Abstract
J Clin Hypertens (Greenwich).
Thiazide‐type diuretics are recommended in national hypertension treatment guidelines, but these guidelines do not give preference to a specific thiazide. Recently, increased use of chlorthalidone has been advocated. The authors reviewed national outpatient prescription data from the Veterans Health Administration from 2003 to 2008 to describe the prescribing trends within the thiazide class, focusing on hydrochlorothiazide and chlorthalidone. Among total thiazide users, the proportion who received hydrochlorothiazide remained stable (95.6% in 2008), but the number of new users of hydrochlorothiazide decreased nearly 30% during this period. In contrast, the proportion of chlorthalidone use among total thiazide users more than doubled (1.1% in 2003 to 2.4% in 2008), and the number of new chlorthalidone users increased by more than 40%. At the time of initiation, chlorthalidone was more likely to be added to an existing antihypertensive regimen; one quarter (25.1%) of new hydrochlorothiazide starts were in patients not receiving concurrent antihypertensive medications, compared with only 12.1% for chlorthalidone (odds ratio, 0.44; 95% confidence interval, 0.42–0.46). Evaluation of national prescribing trends indicates that hydrochlorothiazide remains the most commonly prescribed thiazide, but there appears to be a shift toward more new users of chlorthalidone. J Clin Hypertens (Greenwich). 2010;12:927–934. © 2010 Wiley Periodicals, Inc.
Hypertension affects approximately one in four Americans. 1 A diuretic‐based regimen, using either a thiazide or thiazide‐like agent such as chlorthalidone, is a well‐established strategy to lower blood pressure (BP). Thiazides are not only effective as monotherapy, but they can be combined with other antihypertensives to produce an additive BP‐lowering effect in almost all cases. 2 Accumulated data from several landmark clinical trials, meta‐analyses, and systematic reviews convincingly demonstrate that thiazide‐based regimens significantly reduce rates of stroke, heart failure, and cardiovascular disease (CVD) events. 3 , 4 , 5 Based on the results of these studies, treatment guidelines in the United States such as the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) and the Veteran’s Health Administration/Department of Defense Clinical Practice Guideline on the Management of Hypertension in Primary Care, recommend that a thiazide diuretic such as hydrochlorothiazide (HCTZ) or chlorthalidone be initiated as monotherapy in a stepped‐care approach or as part of a two‐drug combination. 6 , 7
It is noteworthy that guidelines do not give preference to a specific thiazide, even though the substantial clinical trial evidence demonstrating reductions in CVD events with thiazide‐based regimens is primarily derived from studies such as the Hypertension Detection Follow‐Up Program (HDFP), Systolic Hypertension in the Elderly Program (SHEP), and the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), which used chlorthalidone‐based regimens. 8 , 9 , 10 In contrast, studies using the more commonly prescribed HCTZ have shown less robust results, with some finding the regimen (particularly with low‐dose, 12.5–25 mg/d) less effective than the comparator. 11 , 12 Those studies, which have demonstrated benefit of an exclusively HCTZ‐based regimen, used doses (≥50 mg/d) above which are now typically used in practice. 13 , 14
Overall use of thiazides has increased in the period following JNC 7 and ALLHAT. 15 In a study examining antihypertensive medication utilization from 2000 to 2006, initial prescriptions for thiazide‐type diuretics increased from 31.9% to 42.0%, while others reported increases from 30.6% to 39.4% during 2001 to 2004 and from 41.9% to 61.0% during 2002 to 2007 in another registry. 16 , 17 , 18 However, none of these studies examined differences in doses or prescribing rates between specific agents within the class. Given the renewed attention on the importance of chlorthalidone, 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 the purpose of our study was to systematically describe the recent prescribing trends within the thiazide class, focusing specifically on HCTZ and chlorthalidone. Understanding this pattern of use is important for designing interventions to increase the utilization of thiazide‐type diuretics, and chlorthalidone in particular.
Methods
A descriptive analysis was performed using national Veterans Health Administration data obtained from the Austin Information Technology Center for fiscal years (FYs) 2003 to 2008. The project was approved by the University of Iowa institutional review board and the Iowa City Veterans Administration (VA) Research and Development Committee.
Thiazide utilization was identified from prescription fill data contained in the decision support system pharmacy files. Thiazide‐type drugs included HCTZ, chlorthalidone, metolazone, indapamide, and chlorothiazide. Date of birth was taken from the vital status file and used to determine age at the beginning of each FY. Sex was obtained from the patient enrollment file. Race was categorized as white, black, other, and missing and determined by examination of multiple data sources, including the patient treatment files and outpatient care files across multiple FYs. Using this combined resource approach, <5% of patients had missing race information.
Thiazide Utilization Measures
Thiazide utilization was expressed as the number of unique individuals who received an outpatient prescription fill for a thiazide during a given FY. Utilization rates for individual thiazides were expressed as the proportion of patients who received an outpatient prescription fill for that agent from among patients receiving any thiazide medication. Individual thiazide utilization rates were also examined in terms of prescription fill counts and days supply of drug dispensed. The findings were virtually identical to the patient level analysis and are not presented. Variation in chlorthalidone utilization was examined across the 21 Veteran Integrated Service Networks (VISNs) and by US census region (Northeast, Midwest, South, and West). Because HCTZ accounted for the vast majority of thiazide prescriptions, and our research question of interest was primarily focused on chlorthalidone utilization relative to HCTZ, all remaining thiazide‐related analyses were limited to the comparison of HCTZ and chlorthalidone.
Daily thiazide doses were estimated for each patient based on the modal dose received during the course of the FY. First, fills were limited to those with a quantity to days supply ratio equal to 0.5, 1, 1.5, 2, 3, or 4 in order to eliminate fills with nonsense values in these fields. Next, the daily dose was calculated for each remaining thiazide fill by dividing the product of the unit drug strength and quantity dispensed fields by the days supply field. Finally, these individual fill doses were examined at the patient level to determine the modal daily dose that occurred during the course of the FY. In addition to being examined as a continuous value, daily doses were grouped in several analyses according to clinically meaningful categories, including micro‐dose (≤12.5 mg), low‐dose (12.5<×≤25 mg), and high‐dose (>25 mg). Daily doses were contrasted between drug and formulation type (single‐agent, fixed‐dose combination with a potassium‐sparing diuretic, fixed‐dose combination with another antihypertensive drug). Of note, the only chlorthalidone combination product used during the time frame of this study was with the antihypertensive agent, atenolol. Comparisons across categoric dosing levels and formulations were limited to the most recent year of data (FY 2008) to focus on the current state of prescribing.
New Thiazide Users
For several analyses, it was important to restrict the sample to new users of an individual thiazide medication. Changes in prescribing trends are generally more prominent in patients initiating therapy. Prescribers who became new adopters of chlorthalidone during this period would probably not switch patients currently well‐controlled on their existing antihypertensive regimen. 27 Rather, they would preferentially begin the medication in new users. Moreover, the clinical characteristics of prevalent medication users are potentially different than new users since they represent a subgroup that “survived” initial treatment by gaining therapeutic benefit with tolerable side effects. 28 Therefore, we defined a cohort of new users of chlorthalidone and HCTZ who began treatment between FY 2004 and 2007 and characterized these patients in terms of demographic characteristics and concurrent antihypertensive medications. We were limited to this period because the algorithms to define these variables required data from the year prior and the year after initiation and thus complete estimates for FY 2003 and FY 2008 could not be generated.
Initiation of new use was defined by the earliest thiazide drug fill observed in the pharmacy files where the patient received long‐term medications from the VA during the year prior to this initial thiazide fill. Long‐term medication exposure was defined as history of outpatient medication fills where the day’s supply periods spanned at least 240 of 365 days. This year of prior medication exposure was included to eliminate patients transferring care to the VA who may have been taking a thiazide medication outside the VA prior to the first fill observed from the VA.
The initiation date also served as the index date for determining the number of concurrent antihypertensive medications at the time of new thiazide initiation. An antihypertensive medication was considered concurrent if there were prescription fills before and after the thiazide index start date with a gap between fills that was less than a specified number of days. This value was set to twice the day’s supply of the pre‐index antihypertensive fill, with a minimum value of 90 days and a maximum of 180 days. This definition was based on a validated algorithm with an adjustment to the gap period between fills to account for the extended 90‐day fill period that is common within the VA. 29 The purpose of this definition was to exclude antihypertensive medications that were permanently discontinued at thiazide initiation and medications that were stopped and later resumed.
Two additional analyses concerning new thiazide users did not require pharmacy data for the year following initiation, so we were also able to examine data from FY 2008. First, we compared the change in the number of new users of HCTZ and chlorthalidone over time. Second, we determined the proportion of new chlorthalidone users who were switched from regular HCTZ use across FYs. Regular HCTZ use was defined by the presence of at least two HCTZ fills in the year prior to chlorthalidone initiation, and with the additional requirement that the last HCTZ fill be within an interval prior to the chlorthalidone start date of less than twice the day’s supply value of the last fill. This interval was chosen to ensure a reasonable proximity to the date of chlorthalidone initiation, but also allow for patient nonadherence.
Statistical Analysis
Differences in proportions were compared using a chi‐square test. Changes in proportions over time were examined using a Cochran–Armitage trend test. Univariate comparisons of continuous variables were assessed using t tests. HCTZ and chlorthalidone doses were compared over time from FY 2003 through FY 2008 with a generalized linear fixed‐effect model using the SAS procedure Mixed (SAS Institute, Cary, NC). Demographic characteristics predicting new initiation of chlorthalidone vs HCTZ were determined by a generalized linear fixed effect model using the SAS procedure GLIMMIX, controlling for FY.
Results
Thiazide Utilization
The overall number of patients having at least one prescription for a thiazide‐type diuretic in an FY increased during the study period, as shown in Table I. As a percentage of total thiazide users, HCTZ use remained stable (95.0% in FY 2003 to 95.6% in FY 2008). However, chlorthalidone use increased more than three‐fold during this same period (8453 in FY 2003 to 26,165 in FY 2008). Expressed as a percentage of total thiazide prescriptions, chlorthalidone users significantly increased from 1.1% to 2.4% (z=75.6, P<.0001). Use of other thiazides (metolazone, indapamide, chlorothiazide) decreased during the study period, from 34,846 (4.4%) in FY 2003 to 27,837 (2.5%) in FY 2008.
Table I.
Number of Patients Exposed to Thiazides by Fiscal Year
| Fiscal Year | Unique Patients, No. | Hydrochlorothiazide, No. (%) | Chlorthalidone, No. (%) | Other Thiazide, No. (%) |
|---|---|---|---|---|
| 2003 | 792,024 | 752,036 (95.0) | 8453 (1.1) | 34,846 (4.4) |
| 2004 | 908,777 | 864,647 (95.1) | 13,216 (1.5) | 35,305 (3.9) |
| 2005 | 967,342 | 920,556 (95.2) | 16,663 (1.7) | 34,655 (3.6) |
| 2006 | 1,012,588 | 964,445 (95.3) | 19,638 (1.9) | 33,058 (3.3) |
| 2007 | 1,069,718 | 1,021,959 (95.5) | 22,736 (2.1) | 30,033 (2.8) |
| 2008 | 1,098,264 | 1,049,628 (95.6) | 26,165 (2.4) | 27,837 (2.5) |
The sum of percentages across rows exceeds 100% because some patients were exposed to multiple thiazides in a given fiscal year. Other thiazides include metolazone, indapamide, and chlorothiazide.
Chlorthalidone utilization, expressed as percentage of total thiazide users, significantly varied across the 21 VISNs (χ2=2113, df=20, P<.0001) and ranged from 0.4% to 2.1% in FY 2003. Regional rates in FY 2003 ranged from a low of 0.6% in the West, to 1.0% in the South, to 1.3% in the Midwest, and a high of 1.4% in the Northeast (χ2=601, df=3, P<.0001). Chlorthalidone use increased in each of the 21 VISNs from FY 2003 to FY 2008, with relative increases ranging from 46% to 436%. The regional variation pattern persisted through FY 2008, where chlorthalidone utilization ranged from a low of 1.5% in the West, 2.0% in the South, 2.8% in the Midwest, and 2.9% in the Northeast (χ2=1071, df=3, P<.0001).
Thiazide Dosing
A steady downward trend in the mean (standard deviation [SD]) daily dose of HCTZ was noted during the study period, from 24.4 (11.3) mg/d in FY 2003 to 22.4 (9.8) mg/d in FY 2008. This same trend was noted for chlorthalidone, 25.7 (11.5) mg/d in FY 2003 to 24.0 (8.8) mg/d in 2008. Although the dose change over time was significant for both drugs (F=21660, P<.0001), HCTZ doses were significantly lower than chlorthalidone doses across all years (F=1518, P<.0001). Table II shows the distribution of daily doses in FY 2008 according to clinically relevant categories, micro‐dose (≤12.5 mg), low‐dose (12.5<×≤25 mg), and high‐dose (>25 mg). More than 90% of patients taking either drug received doses of ≤25 mg/d. Importantly, HCTZ users were significantly more likely to receive micro‐dose therapy (34.8%) than chlorthalidone users (20.6%) (odds ratio [OR], 2.06; 95% confidence interval [CI], 1.99–2.12; P<.0001).
Table II.
Distribution of Daily Thiazide Doses by Formulation Type, Fiscal Year 2008
| Hydrochlorothiazide | Chlorthalidone | ||||||
|---|---|---|---|---|---|---|---|
| All Products (n=1,040,608), % | Single‐Agent (n=708,022), % | Antihypertensive Combination (n=215,565), % | Diuretic Combinationa (n=117,021), % | All Products (n=24,824), % | Single‐Agent (n=11,692), % | Antihypertensive Combination (n=13,132), % | |
| Micro‐dose (≤12.5 mg) | 34.8 | 33.0 | 55.4 | 7.4 | 20.6 | 26.4 | 15.4 |
| Low‐dose (12.5<×≤25 mg) | 58.3 | 61.5 | 41.5 | 69.4 | 73.6 | 64.3 | 81.9 |
| High‐dose (>25 mg) | 7.0 | 5.5 | 3.1 | 23.2 | 5.8 | 9.3 | 2.7 |
aCombination with potassium‐sparing diuretics, such as amiloride, triamterene, and spironolactone.
Significant differences in daily dose were also noted across single‐agent and fixed‐dose combination thiazide formulations. In FY 2008, 68.0% of HCTZ users received the drug as a single‐agent formulation, compared with only 47.1% of chlorthalidone users (χ2=4850, df=1, P<.0001). The remaining HCTZ users received the drug in a fixed‐dose combination with another antihypertensive agent (20.7%) or with a potassium‐sparing diuretic (11.3%). All remaining chlorthalidone users (52.9%) received the drug as a combination product with another antihypertensive agent (atenolol), since combination formulations with potassium‐sparing diuretics are not available.
The mean (SD) daily doses of HCTZ across different formulations were 22.3 (9.0) mg for single‐agent formulations, 18.7 (8.4) mg for fixed‐dose combinations with another antihypertensive, and 30.0 (12.2) mg in combination with a potassium‐sparing diuretic. These differences were statistically significant, where HCTZ doses were lower in both single‐agent (t=169, df=382278, P<.0001) and potassium‐sparing diuretic combination products (t=234, df=133831, P<.0001) compared with fixed‐dose antihypertensive combinations. Although statistically different, mean chlorthalidone doses were clinically similar when used as a single‐agent or in a fixed‐dose combination formulation with atenolol (24.2 mg, SD=10.9 vs 23.7 mg, SD=6.3; t=4.0, df=18237, P<.0001). Underlying the differences in mean doses across formulations was the proportion of patients receiving thiazide treatment in the micro‐dose range (Table II). For single‐agent formulations, the frequency of micro‐dose treatment was only marginally higher for HCTZ (33.0%) than chlorthalidone (26.4%). However, patients taking antihypertensive combinations were more than three times more likely to receive micro‐dose treatment if taking HCTZ (55.4%) rather than chlorthalidone (15.4%).
Characteristics of New Chlorthalidone Users
There were 456,425 new users of HCTZ and 17,613 new users of chlorthalidone during FY 2004 through FY 2007. New chlorthalidone users were somewhat older (mean=65.9 years) than new HCTZ users (mean=65.1 years), more likely to be black (20.5% vs 13.0%), and did not differ by frequency of male sex (96.0% vs 95.8%). However, the demographic characteristics were interrelated, and, in multivariate analyses adjusting for year of initiation, new chlorthalidone use remained significantly more frequent among blacks (OR, 1.7; P<.0001) compared with whites, but was found to be less frequent among women (OR, 0.90; P=.0094) and unaffected by age.
At the time of initiation, chlorthalidone was more likely to be added to an existing antihypertensive regimen (Table III). One quarter (25.1%) of new HCTZ starts were in patients not receiving concurrent antihypertensive medications, compared with only 12.1% for chlorthalidone (OR, 0.44; 95% CI, 0.42–0.46). Of new thiazide starters, the distributions of HCTZ and chlorthalidone were similar for those patients already receiving one or two antihypertensives. However, 16.2% of new chlorthalidone starts occurred in patients already receiving ≥3 antihypertensives, compared with only 8.7% for HCTZ (OR, 2.0; 95% CI, 1.9–2.1).
Table III.
Concurrent Antihypertensive Medications at Thiazide Initiation, Fiscal Years 2003 to 2007
| No. of Concurrent Antihypertensives | Hydrochlorothiazide, No. (%) | Chlorthalidone, No. (%) |
|---|---|---|
| None | 114,411 (25.1) | 2273 (12.9) |
| 1 | 188,577 (41.3) | 7225 (41.0) |
| 2 | 114,969 (25.2) | 5360 (30.4) |
| ≥3 | 38,468 (8.4) | 2755 (15.6) |
The annual number of new users of chlorthalidone and HCTZ for FY 2004 through FY 2008 is illustrated in the Figure. While the number of new users of HCTZ decreased nearly 30% during this period, new chlorthalidone users increased by more than 40%. Among the new chlorthalidone users, we also found that prior HCTZ use was common. The proportion of new chlorthalidone users directly switched from HCTZ increased over time, from 27.8% in 2004 to 33.8% in 2008 (z=6.1, P<.0001).
Figure.
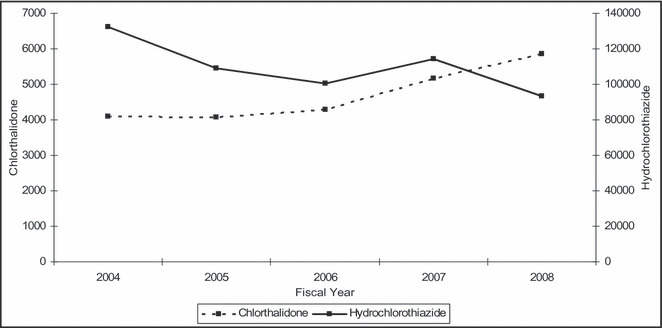
Annual number of new users of hydrochlorothiazide and chlorthalidone, fiscal years 2004 to 2008.
Discussion
The principle finding of our study is that utilization of chlorthalidone among veterans receiving prescriptions for thiazide‐type diuretics has significantly increased since 2003 but continues to account for a very small portion of total thiazide prescriptions. Although the magnitude of change varied by VISN and US census region, use of chlorthalidone increased similarly in all areas. During this same period, overall utilization of thiazide‐type diuretics steadily increased in the population; however, the rate of growth of new chlorthalidone use outpaced that of HCTZ. When restricting the sample to new thiazide users, the number of new users of HCTZ actually decreased nearly 30% during this period, while new chlorthalidone users increased by more than 40%.
Another notable observation in our study is the increased frequency of prior thiazide use among new chlorthalidone starters. This finding indicates that clinicians may be switching within the thiazide class. Presumably, the switch to chlorthalidone would only be undertaken in those with uncontrolled BP in an attempt to obtain better control, but we were not able to specifically ascertain the reasoning. Our finding that HCTZ is more likely to be started as a monotherapy, whereas chlorthalidone is more commonly added to an existing regimen, lends plausibility to this hypothesis. There is some evidence to suggest that, on a milligram‐per‐milligram basis, chlorthalidone is more effective in lowering BP than HCTZ. 19 , 20 , 30 These findings, as well as renewed calls for increased use of chlorthalidone, may be responsible in part for some of the shift in prescribing observed. 21 , 22 , 31 In addition, the recent American Heart Association guidelines for the treatment of resistant hypertension specifically recommend using chlorthalidone in patients with difficult‐to‐control BP. 23
The finding of differences in the thiazide doses used is of clinical interest. In our study, there was a slight downward trend in the mean doses used during the study period, with HCTZ remaining significantly lower than chlorthalidone in all years. This may reflect the higher use of fixed‐dose combination products for HCTZ, nearly all of which employ doses of 12.5 or 25 mg, while chlorthalidone is not available in any formulation (single‐agent or in fixed‐dose combination) in a dose <25 mg.
The difference in doses may have important implications for BP control. Chlorthalidone and HCTZ have traditionally been viewed as equipotent, although more recent evidence suggests that 12.5 to 25 mg of chlorthalidone may be similar to 25 to 50 mg of HCTZ. 19 , 20 , 30 In our present study, HCTZ users were significantly more likely to receive micro‐dose therapy (≤12.5 mg) than chlorthalidone users. The trend toward using lower doses of HCTZ may leave some patients without adequate reduction in volume status, failing to reach lower BPs as a consequence. 32
Limitations
Several limitations are recognized with our study. First, both HCTZ and chlorthalidone are generically available. Given their inclusion in Medicare Part D prescription coverage programs as well as many discounted pharmacy price lists, it is likely that some veterans obtain these prescriptions outside of the VA system. This could have led to an underestimation of the absolute rates of utilization of the two drugs. If so, we would have expected to see a shift in the prescription patterns occurring in 2006 to 2007 when these programs began, but there were no notable deviations in thiazide utilization. Nevertheless, the potential impact of non‐VA medication use cannot be determined. Second, our analyses are based in a population of older veterans, which may not be representative of the general population. However, prescription rates of thiazides in the VA are similar to the general population, 15 , 16 , 17 leading us to believe that the increased use of chlorthalidone is probably not specific to the VA health system. Last, we did not restrict our analysis to patients with hypertension. Imposing this restriction would require identifying patients with hypertension using diagnostic codes from inpatient and outpatient treatment files. This approach may have had low sensitivity and thus decreased the power and generalizability of our findings. In addition, patients with hypertension who have a diagnosis coded in the VA administrative data could be systematically different than patients with hypertension who are not coded, and thus introduce a selection bias in our sample. Given the high specificity of thiazide diuretics for the treatment of hypertension, we felt that omitting any sample restriction based on diagnostic codes for hypertension would provide the most accurate assessment of current prescribing patterns.
Strengths
A strength of our study is that it provides the first systematic comparison of prescribing rates of HCTZ and chlorthalidone. In doing so, it supplements recent analyses that reported general utilization measures for thiazide diuretics but which lacked examination of specific frequency and dosing trends within the class. 15 , 16 , 17 , 18 In addition to being able to examine these within‐class trends, we were also able to separate out a cohort of new thiazide users, which allowed us to better characterize the scope of chlorthalidone use. The ability to examine new users is increasingly important in observational drug studies and affords additional evidence in determining whether a true shift in prescribing is occurring. 27 Given the prominent role that thiazide diuretics play in the treatment of hypertension, potential differences in clinical effectiveness between HCTZ and chlorthalidone would have major public health significance. Comparative effectiveness studies involving these two agents are needed.
Conclusions
Despite a two‐fold increase in chlorthalidone use from 2003 to 2008, chlorthalidone still accounts for <5% of total thiazide users use in the Veterans Health Administration. Evaluation of prescribing trends indicates that HCTZ remains the most commonly prescribed thiazide, but there appears to be a shift toward more new users of chlorthalidone. Continued efforts are necessary to improve knowledge and utilization of thiazides, and, particularly, in promoting increased use of chlorthalidone.
Acknowledgments
Acknowledgement: The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service through the Center for Research in the Implementation of Innovative Strategies in Practice (CRIISP) (REA 09‐220) at the Iowa City VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988‐2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 2. Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361:2153–2164. [DOI] [PubMed] [Google Scholar]
- 3. Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first‐line agents. A network meta‐analysis. JAMA. 2003;289:2534–2544. [DOI] [PubMed] [Google Scholar]
- 4. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomized trials in the context of expectations from prospective epidemiologic studies. BMJ. 2009;338:b1665. Published online 2009 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database Syst Rev. 2009;3:CD001841. [DOI] [PubMed] [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 7. VHA/DoD Clinical Practice Guideline for the Diagnosis and Management of Hypertension in the Primary Care Setting. Washington, DC: Office of Quality and Performance and the Veterans Affairs and Department of Defense Development Work Group, Veterans Health Administration, Department of Veterans Affairs; 1999. Update 2004. [Google Scholar]
- 8. Hypertension Detection and Follow‐up Program Cooperative Group . The effect of treatment on mild hypertension: results of the hypertension detection and follow‐up program. N Engl J Med. 1982;307:976–980. [DOI] [PubMed] [Google Scholar]
- 9. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 10. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 11. Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin‐converting enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. [DOI] [PubMed] [Google Scholar]
- 12. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 13. Amery A, Birkenhager W, Brixko P, et al. Mortality and morbidity from the European Working Party on High Blood Pressure in the Elderly trial. Lancet. 1985;1:1349–1354. [DOI] [PubMed] [Google Scholar]
- 14. Medical Research Council Working Party . Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ. 1992;304:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stafford RS, Bartholomew LK, Cushman WC, et al. Impact of ALLHAT/JNC7 dissemination project on thiazide‐type diuretic use. Arch Intern Med. 2010;170:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furmaga EM, Cunningham FE, Cushman WC, et al. National utilization of antihypertensive medications from 2000 to 2006 in the Veterans Health Administration: focus on thiazide diuretics. J Clin Hypertens (Greenwich). 2008;10:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muntner P, Krousel‐Wood M, Hyre AD, et al. Antihypertensive prescriptions for newly treated patients before and after the main antihypertensive and lipid‐lowering treatment to prevent heart attack trial results and seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure guidelines. Hypertension. 2009;53:617–623. [DOI] [PubMed] [Google Scholar]
- 18. Ho PM, Zeng C, Tavel HM, et al. Trends in first‐line therapy for hypertension in the cardiovascular research network hypertension registry, 2002‐2007. Arch Intern Med. 2010;170:912–913. [DOI] [PubMed] [Google Scholar]
- 19. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4–9. [DOI] [PubMed] [Google Scholar]
- 20. Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. [DOI] [PubMed] [Google Scholar]
- 21. Elliott WJ, Grimm RH Jr. Using diuretics in practice – one opinion. J Clin Hypertens (Greenwich). 2008;10:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaplan NM. The choice of thiazide diuretics: why chlorthalidone may replace hydrochlorothiazide. Hypertension. 2009;54:951–953. [DOI] [PubMed] [Google Scholar]
- 23. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 24. Sica DA. Chlorthalidone. Has it always been the best thiazide‐type diuretic? Hypertension. 2006;47:321–322. [DOI] [PubMed] [Google Scholar]
- 25. Taler SJ. Should chlorthalidone be the diuretic of choice for antihypertensive therapy? Curr Hypertens Rep. 2008;10:293–297. [DOI] [PubMed] [Google Scholar]
- 26. Saklayen MG. Which diuretic should be used for treatment of hypertension? Am Fam Physician. 2008;78:445–446. [PubMed] [Google Scholar]
- 27. Avorn J. Transforming trial results into practice change. The final translational hurdle. Arch Intern Med. 2010;170:858–860. [DOI] [PubMed] [Google Scholar]
- 28. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 29. Lund BC, Crischilles EA, Carter BL, et al. Development of a computer algorithm for defining an active drug list using an automated pharmacy database. J Clin Epidemiol. 2003;56:802–806. [DOI] [PubMed] [Google Scholar]
- 30. Ernst ME, Carter BL, Zheng S, et al. Meta‐analysis of dose‐response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23:440–446. [DOI] [PubMed] [Google Scholar]
- 31. Ernst ME, Carter BL, Basile JN. All thiazide‐like diuretics are not chlorthalidone: putting the ACCOMPLISH trial into perspective. J Clin Hypertens (Greenwich). 2009;11:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39:982–988. [DOI] [PubMed] [Google Scholar]


