Abstract
Cardiometabolic syndrome has been associated with increased likelihood and extent of coronary artery calcium (CAC). The authors examined the relationship of cardiometabolic syndrome to CAC progression in 200 healthy men who volunteered to undergo repeated electron beam tomography separated by 4.2±1.3 years. Prediction of clinically significant CAC progression (≥15% per year) was evaluated using multivariable logistic regression models and principal component analysis. Clinically significant CAC progression was observed in 52.5% of the cohort, with the mean and median rate of annual progression 41.3% and 18.3%, respectively. The cardiometabolic syndrome in clinically significant CAC progression participants was significantly higher compared with those without CAC progression (24.8% vs 11.6%; P=.016). Cardiometabolic syndrome was a significant independent predictor of clinically significant CAC progression (odds ratio, 2.65; P=.022). Cardiometabolic syndrome is associated with the baseline CAC score, and independently associated with the progression of CAC over 4 years.
T he cardiometabolic syndrome (CMS) is prevalent 1 and associated with increased risk of cardiovascular events and death as summarized in a metaanalysis including 43 cohorts and 172,573 individuals showing CMS was associated with a relative risk of 1.78 for cardiovascular events and death. 2 In the National Cholesterol Education Program Adult Treatment Panel III guidelines (NCEP), CMS was recommended as a secondary target of coronary risk reduction. 3
A separate marker of cardiovascular risk is coronary artery calcium (CAC), which has also been associated with the presence and extent of CAC. Furthermore, measuring the progression of CAC may augment the coronary risk assessment. 4 , 5 , 6 Thus understanding the determinants of CAC progression may aid the selection of patients for serial CAC testing. The purpose of this study was to evaluate the relationships between the CMS and CAC progression towards identification of optimal target populations for serial monitoring of CAC progression.
Methods
The Prospective Army Coronary Calcium (PACC) Project is a prospective cohort study of US Army personnel examining the role of coronary computed tomography for the detection of CAC. Begun in 1998, we enrolled healthy, asymptomatic men and women between the ages of 40 and 50 years who were presenting for a periodic physical examination. The methods of the PACC project have been previously published. 7 The baseline coronary risk factors and their relationships to CAC in the PACC Project have been previously reported. 8 , 9 In 2005, a companion study, the PACC Rescan Project, was begun. This study is a hypothesis‐directed investigation of the relationships of CAC progression and coronary risk factors in a recruited sample from the original cohort of the PACC Project. A primary hypothesis of PACC Rescan involved investigating the relationship between CMS and CAC progression. The goal of this proposed study was to test the null hypothesis that the rate of CAC progression is identical in participants with and without CMS.
Two‐hundred volunteer patients who had coronary calcium detected on their original study‐related electron‐beam computed tomography (EBCT) scan were enrolled to undergo repeat PACC procedures including a second EBCT scan and assessment of coronary risk factors. All repeat measurements were evaluated blinded to prior clinical data. EBCT was performed in standard fashion using an EBCT scanner with axial prospective electrocardiogram gating using a 3 mm slice thickness during a single breathhold. Fasting blood was collected for the serologic measurement of total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, serum glucose, glycosylated hemoglobin, lipoprotein (a), high‐sensitivity C‐reactive protein (hsCRP), homocysteine, fibrinogen, and insulin. Serum hsCRP was measured using a particle‐enhanced immunoturbidometric latex agglutination assay. Three patients with acute phase reactant levels of hsCRP (>1 mg/L) were excluded from this subgroup analysis.
Cardiometabolic Syndrome
We prospectively elected to follow the definition of cardiometabolic syndrome as defined by the NCEP. 3 This designation includes the presence of 3 or more of the following variables: (1) hypertension, defined as either a history of treated or untreated hypertension, or an average systolic blood pressure above 135 mm Hg or diastolic blood pressure above 85 mm Hg on 3 consecutive automated blood pressure measurements taken 5 minutes apart in the seated position; (2) abdominal obesity, defined as a waist girth ≥102 cm for men and 88 cm for women on a single taped measurement to the nearest centimeter on exposed skin at the level of the umbilicus in a standing position with arms and shoulders relaxed; (3) elevated fasting serum glucose, defined as a fasting value ≥110 mg/dL; (4) elevated triglycerides, defined as a fasting value ≥150 mg/dL; (5) reduced HDL cholesterol, defined as a fasting serum HDL cholesterol <50 mg/dL in women and <40 mg/dL in men. For each patient, the number of individual CMS component variables present (the “metabolic score”) was calculated (range 0–5). Participants with a metabolic score of ≥3 were defined as having the CMS.
Statistical Analysis
The primary, prespecified analysis of PACC Rescan was the relationship between CAC progression and CMS in this prospective, cross‐sectional study. For univariate analyses, continuous variables were compared using a t test for independent groups and categoric variables were compared using the chi‐square test or Fisher exact test where appropriate. Data are expressed as mean ± 1 standard deviation. A 2‐tailed P value of ≤.05 was considered statistically significant. Because the distribution of the fasting serum insulin levels and CRP levels were not normally distributed as evaluated by the one‐sample Kolmogorov–Smirnov test, these variables were natural log transformed to best approximate conditional normality. Clinically significant CAC progression was defined as ≥15% per year, as reported by Raggi and colleagues 4 , 5 , 6 The annualized percent changes in the coronary calcium score (CCS) were calculated using the formula: ([CCSrescan−CCSoriginal]/CCSoriginal)/follow‐up interval (years). Individuals with a decrease in score were considered in the <15% per year group. The bivariate correlation between fasting serum insulin levels and the cardiometabolic score was tested using Spearman’s rho. Factor analysis using 15 variables was performed to assess the clustering of cardiovascular risk factors such as the insulin resistance factors and other traditional risk factors. Based on the results of the factor analysis and clinical judgments, we selected CMS and other high loading risk factors in the multivariate logistic regression analysis to find the significant predictors of CAC progression. Goodness of fit for the multivariate logistic regression model was evaluated by the Hosmer and Lemeshow test. Variables in the final model were tested for confounding, interactions and linearity. All analyses were performed using SPSS version 13.0 (SPSS Inc, Chicago, IL).
Results
The clinical characteristics of the 200 participants are shown in Table I. Caucasians comprised the majority of the cohort (84.0%). The most prevalent cardiac risk factors were hypertension (35%) and either a first or second degree family history of coronary heart disease (37.6%). The CMS was present in 18.5% of the cohort. The mean 10 year Framingham risk score (FRS) for coronary heart disease (CHD) was 5.2%±2.6%. The mean baseline CAC score was 90.8±247.3. After a mean interscan interval of 4.2±1.3 years (range, 1.5–6.6 years), the mean and median of the rate of CAC progression per year were 41.3% and 18.3%, respectively. An annual CAC progression rate ≥15% was observed in 52.5% of participants (n=108). Interquartile ranges for baseline and follow‐up calcium scores in white patients were 7 to 67 and 19 to 137. Respective values in black patients were 5 to 36 and 12 to 72.
Table I.
Clinical Characteristics of the Study Population
| Variable | Values (n=200) |
|---|---|
| Age, y, mean ± SD | 47.8±2.8 |
| Caucasian race, % | 84.0 |
| Military rank, pay grade | 8.1±2.1 |
| College educated, % | 82.2 |
| Statin use, % | 40.4 |
| Aspirin use, % | 52.8 |
| Antihypertension medication use, % | 9.5 |
| Baseline CAC score | 90.8±247.3 |
| Cardiac risk factors, % | |
| Hypertension | 35.0 |
| Family history of CHD | 37.6 |
| Metabolic syndrome | 17.5 |
| Current tobacco use | 5.6 |
| Diabetes mellitus | 3.3 |
| Framingham CHD risk, % | 5.2±2.6 |
| Body mass index, kg/m2 | 28.3±2.0 |
| Waist girth, cm | 96.7±9.1 |
| Systolic blood pressure, mm Hg | 124.9±11.8 |
| Diastolic blood pressure, mm Hg | 78.7±8.0 |
| Total cholesterol, mg/dL | 213.4±39.0 |
| LDL cholesterol, mg/dL | 135.1±33.4 |
| HDL cholesterol, mg/dL | 49.0±11.0 |
| Triglycerides, mg/dL | 150.8±114.3 |
| Fasting glucose, mg/dL | 91.9±9.5 |
| Hemoglobin A1C, % | 5.4±0.5 |
| C‐reactive protein, mg/dL | 1.7±1.4 |
| Fibrinogen, mg/dL | 321.3±66.1 |
| Lipoprotein(a), mg/dL | 36.7±40.7 |
| Homocysteine, μmol/L | 9.6±2.5 |
| Serum insulin, μU/mol | 8.9±6.1 |
| HOMA‐IR | 36.0±31.7 |
Abbreviations: CAC, coronary artery calcium; CHD, coronary heart disease; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment of insulin resistance; LDL, low density lipoprotein.
The distribution of the metabolic score and its association with mean serum insulin levels are shown in Figure 1. Fasting serum insulin levels significantly increased in the presence of an increasing number of CMS risk variables (P<.0001). There was a significant correlation between the metabolic score and insulin level (correlation coefficient [Spearman’s rho] r=.51, P<.0001).
Figure 1.
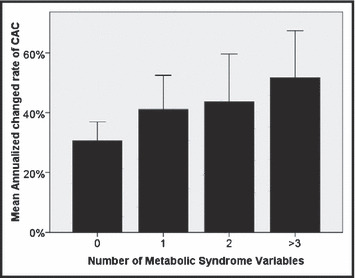
Annualized rate of change in coronary artery calcium (CAC) score according to the number of metabolic syndrome variables present.
In univariate analysis (II, III), CMS, Framingham CHD risk, total cholesterol, LDL cholesterol, and triglycerides were significantly related to CAC progression. CRP level had a correlation with CAC progression of borderline statistical significance. Participants with CMS were more likely to have a clinically significant (CAC progression ≥15% per year), 70.3% (26 of 37) compared with those without, 48.5% (79 of 163) (P=.016) (Figure 2). The annualized rate of change of CAC significantly increased in relation to the number of CMS components present, ranging from no CMS variables (30.6%), 1 CMS variable (41.1%), 2 CMS variables (43.6%), and ≥3 CMS variables (58.7%).
Table II.
Univariate Analysis for the Associations With Coronary Artery Calcification Progression
| <15%per Year | ≥15%per Year | P Value | |
|---|---|---|---|
| Number | 95 | 105 | |
| Age, y, mean ± SD | 48.0±2.8 | 47.7±2.8 | .51 |
| African American, % | 12 | 16.2 | .43 |
| Statin use, % | 47.5 | 52.5 | .56 |
| Aspirin use, % | 51.5 | 48.5 | .26 |
| Cardiac risk factors, % | |||
| Hypertension | 29.3 | 39 | .18 |
| Family history of CHD | 30.1 | 44.2 | .04 |
| Metabolic syndrome | 11.6 | 24.8 | .02 |
| Current tobacco use | 6.7 | 5.7 | .99 |
| Framingham CHD risk, % | 4.8±2.6 | 5.6±2.6 | .03 |
| Body mass index, kg/m2 | 28.4±3.0 | 28.1±2.9 | .47 |
| Waist girth, cm | 96.1±10.6 | 97.0±7.6 | .51 |
| Systolic blood pressure, mm Hg | 124.3±12.0 | 125.4±11.5 | .28 |
| Diastolic blood pressure, mm Hg | 78.5±8.6 | 78.8±7.4 | .47 |
| Total cholesterol, mg/dL | 208.8±39.8 | 217.5±38.0 | .05 |
| LDL cholesterol, mg/dL | 130.5±32.1 | 139.3±34.2 | .03 |
| HDL cholesterol, mg/dL | 50.4±9.7 | 47.8±11.9 | .12 |
| Triglycerides, mg/dL | 136.8±105.9 | 163.5±120.5 | .04 |
| Fasting glucose, mg/dL | 91.2±9.5 | 92.5±9.4 | .19 |
| C‐reactive protein, mg/dL | 1.5±1.2 | 1.8±1.5 | .09 |
| HOMA‐IR | 36.3±23.2 | 40.4±32.0 | .15 |
| Hemoglobin A1C, % | 5.3±0.51 | 5.4±0.49 | .36 |
Abbreviations: CHD, coronary heart disease; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment of insulin resistance; LDL, low‐density lipoprotein.
Table III.
Factor Analysis of Cardiovascular Risk Factors
| Components (Rotated Component Matrix) | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age | −.146 | .194 | .624 | −.103 | −.262 |
| Total cholesterol | .949 | .161 | .057 | .047 | −.021 |
| Low‐density lipoprotein | .949 | .090 | −.039 | .065 | .050 |
| Military rank | .088 | −.031 | .806 | .018 | .276 |
| hsCRP, log | .206 | .485 | −.002 | .137 | −.446 |
| Waist girth | .124 | .694 | −.215 | .092 | .085 |
| Systolic blood pressure | .046 | .084 | −.027 | .898 | −.054 |
| Diastolic blood pressure | .064 | .154 | .012 | .879 | −.041 |
| Triglycerides | .119 | .520 | .152 | −.007 | .092 |
| Fasting plasma glucose | −.237 | .502 | .157 | .212 | −.019 |
| Tobacco using | −.043 | .008 | −.043 | −.060 | −.617 |
| Education level | .043 | −.144 | .786 | .057 | .222 |
| Family history | .072 | .250 | .030 | −.196 | .433 |
| Race | −.034 | .042 | .152 | −.031 | .747 |
| Fasting insulin, log | .084 | .726 | −.096 | .033 | .018 |
Abbreviation: hsCRP, high‐sensitivity C‐reactive protein.
Figure 2.
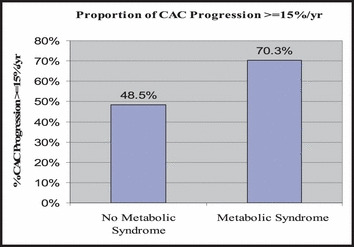
The prevalence of the cardiometabolic syndrome was 18.5% (37 of 200). Participants with metabolic syndrome were more likely to have a clinically significant coronary artery calcium (CAC) progression 70.3% (26 of 37) compared with those without 48.5% (79 of 163) (P=.016).
The Varimax rotated factors and their loadings on original variables from factor analysis are shown in Table III. Five factors transformed (rotated) into interpretable factors were identified by use of the principle component analysis. These 5 factors account for more than 60% of the total variance of CAC progression. Factor 1 is mainly correlated with lipids; factor 2 is mainly correlated with the CMS; factor 3 is mainly correlated with age, education, and socioeconomic status; factor 4 is mainly correlated with blood pressure; factor 5 is mainly correlated with race, family history, log (hsCRP), and tobacco use.
Based on the results of the factor analysis and clinical relevance, we selected CMS, age, race, hsCRP, LDL, and educational level into a multivariate logistic regression model to evaluate independent predictors of CAC progression. In the final multivariate logistic regression analysis (Table IV), CMS was significantly associated with CAC progression after controlling for age, race, hsCRP, LDL, and educational level. Overall, CMS was associated with a 165% increase in the odds of clinically significant CAC progression (odds ratio=2.65; 95% confidence interval [CI], 1.15–6.11; P=.022).
Table IV.
Multivariate Logistic Regression Analyses for the Predictors of Coronary Artery Calcification Progression
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age | 0.95 | 0.85–1.05 | .307 |
| Race (white vs black) | 0.91 | 0.39–2.14 | .832 |
| Metabolic syndrome | 2.65 | 1.15–6.11 | .022 |
| hsCRP, log | 1.21 | 0.75–1.96 | .430 |
| LDL | 1.01 | 1.00–1.02 | .218 |
| Educational level | 1.24 | 0.81–1.90 | .324 |
Abbreviations: CI, confidence interval; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein.
Discussion
The results of this prespecified analysis from the Prospective Army Coronary Calcium Rescan Project demonstrate a significant, independent relationship between CMS and progression of calcified atherosclerosis. Specifically, among middle‐aged men with coronary calcium, CMS was strongly associated with CAC progression over 4 years after controlling for standard coronary risk factors. This association of clinically clustered variables that comprise the CMS with CAC progression provides insights into the determinants of CAC progression and suggests that individuals with CMS may be an optimal population for serial CAC monitoring for progression.
CMS, also termed the insulin resistance syndrome, is a clustering of cardiovascular risk factors associated with multiple metabolic abnormalities and pathophysiology. Due to an increased prevalence of obesity associated with the sedentary lifestyle, diet, and genetic factors, the prevalence of CMS is increasing and has become a global public health problem. Several definitions exist for the CMS and generally overlap: World Health Organization (WHO), 10 European Group for the Study of Insulin Resistance (EGIR), 11 NCEP Adult Treatment Panel III (NCEP ATP III), 3 International Diabetes Federation (IDF), 12 and the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI). 13 Regardless of the definition of CMS employed, survey studies in different countries and different populations show the prevalence of CMS ranges from 15.0% to 48.0%.
The relationship of CMS to CHD outcomes has been controversial, however a recent metaanalysis of 172,573 individuals summarized the available data finding a modest incremental CHD risk. 2 An additional association of CMS is the presence and extent of subclinical atherosclerosis, including CAC. In a prospective cardiovascular risk assessment study of 1000 asymptomatic individuals, we reported that participants with the CMS were more likely to have a positive CAC score: 24.7% compared to those without CMS, 16.5% (P<.05), a finding which was independent of serum LDL. 14 Wong and colleagues 15 also reported in a CAC screening study of 1823 persons (36% female) aged 20 to 79 years that people with CMS had an increased likelihood of CAC compared to those without. The risk factor‐adjusted odds for the presence of CAC was 1.40 (95% CI 1.05–1.87). In the NHLBI Family Heart Study among 3166 white and African American men and women, Ellison 16 found the CMS and most of its components were associated with a higher prevalence of calcified atherosclerotic plaque in the coronary arteries and abdominal aorta. The odds ratios and 95% CI for a CAC score >100 for patients with CMS were 1.7 (95% CI 1.3–2.3) for men and 1.6 (95% CI 1.2–2.1) for women, respectively.
Data from Raggi and colleagues 5 , 6 suggest that CAC progression may be associated with increasing risk for CHD outcomes. However, in 2007, the American College of Cardiology released an expert consensus document recommending against serial testing for CAC progression, in part because the determinations and clinical importance of CAC progression are uncertain. 17 This uncertainty includes the absence of identified target populations. Scientific statements will be unlikely to endorse serial CAC screening without definition of optimal candidates for such testing, and the management implications of the findings are understood. Our results improve our study of CAC progression through demonstration that CMS is strongly associated with the progression of CAC. The CMS components were clearly identified on factor analysis, and individuals with CMS had a 165% increase in the odds of clinically significant CAC progression (odds ratio=2.65; 95% CI 1.15–6.11; P=.022). The identification of CMS along with other risk factors during screening and follow up for CAC progression may help identify these individuals as an optimal target population for serial CAC monitoring for progression.
Limitations
The data within this study are generalizable to healthy, physically active white and black men. Further studies addressing this question as well as involving women and ethnic minorities are needed to extend these data. Our study inclusion criteria were limited to men with previously positive calcium scores, thus further study of the association between CMS and conversion from 0 to positive scores is needed. The choice of scale for the analysis of CAC progression is challenging for those patients with CAC present. Many other methodologies for CAC progression have been suggested (eg, raw change vs percentage change vs change in log calcium plus a constant vs change in score square root transformed). 18 , 19 None is universally accepted and only the definitions as applied by Raggi and colleagues (as applied in this study) have been related to clinical outcomes. Given uncertainty in the statistical methods and error in the measurements themselves, there is a modest potential for misclassification bias which precludes current application of serial CAC scanning in individual patients.
Conclusions
Among asymptomatic, middle‐aged men with coronary calcium, progression of CAC is common and has a limited relationship to standard cardiovascular risk factors. The CMS is associated with the extent of CAC and its progression over 4 years. Further work is warranted that will evaluate the potential of individuals with cardiometabolic risk factors to derive clinical benefit from serial CAC monitoring.
Acknowledgments
Disclosure: The opinions or assertions herein are the private views of the authors and are not to be construed as reflecting the views of the Department of the Army or the Department of Defense. Supported by the Congressionally‐directed, Peer Reviewed Medical Research Program, grant number ERMS 00239017‐00216.
References
- 1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. [DOI] [PubMed] [Google Scholar]
- 2. Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta‐analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. [DOI] [PubMed] [Google Scholar]
- 3. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) . Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. [DOI] [PubMed] [Google Scholar]
- 4. Raggi P, Cooil B, Shaw LJ, et al. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol. 2003;92(7):827–829. [DOI] [PubMed] [Google Scholar]
- 5. Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol‐lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24(7):1272–1277. [DOI] [PubMed] [Google Scholar]
- 6. Raggi P, Cooil B, Ratti C, et al. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension. 2005;46(1):238–243. [DOI] [PubMed] [Google Scholar]
- 7. O’Malley PG, Taylor AJ, Gibbons RV, et al. Rationale and design of the Prospective Army Coronary Calcium (PACC) Study: utility of electron beam computed tomography as a screening test for coronary artery disease and as an intervention for risk factor modification among young, asymptomatic, active‐duty United States Army Personnel. Am Heart J. 1999;137(5):932–941. [DOI] [PubMed] [Google Scholar]
- 8. Taylor AJ, Feuerstein IM, Wong H, et al. Do conventional risk factors predict subclinical coronary artery disease?: results from the Prospective Army Coronary Calcium Project. Am Heart J. 2001;141(3):463–468. [DOI] [PubMed] [Google Scholar]
- 9. Taylor AJ, Arora NS, Bindeman J, et al. Conventional, emerging, heredity, lifestyle, and psychosocial coronary risk factors: relationships to subclinical atherosclerosis. Prev Cardiol. 2006;9(1):25–32. [DOI] [PubMed] [Google Scholar]
- 10. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 11. Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999;16(5):442–443. [DOI] [PubMed] [Google Scholar]
- 12. Adams RJ, Appleton S, Wilson DH, et al. Population comparison of two clinical approaches to the metabolic syndrome: implications of the new International Diabetes Federation consensus definition. Diabetes Care. 2005;28(11):2777–2779. [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement 7. Circulation. 2005;112(17):2735–2752. [DOI] [PubMed] [Google Scholar]
- 14. Hunt ME, O’Malley PG, Feuerstein I, et al. The relationship between the ‘metabolic score’ and sub‐clinical atherosclerosis detected with electron beam computed tomography. Coron Artery Dis. 2003;14(4):317–322. [DOI] [PubMed] [Google Scholar]
- 15. Wong ND, Sciammarella MG, Polk D, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41(9):1547–1553. [DOI] [PubMed] [Google Scholar]
- 16. Ellison RC, Zhang Y, Wagenknecht LE, et al. Relation of the metabolic syndrome to calcified atherosclerotic plaque in the coronary arteries and aorta. Am J Cardiol. 2005;95(10):1180–1186. [DOI] [PubMed] [Google Scholar]
- 17. Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49(3):378–402. [DOI] [PubMed] [Google Scholar]
- 18. Hokanson JE, MacKenzie T, Kinney G, et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. Am J Roentgenol. 2004;182(5):1327–1332. [DOI] [PubMed] [Google Scholar]
- 19. Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115(21):2722–2730. [DOI] [PubMed] [Google Scholar]


