4% for TG, TC, HDL‐C, and glucose, respectively). Cholesterol in HDL particles was determined using a modified heparin‐MnCl2 method previously described 18 and LDL‐C was calculated using the Friedewald equation. 19 Insulin was measured using a chemiluminescent technique (intra‐assay cv of 3.1%). Insulin resistance and sensitivity were estimated by measures of fasting glucose and insulin and calculation of the homeostasis model assessment (HOMA) 20 and quantitative insulin‐sensitivity check index (QUICKI). 21
Statistical Analysis
A one‐way analysis of variance with repeated measures (time) was used to analyze outcome variables. All data were analyzed using SPSS statistical software (SPSS version 11.0; Chicago, IL). Significant main effects were followed up with Bonferroni post hoc analyses to locate specific differences over time. Values are expressed as means ± standard error of the mean and are considered significant at P≤.05.
Results
Dietary and Exercise Compliance
Nine subjects (1 man and 8 women) completed the study. Patients lost 6.0%±0.3% (5.72±0.43 kg) of their initial body weight during the WL phase and regained 48.3%±3.3% (2.74±0.25 kg) of the lost weight during the WR phase. Mean dietary intake was 1993±50 kcal/d, 1402±98 kcal/d, and 2460±282 kcal/d at baseline, post‐WL, and post‐WR, respectively. There were no significant changes in the macronutrient composition of the diet throughout the study (percent fat was 36.5%, 29.6%, and 36.1%; percent protein was 15.6%, 17.2%, and 16.5%; and percent carbohydrate was 48.9%, 53.2%, and 48.4% at baseline, post‐WL, and post‐WR, respectively; all P>.05). Aerobic training compliance during the WL phase was 96.6%, and 98.3% of the aerobic training sessions were supervised (performed in the Exercise Physiology Fitness Center). RT compliance during the WR phase was 96.5%, and 98.9% of the RT sessions were supervised.
VO2max, BP, and Muscular Strength
VO2max, BP, and muscular strength results are presented in Table II. As expected, VO2max significantly increased following 8 to 12 weeks of aerobic training during the WL phase. Interestingly, improvements in VO2max were maintained with 8 to 12 weeks of RT (post‐WR VO2max values remained significantly greater than baseline values, P<.05). Systolic BP was significantly reduced at post‐WL and did not increase significantly during WR. There were no significant changes in diastolic BP throughout the study. Knee extension peak torque was unchanged from baseline to post‐WL but increased significantly following RT. Knee flexion was significantly increased from baseline to post‐WL and was further increased following RT. Additionally, both upper and lower body strength, as determined by 1RM testing, increased significantly following the RT program (14%, 11%, and 23% increase in bench press, bicep curl, and leg extension strength, respectively).
Table II.
Cardiorespiratory Fitness and Muscular Strength
| Baseline | Post‐WL | Post‐WR | |
|---|---|---|---|
| VO2max, L/min | 2.44±0.15 | 2.67±0.17a | 2.89±0.15a |
| Systolic BP, mmHg | 116±3 | 108±2a | 112±3 |
| Diastolic BP, mmHg | 74±2 | 68±2 | 73±2 |
| Knee extension, Nm | 105.11±8.98 | 112.89±9.91 | 129.18±12.96a,b |
| Knee flexion, Nm | 52.75±6.51 | 61.09±6.05 | 71.17±7.50a,b |
| 1RM bench press, kg | 47.6±6.0 | 43.0±7.0 | 50.0±9.0b |
| 1RM bicep curl, kg | 25.0±3.0 | 24.0±3.0 | 27.0±3.0b |
| 1RM leg extension, kg | 61.0±3.0 | 60.0±4.0 | 78.0±4.0a,b |
| 1RM leg curl, kg | 47.0±2.0 | 46.0±3.0 | 54.0±4.0 |
Abbreviations: BP, blood pressure; 1RM, one repetition maximum; VO2max, maximal oxygen consumption; WL, weight loss; WR, weight regain. aSignificantly different from baseline, P<.05. bSignificantly different from post‐WL, P<.05.
Body Composition and Abdominal AT Distribution
Following the WL phase, BMI, body fat percentage, WC, and all abdominal AT depots were significantly reduced (1, 2). TATthigh and SATthigh were also significantly reduced (Table III). During WR, body fat percentage and WC were maintained (P<.05 baseline vs post‐WR), and lean body mass significantly increased. Additionally, although not significantly different from baseline values, all abdominal and total and subcutaneous thigh AT depots did not significantly increase during WR (post‐WL vs post‐WR, P>.05).
Figure 1.
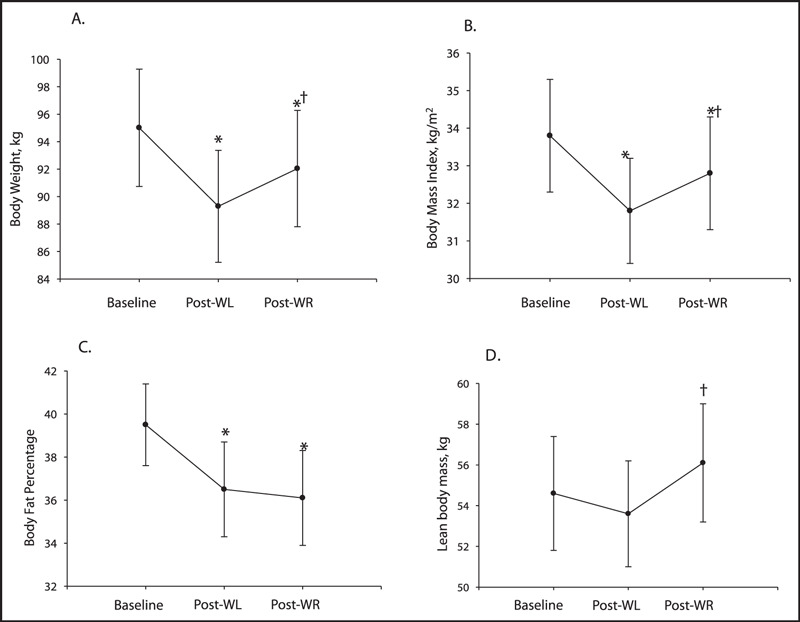
Body composition during weight loss (WL) and weight regain (WR) (n=9): (A) body weight, (B) body mass index, (C) body fat percentage, and (D) lean body mass. *Significantly different from baseline (P<.05). †Significantly different from post‐WL (P<.05).
Figure 2.
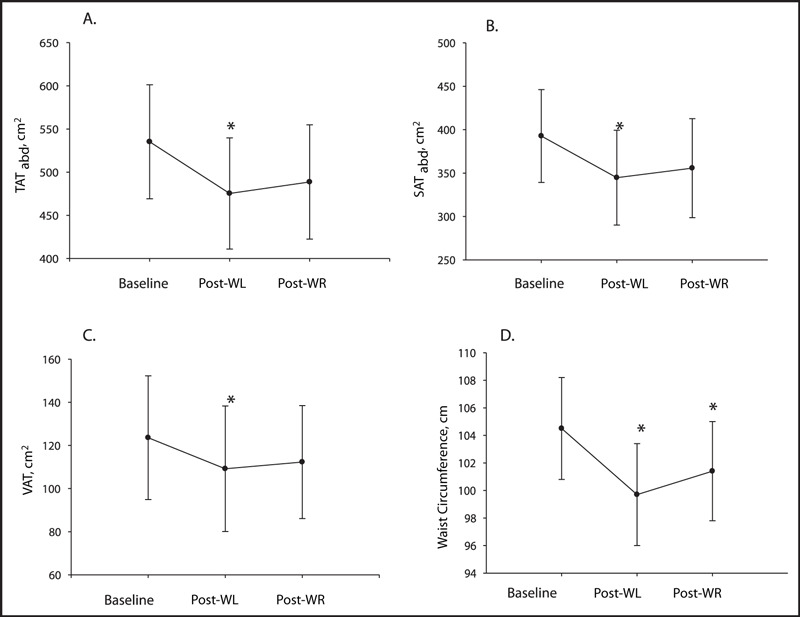
Abdominal adipose tissue depots and waist circumference during weight loss (WL) and weight regain (WR) (n=9): (A) total abdominal adipose tissue (TATabd), (B) subcutaneous abdominal adipose tissue (SATabd), (C) visceral adipose tissue (VAT), and (D) waist circumference. *Significantly different from baseline (P<.05).
Table III.
Thigh Adipose Tissue Distributiona
| Baseline | Post‐WL | Post‐WR | |
|---|---|---|---|
| TATthigh, cm2 | 195.6±20.0 | 176.8±20.3b | 189.4±21.0 |
| SATthigh, cm2 | 174.1±20.5 | 157.0±20.1b | 167.9±21.1 |
| IMAT, cm2 | 21.5±2.1 | 19.8±1.7 | 21.6±1.7 |
| TM, cm2 | 138.1±10.8 | 136.9±11.0 | 141.0±10.5 |
| HDM, cm2 | 107.7±8.1 | 106.2±8.5 | 111.5±7.0 |
| LDM, cm2 | 30.4±4.8 | 30.7±4.7 | 29.5±4.8 |
Abbreviations: HDM, high‐density muscle; IMAT, intermuscular adipose tissue; LDM, low‐density muscle; SATthigh, subcutaneous adipose tissue; TATthigh, total adipose tissue; TM, total muscle; WL, weight loss; WR, weight regain. aAll obtained at mid‐thigh. bSignificantly different from baseline, P<.05.
Plasma Variables
There were no significant changes in plasma TGs, HDL‐C, or glucose. However, TC, LDL‐C, insulin, and HOMA were significantly reduced (P<.05), and QUICKI was significantly increased during the WL phase (P<.05) (Figure 3 and Table IV). During WR, the improvements in TC and LDL‐C were not maintained. There was a significant increase in TC during the WR phase and both TC and LDL‐C values returned to baseline concentrations (Table IV). Although not significantly different from baseline values, plasma insulin (Table IV), HOMA, and QUICKI (Figure 3) did not significantly change during WR (post‐WL vs post‐WR, P>.05).
Figure 3.
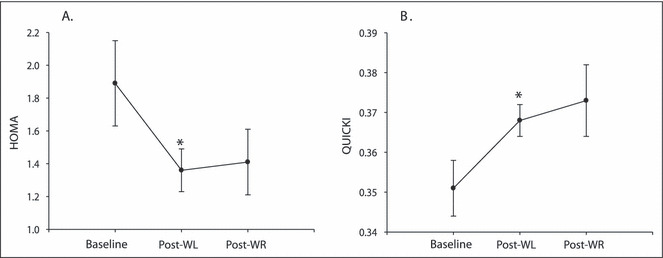
Measures of insulin resistance and insulin sensitivity during weight loss (WL) and weight regain (WR) (n=9): (A) homeostasis model assessment (HOMA) and (B) quantitative insulin‐sensitivity check index (QUICKI). *Significantly different from baseline (P<.05).
Table IV.
Plasma Lipids, Glucose, and Insulin Values
| Baseline | Post‐WL | Post‐WR | |
|---|---|---|---|
| TGs, mg/dL | 111.1±11.2 | 102.8±9.2 | 107.8±8.3 |
| TC, mg/dL | 179.5±8.7 | 162.2±7.8a | 180.5±6.1b |
| HDL‐C, mg/dL | 50.9±2.0 | 47.9±1.4 | 52.0±1.6 |
| LDL‐C, mg/dL | 106.4±6.2 | 93.7±6.8a | 107.0±5.6 |
| Glucose, mg/dL | 89.2±2.7 | 86.4±2.0 | 86.5±2.3 |
| Insulin, μIU/mL | 8.45±1.06 | 6.41±0.63a | 6.50±0.89 |
Abbreviations: HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; WL, weight loss; WR, weight regain. aSignificantly different from baseline, P<.05. bSignificantly different from post‐WL, P<.05.
Discussion
An 8‐ to 12‐week diet‐ and aerobic exercise–induced WL of approximately 6.0% improved cardiometabolic syndrome risk factors and cardiorespiratory fitness. The main findings of this study were that RT significantly increased lean body mass and muscular strength and maintained some of the diet‐ and aerobic exercise–induced improvements in metabolic health during a period of WR. Cardiorespiratory fitness is inversely related to all‐cause mortality 22 and has long been linked to a lower risk of chronic disease, 23 while muscular strength has been inversely associated with the incidence of the metabolic syndrome. 13 The cessation of aerobic training can result in a reversal of improvements in VO2max in as few as 12 weeks after completion of an aerobic training program. 24 The results of the present study suggest that improvements in VO2max can be maintained after cessation of an aerobic training program by performing moderate‐ to high‐intensity whole‐body RT. Additionally, Hagerman and colleagues 25 found that 16 weeks of high‐intensity RT significantly improves VO2max in untrained men. The results of the present study suggest RT to be an effective means by which to maintain cardiorespiratory fitness and improve muscular strength, and therefore could be a vital component of the exercise prescription for reducing the risk of cardiometabolic syndrome and other chronic diseases.
The major aim of this study sought to determine the ability of RT to maintain metabolic health during a period of WR. One recent animal study showed that RT could prevent detrimental changes in body composition during a period of partial WR in ovariectomized rats. 26 The return to ad libitum feeding (5 weeks) following caloric restriction (26% for 8 weeks) resulted in a partial regain of body weight, intra‐abdominal fat pad weight, and liver TG content in the sedentary rats; however, the effects were abated when the animals performed RT. 26 The results of the present study demonstrate that RT during a period of weight gain maintains body fat percentage in humans, which is most likely attributed to the increase in lean body mass as a result of the RT program. A number of studies have shown that moderate‐ to high‐intensity RT programs result in significant increases in lean body mass in a variety of populations. 11 , 27 , 28 Hunter and colleagues 11 found that 26 weeks of RT at an intensity equivalent to 65% to 80% 1RM resulted in a 2‐kg increase in fat‐free mass and a 36% increase in muscular strength in healthy nonresistance‐trained older adults. In addition, Castaneda and colleagues 27 found that 16 weeks of RT at 60% to 80% 1RM significantly increased whole‐body lean tissue mass in type 2 diabetics. The RT program also led to a reduction of plasma glycosylated hemoglobin concentrations and a reduction in the dose of prescribed diabetes medication. 27
Skeletal muscle is the major consumer of blood glucose and is quantitatively the most important site for insulin resistance in cardiometabolic syndrome and type II diabetes mellitus patients. 12 Therefore, it is possible that an RT program resulting in a significant increase in skeletal muscle mass might also improve insulin sensitivity in addition to body composition. The RT program performed in the present study did result in a significant increase in lean body mass and it is possible that this was responsible for the maintenance of glucose metabolism during a period of increased caloric consumption and weight gain.
Previous studies showed that both an acute bout of RT 14 and a 26‐week RT program 11 increased fat oxidation. In addition, the above‐mentioned studies 11 , 14 also demonstrated an increase in energy expenditure following RT exercise. Moreover, an acute bout of resistance exercise was shown to enhance lipolysis in abdominal SAT. 14 Therefore, it is plausible that the combined effects of enhanced AT lipolysis and increased fat oxidation and energy expenditure are responsible for the beneficial effects of RT on body composition and metabolic health even during a period of body weight gain.
Previous studies have shown that RT reduces both abdominal subcutaneous 8 and visceral 8 , 9 adiposity in the absence of body weight changes. In the present study, WC was reduced following the WL phase and was maintained (significantly less than baseline, P<.05) following WR. While the results of the present study provide evidence that RT during a period of WR can maintain improvements in important variables such as cardiorespiratory fitness, body fat percentage, and WC, the evidence is less compelling for abdominal adiposity. All abdominal AT depots were significantly reduced following short‐term WL and were not significantly increased after WR. However, abdominal adiposity as determined by CT was not significantly different than baseline following the RT WR period. Based on this pilot data, further investigation of the maintenance of abdominal adiposity with RT during weight gain of longer duration is warranted. The ability to maintain abdominal AT values similar to that following WL during a period of excess caloric intake and significant body weight increases could be of great importance as abdominal adiposity is a key feature of the cardiometabolic syndrome.
The changes in plasma lipids were somewhat unremarkable. There were no changes in either TGs or HDL‐C in response to either phase of the study. TC and LDL‐C were reduced with WL; however, both returned to baseline values following WR. These results are in accordance with previous studies that have failed to show changes in plasma lipids following RT. 9 , 10 , 27 However, Mirsa and colleagues 29 found that 12 weeks of moderate‐intensity RT significantly reduces TC, very LDL‐C, and plasma TGs in type 2 diabetics. Although the duration and intensity of the RT was similar to that of the present study, the participants in the Mirsa study were type 2 diabetics with poorer baseline plasma TG and glucose levels. Baseline lipid values in the present study were within the normal ranges, and this difference in study populations may be the reason for the discrepancy in lipid outcomes.
Limitations
One limitation of this study is the small sample size; however, the observed power for most variables was adequate to detect changes over time. Observed power ranged from 0.52 to 1.00 for all variables except glucose and TGs. The major limitation of this study is the lack of a comparison group. Data obtained from a similar study conducted in our laboratory revealed WR without exercise following a period of diet‐ and aerobic exercise–induced WL to result in a reversal of nearly all aspects of metabolic health tested. 30 Additionally, aerobic training during the WR phase had many beneficial effects including the maintenance of cardiorespiratory fitness, oxidized LDL‐C, BP, and insulin sensitivity. 30 Although the study population, design, and outcome measures were similar to that of the current study, the magnitude of WL and WR was greater (10% WL followed by a regain of ∼50% of lost weight) and the duration was longer (16–24 weeks per phase vs 8–12 weeks) disallowing a direct comparison among groups. However, the results obtained from the current study suggest that RT during WR has beneficial effects that could be directly attributable to the RT and may compliment or add to what can be obtained with aerobic training. Further investigation is warranted and should include a combination of aerobic training and RT during WR.
Conclusions
RT was effective in maintaining important diet‐ and aerobic exercise–induced improvements in metabolic health during a period of WR. Many at‐risk individuals struggle with WL maintenance, and the results of this study suggest that RT may be beneficial in preventing the detrimental metabolic consequences of WR.
Acknowledgments
Acknowledgements and disclosures: The authors thank David R. Huyette, MD, for his expertise and assistance in the collection and analysis of the computed tomography data. No coauthor had any financial or proprietary interest in the subject matter or materials discussed in the manuscript. This study was supported by grant R01 DK067036 from the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases and grant T32 AR048523 from the National Heart Lung and Blood Institute.
References
- 1. Flegal KM, Carroll MD, Ogden CL, et al; Prevalence and trends in obesity among US adults, 1999–2000 [see comment]. JAMA. 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 2. Park Y‐W, Shankuan Z, Palaniappan L, et al; The metabolic syndrome. Prevalence and associated risk factor findings in the US population from the third national health and nutrition examination survey, 1988–1994. Arch Intern Med. 2003;163:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen I, Fortier A, Hudson R, et al; Effects of an energy‐restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. [DOI] [PubMed] [Google Scholar]
- 4. Despres JP, Pouliot MC, Moorjani S, et al; Loss of abdominal fat and metabolic response to exercise training in obese women. Am J Physiol. 1991;261(2 Pt 1):E159–E167. [DOI] [PubMed] [Google Scholar]
- 5. Ross R, Dagnone D, Jones PJH, et al; Reduction in obesity and related comorbid conditions after diet‐induced weight loss or exercise‐induced weight loss in men. Ann Intern Med. 2000;133:92–103. [DOI] [PubMed] [Google Scholar]
- 6. Goodpaster BH, Kelley DE, Wing RR, et al; Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. [DOI] [PubMed] [Google Scholar]
- 7. Wadden TA, Vogt RA, Foster GD, et al; Exercise and the maintenance of weight loss: 1‐year follow‐up of a controlled clinical trial. J Consult Clin Psychol. 1998;66:429–433. [DOI] [PubMed] [Google Scholar]
- 8. Ibanez J, Izquierdo M, Arguelles I, et al; Twice‐weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28:662–667. [DOI] [PubMed] [Google Scholar]
- 9. Treuth MS, Hunter GR, Kekes‐Szabo T, et al; Reduction in intra‐abdominal adipose tissue after strength training in older women. J Appl Physiol. 1995;78:1425–1431. [DOI] [PubMed] [Google Scholar]
- 10. Klimcakova E, Polak J, Moro C, et al; Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91:5107–5112. [DOI] [PubMed] [Google Scholar]
- 11. Hunter GR, Wetzstein CJ, Fields DA, et al; Resistance training increases total energy expenditure and free‐living physical activity in older adults. J Appl Physiol. 2000;89:977–984. [DOI] [PubMed] [Google Scholar]
- 12. DeFronzo RA, Gunnarsson R, Bjorkman O, et al; Effects of insulin on peripheral and splancnic glucose metabolism in non‐insulin‐dependent (Type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jurca R, Lamonte MJ, Church TS, et al; Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36:1301–1307. [DOI] [PubMed] [Google Scholar]
- 14. Ormsbee MJ, Thyfault JP, Johnson EA, et al; Fat metabolism and acute resistance exercise in trained men. J Appl Physiol. 2007;102:1767–1772. [DOI] [PubMed] [Google Scholar]
- 15. Ainsworth BE, Haskell WL, Whitt MC, et al; Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S516. [DOI] [PubMed] [Google Scholar]
- 16. ACSM (ed). American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 17. Kelley DE, Thaete FL, Troost F, et al; Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. [DOI] [PubMed] [Google Scholar]
- 18. Zhang JQ, Thomas TR, Ball SD. Effect of exercise timing on postprandial lipemia and HDL cholesterol subfractions. J Appl Physiol. 1998;85:1516–1522. [DOI] [PubMed] [Google Scholar]
- 19. Rifai N, Warnick GR, McNamara JR, et al; Measurement of low‐density‐lipoprotein cholesterol in serum: a status report. Clin Chem. 1992;38:150–160. [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, et al; Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 21. Katz A, Nambi SS, Mather K, et al; Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 22. Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, et al; Physical fitness and all‐cause mortality. A prospective study of healthy men and women [see comment]. JAMA. 1989;262:2395–2401. [DOI] [PubMed] [Google Scholar]
- 23. Blair SN, Morris JN. Healthy hearts – and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–256. [DOI] [PubMed] [Google Scholar]
- 24. Orio F, Giallauriat F, Palomba S, et al; Metabolic and cariopulmonary effects of detraining after a structured exercise training programme in young PCOS women. Clin Endocrinol. 2008;68:976–981. [DOI] [PubMed] [Google Scholar]
- 25. Hagerman FC, Walsh SJ, Staron RS, et al; Effects of high‐intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci. 2000;55:B336–B346. [DOI] [PubMed] [Google Scholar]
- 26. Pighon A, Paquette A, Barsalani R, et al; Substituting food restriction by resistance training prevents liver and body fat regain in ovariectomized rats. Climacteric. 2009;12:153–164. [DOI] [PubMed] [Google Scholar]
- 27. Castaneda C, Layne JE, Munoz‐Orians L, et al; A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. [DOI] [PubMed] [Google Scholar]
- 28. McGuigan MR, Tatasciore M, Newton RU, et al; Eight weeks of resistance training can significantly alter body composition in children who are overweight or obese. J Strength Cond Res. 2009;23:80–85. [DOI] [PubMed] [Google Scholar]
- 29. Misra A, Alappan NK, Vikram NK, et al; Effect of supervised progressive resistance‐exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care. 2008;31:1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas TR, Warner SO, Liu Y, et al; Exercise and the metabolic syndrome with weight regain [abstract]. Med Sci Sports Exerc. 2009;41:S588. [Google Scholar]


