Abstract
J Clin Hypertens (Greenwich). 2010;12:721–726. ©2010 Wiley Periodicals, Inc.
Isometric exercise is associated with acute hemodynamic changes consisting of increases in systolic, diastolic, and mean arterial pressure and also an increase in heart rate and cardiac output. The peripheral vascular resistance is either not changed or decreased. These hemodynamic changes return to baseline values soon after the completion of exercise. Since isometric exercise is not an aerobic exercise, it was not recommended to hypertensive patients by national and international committee guidelines. Recent studies and meta‐analyses of the subject have demonstrated, however, that isometric or resistance exercise does not raise resting blood pressure and frequently leads to a small decrease, which could be enhanced with the concomitant administration of antihypertensive drugs. Besides blood pressure, isometric exercise is associated with other beneficial effects consisting of an increase in muscle bulk, upper and lower body strength, increase in bone density, and a decrease in bone fractures. These changes are extremely beneficial to older patients by making them more mobile and increasing their quality of life. Based on these changes, the authors believe that isometric exercise, whether alone or in combination with dynamic exercise, should be recommended to hypertensive patients and be part of a comprehensive treatment regimen.
Dynamic exercise has been advocated by national and international committees as part of a comprehensive regimen for the treatment of hypertension. 1 , 2 There has been a paucity of data, however, regarding recommendations for isometric or resistance exercise. The fear has been that this type of exercise increases blood pressure (BP) and is not a conditioning exercise, like the dynamic exercise, which is a conditioning exercise, and lowers resting BP and heart rate. 3 Recently there has been a craze in which body fitness and isometric exercises are performed daily by many people in fitness centers or at home regardless of BP levels. Also, daily activities, such as lifting, holding, and carrying a suitcase involve isometric exercises. Although the hemodynamic effects of dynamic exercise have been well studied, 3 there is limited knowledge regarding the hemodynamic, circulatory, and BP effects of isometric exercise in hypertensive patients. An updated Medline search was conducted from 2000 to 2009, since the subject was reviewed by Kelley and coworkers in 2000. 4 Using the terms isometric and resistance exercise, we identified 82 articles. Of these, 6 were selected that included normotensive and hypertensive patients. Thus, in this concise review, the hemodynamic and circulatory effects of isometric or resistance exercise in hypertensive patients and its merits as complimentary to antihypertensive treatment will be discussed.
Underlying Hemodynamic and Circulatory Mechanisms of Isometric Exercise in Normotensive and Hypertensive Patients
Normotensive Patients
The immediate response to a sustained handgrip (SHG) is a rise in heart rate and BP. 4 , 5 , 6 , 7 Studies in normotensive young adults have shown that the initial increase in heart rate and BP to an SHG for 3 minutes at 30% maximal voluntary contraction (MVC) was due to vagal withdrawal, since this response was blocked with pretreatment with atropine. The late response was due to sympathetic nervous system stimulation, because this action was not blocked by previous atropinization of the patients, but only by the combined administration of atropine and propranolol. 5 In our studies of normotensive patients, 7 an SHG for 2 minutes at 30% MVC was associated with a rapid rise of heart rate, BP, and cardiac output and no change in peripheral vascular resistance (PVR), as depicted in 1, 2, 3. The hemodynamic studies were performed in the morning after an overnight fast without premedication. Briefly, small segments of polyethylene tubing were percutaneously introduced into the antecubital vein and brachial artery up to the shoulder level. This enabled continuous direct recording at 25 mm/s and 100 mm/s paper speeds of arterial and venous pressures simultaneously with the electrocardiogram (LII). After a 15‐minute supine rest on a tilt table, the control hemodynamic measurements were taken. The cardiac output, in triplicate, was obtained with 5 mL of indocyanine green (cardiogreen) injected intravenously, followed by 5 mL of normal saline flush. After inscription of each indicator dilution curve, the withdrawn blood was returned to the patient. These curves were plotted semilogarithmically to calculate the cardiac output per minute. The cardiac output was repeated after an SHG at 30% and MVC for 1 minute before and during the hemodynamic measurements. The 30% MVC was previously determined by having the patient grip a hand ergometer (Stoelting Co, Chicago, IL) to his MVC. From this value, the 30% MVC of SHG was calculated for the individual patient. After 15 minutes of supine rest, the Valsalva maneuver was performed by having the patient blow against a resistance of 40 mm Hg for 30 seconds, and the hemodynamic measurements were repeated. After another 15 minutes of supine rest, the patient was tilted at 50 degrees upright for 5 minutes, and the hemodynamic measurements were repeated. From these measurements, the following hemodynamic parameters were calculated: (1) cardiac index by dividing the cardiac output by the body surface area in m2; (2) mean arterial pressure by adding to the diastolic arterial pressure one third of pulse pressure; (3) peripheral vascular resistance (PVR) index by dividing the PVR by the body surface area in m2 and expressed in arbitrary units; and (4) the percent of diastolic arterial pressure overshoot of the Valsalva maneuver by dividing the difference of diastolic arterial pressure pre‐ and post‐Valsalva maneuver by the pre‐Valsalva maneuver diastolic arterial pressure and multiplying it by 100. The same hemodynamic measurements were performed in both normotensive and hypertensive patients. The patients had either never received medications or had discontinued their medication for 2 weeks. Their baseline clinical characteristics are listed in the Table. Similar findings to ours have been demonstrated by other investigators. 5 , 8 , 9 , 10 Our knowledge regarding the circulatory responses to isometric exercise comes from earlier studies, 11 which show that the blood supply to a contracting muscle is a compromise of two opposing events. One event concerns the dilatation of the blood vessels in an effort to increase flow and the second event represents the throttling effect on these vessels from the mechanical compression of the contracting muscles. It was found that the blood flow to the calf muscles was cut off during sustained contractions at a tension of 20% of MVC. 11 Other investigators studied this phenomenon by having their patients grip a hand dynamometer at 30% MVC. 5 , 12 , 13 They found that the arterial pressure reached 200/135 mm Hg and quickly returned to control levels after the exercise. In these studies, like in ours, the BP rise during the handgrip was caused by an increase in cardiac output, because the heart rate and stroke volume both increased and the PVR either did not change or showed a slight decrease. Also, activities involving lifting, holding, and carrying weights, brought about the same sort of responses, whether the forearm muscles are used to grip the handle of the weight or whether the weight is allowed to hang from the crook of the fingers. The holding of a 20‐kg weight (equivalent to a moderately heavy suitcase), produced a mean increase in BP in 4 patients of 45/30 mm Hg, systolic/diastolic, and a heart rate increase of 24 beats per minute after only 2 minutes. 14 Heavier weight lifting can lead to extremely high levels of BP. A study in professional weight lifters showed that bench pressing at 80%, 90%, 95%, and 100% MVC resulted in a mean BP increase at peak exercise of 320/250 mm Hg and, in one patient, 480/350 mm Hg. 15 The BP returned to control levels shortly after completion of exercise, however.
Figure 1.
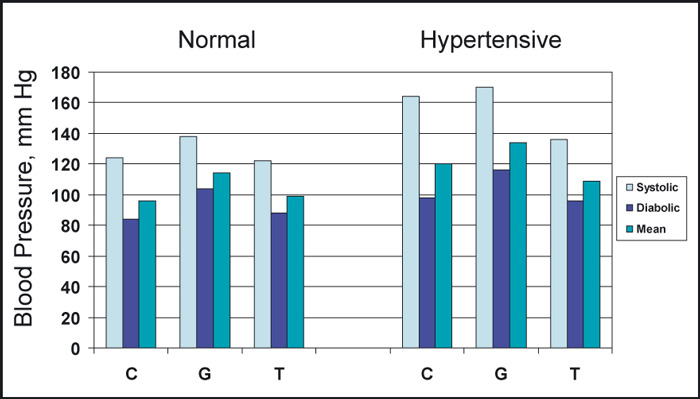
The acute blood pressure response to sustained handgrip and upright tilt in 26 normotensive and 18 hypertensive patients. C indicates control; G, handgrip; T, tilt. Adapted with permission from Chrysant. 7
Figure 2.
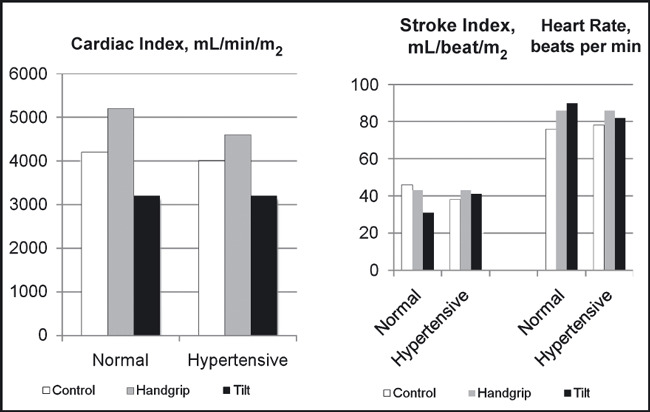
The acute effects of sustained handgrip and upright tilt on cardiac index, stroke index, and heart rate in 26 normotensive and 18 hypertensive patients. Adapted with permission from Chrysant. 7
Figure 3.
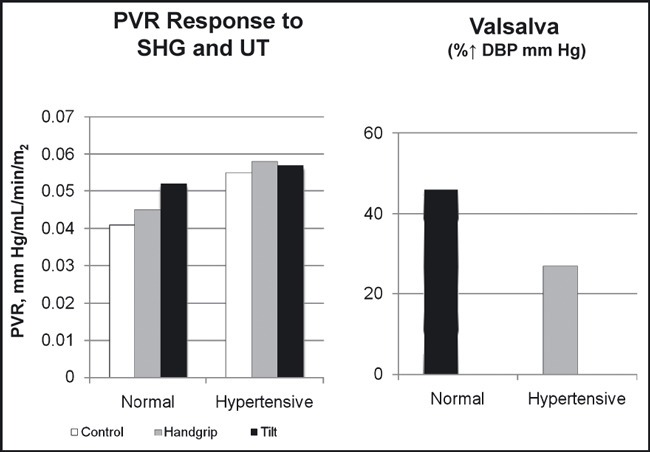
The acute effects of sustained handgrip (SHG) and upright tilt (UT) on peripheral vascular resistance (PVR) index and the diastolic blood pressure (DBP) response to the Valsalva maneuver in 26 normotensive and 18 hypertensive patients. Adapted with permission from Chrysant. 7
Table.
Baseline Clinical Characteristics of the Two Groups of Men
| Normotensive Patients | Hypertensive Patients | |
|---|---|---|
| No. of patients | 26 | 18 |
| Age, y | 47±2 | 51±2 |
| SAP, mm Hg | 124±2 | 164±2 |
| DAP, mm Hg | 82±1 | 98±1 |
| MAP, mm Hg | 96±1 | 120±3 |
| HR, beats per min | 71±2 | 74±2 |
Abbreviations: DAP, diastolic arterial pressure; HR, heart rate; MAP, mean arterial pressure; SAP, systolic arterial pressure. Values are expressed as mean ± standard error of the mean.
Hypertensive Patients
Studies by previous investigators have demonstrated that increased arterial pressure is associated with an alteration of the baroreceptor reflex function. 5 , 16 , 17 Otherwise, persistent bradycardia would have been the characteristic finding in hypertensive patients due to continued high levels of reflex activity. Therefore, the normal heart rate found in most hypertensive patients reflects an adaptation of the baroreflex systems to higher pressures. Keeping with this observation, our studies showed that the responses of arterial pressure, heart rate, cardiac output, and PVR to SHG at 30% MVC were similar in both normotensive and hypertensive patients, 7 even though the responses of the latter patients to upright tilt and the Valsalva maneuver were attenuated (1, 2, 3). Upright tilt in normotensive individuals results in activation of the sympathetic nervous system through the baroreceptors, which, in turn, increase heart rate, diastolic BP, and PVR from arterial vasoconstriction. These sympathetic adjustments attempt to maintain adequate BP and blood flow in the face of falling cardiac output from peripheral venous pooling. The Valsalva maneuver in the same group of patients also results in an overshoot of the diastolic BP through a sudden release of blood into the right ventricle. These autonomic sympathetic adjustments are usually attenuated in hypertensive patients, and such patients frequently fail to compensate and maintain adequate BP and blood flow to vital organs when they suddenly assume the upright posture and may faint. In our studies, normotensive individuals responded to the upright tilt with an increase in the diastolic and mean BP and PVR, in contrast to hypertensive patients who responded with a decrease in diastolic and mean BP and no change in PVR (1, 2, 3). These defective autonomic sympathetic adjustments are aggravated with sympatholytic drugs, which further cripple a sluggish sympathetic system. For this reason, the administration of sympatholytic drugs in such patients should be carefully titrated. It was interesting to see that these patients responded similarly to SHG with the normotensive patients with intact autonomic sympathetic reflexes. In the hypertensive patients, as in the normotensive patients, the rise in systolic, diastolic, and mean arterial pressure to SHG was due to an increase in the cardiac output, since the PVR did not change in both groups. These hemodynamic changes returned to baseline levels soon after completion of the exercise. Similar findings have been reported by other investigators. 18 Isometric exercise in weight lifters and hypertensive patients with left ventricular hypertrophy is associated with abnormal response to left ventricular filling. 19 Both weight lifters and hypertensive patients demonstrated a sharp increase in BP, which was associated with a decrease in diastolic left ventricular filling, decrease in E velocity, increase in A velocity, and decrease in the E/A ratio after a 90‐second SHG at 50% MVC. 19 Abnormal left ventricular hemodynamic changes to isometric exercise have also been reported in patients with coronary heart disease. 8 , 10 Such patients respond to SHG with an increase in BP and left ventricular end‐diastolic pressure. 8 , 10 Because of these hemodynamic changes associated with isometric exercise, such exercise was not recommended in patients with hypertension. 1 , 2 Other studies have shown, however, that mild repetitive isometric exercises are associated with mild decrease in resting BP and can be performed by young and old patients with hypertension. 20 , 21 , 22 , 23 In one study, 22 patients aged 70 to 79 years with mild to moderate hypertension were assigned to resistance training on a Nautilus machine doing 8 to 12 repetitions 3 times per week for 6 months. At the end of resistance exercise training, there was no change in the resting systolic, diastolic, and mean BP, in contrast to endurance exercise groups, which showed an 8‐, 9‐, and 4‐mm Hg reduction in their systolic, diastolic, and mean BP, respectively. 22 There were no statistically significant hemodynamic (heart rate, cardiac output, PVR) or hormonal (epinephrine, no epinephrine, or angiotensin II) changes with resistance training. There was a substantial improvement, however, in upper and lower body muscular strength with resistance exercise. 22 Similar findings were observed in 10 hypertensive patients aged 24 to 40 years who participated in circuit weight training 3 days per week for 9 weeks. At the end of the training, resting BP and heart rate were significantly lower from baseline. In addition, the resistance exercise increased body weight and lean body mass by 1.2% and 2.2%, respectively, and also upper and lower body strength. 21 In another study, 17 hypertensive patients aged 60 to 80 years participated in isometric exercises by performing 4 isometric contractions at 30% MVC for 2 minutes 3 times per week for 10 weeks. 23 At the end of the exercise training, there was a significant reduction in resting systolic BP by 19 mm Hg (P<.001) and mean BP by 11 mm Hg (P<.009) and a 7‐mm Hg reduction in resting diastolic BP (P=not significant). The resting heart rate was not changed, but the isometric exercise was associated with a trend toward lower low‐frequency/high‐frequency ratio for heart rate, although not statistically significant for the group. 23 Recent studies and a meta‐analysis also show significant additional BP‐lowering effects of isometric exercise to antihypertensive treatment, ranging from 11.0 mm Hg to 14.8 mm Hg for systolic BP and 3.3 mm Hg to 10.3 mm Hg for diastolic BP. 24 , 25 , 26
Discussion
The data presented in this concise review suggest that isometric or resistance exercise results in either no change or small reductions in resting systolic and diastolic BP and, more important, does not appear to raise it, as it was feared in the past. For this reason, it was not recommended to hypertensive patients by national and international committee guidelines. 1 , 2 The rise in BP during the performance of isometric exercise is due mostly to the increase in cardiac output and not to PVR 7 and returns quickly to baseline levels after the completion of exercise. Similar findings have been reported by recent meta‐analyses on the subject. 4 , 27 , 28 This suggests that the repeated performance of isometric exercise leads to resetting of the baroreceptors, leading to lower BP in the long‐term, and may enhance the action of antihypertensive drugs. 29 Additional benefits of the isometric or resistance exercise are the increase in muscle bulk, upper and lower body strength, decrease in body fat, increase in bone density, decrease in bone fractures, and increase in quality of life, especially in older persons. 4 , 27 , 28 , 30 These are very important benefits despite the modest decreases in BP. According to recent meta‐analyses and the review of published studies, the modest decreases in systolic and diastolic BP were due to the inclusion in these studies of mostly normotensive patients and very few hypertensive patients. In addition, these studies were small and of short duration to see long‐term benefits. Therefore, large, long‐term, randomized, prospective studies, restricted to hypertensive patients, are needed to provide more definitive information on the effects of isometric exercise in resting BP, alone and in combination with antihypertensive drugs. It is now time to recommend individualized training with isometric exercise in patients with hypertension. Therefore, based on the available evidence, we believe that mild to moderate isometric exercise, either alone or in combination with dynamic exercise, should be recommended to patients with hypertension and be part of a comprehensive treatment regimen. 3 , 4 , 27 , 28 A new hand‐held dynamometer (Zona Plus; Zona Health, Boise, ID) has been approved by the US Food and Drug Administration, which can be used at home by patients who are able to ambulate or not and has been shown to reduce BP. 24 , 25 , 31 The Zona Plus dynamometer looks like an electric razor (Figure 4), and can be held by the right or left hand. Exercises can be performed at home while the person is sitting down watching TV. It is fully automated and, according to their instructions, the person must squeeze as hard as possible for 5 seconds. The device measures the maximal strength of the person’s squeeze and calculates the 30% MVC. The device then prompts the individual through four 2‐minute bouts of squeezing, with a minute rest between squeezes. It also informs the patient whether the grip is kept at 30% MVC and will count down the time for the user. The entire session lasts about 12 minutes and should be performed at least 3 times per week. Results become evident after 1 to 2 weeks of training. The BP effects, like drugs, are not permanent. They last as long as the exercises are performed. The cost of the device is $300 and is not reimbursable as yet by Medicare or other health insurances, but is a good investment long‐term.
Figure 4.
The Zona Plus hand‐held dynamometer (Zona Health, Boise, ID).
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Debacker G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 3. Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension. Sports Med. 2000;30:193–206. [DOI] [PubMed] [Google Scholar]
- 4. Kelley GA, Sharpe Kelley K. Progressive resistance exercise and resting blood pressure. A meta‐analysis of randomized control trials. Hypertension. 2000;35:838–843. [DOI] [PubMed] [Google Scholar]
- 5. Martin CE, Shaver JA, Leon DF, et al. Autonomic mechanisms in hemodynamic responses to isometric exercise. J Clin Invest. 1974;54:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freyschuss U. Cardiovascular adjustments to somatomotor activation: elicitation of increments in heart rate, aortic pressure and vasomotor tone with institution of muscle contraction. Acta Physiol Scand. 1970;1:1–63. [PubMed] [Google Scholar]
- 7. Chrysant SG. Hemodynamic effects of isometric exercise in normotensive and hypertensive subjects. Angiology. 1978;29:379–385. [DOI] [PubMed] [Google Scholar]
- 8. Kivowitz C, Parmley WW, Donoso R, et al. Effects of isometric exercise on cardiac performance. The grip test. Circulation. 1971;44:994–1002. [DOI] [PubMed] [Google Scholar]
- 9. Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helfant RH, DeVilla MA, Meister SG. Effects of isometric handgrip exercise on left ventricular performance. Circulation. 1971;44:982–993. [DOI] [PubMed] [Google Scholar]
- 11. Barcroft H, Millen JL. Blood flow through the muscle during sustained contractions. J Physiol. 1939;97:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson DH, Reeves TJ, Sheffield LT, et al. Isometric effects on treadmill exercise response in healthy young men. Am J Cardiol. 1973;31:344–350. [DOI] [PubMed] [Google Scholar]
- 13. Nutter DO, Schlant RC, Hurst JR. Isometric exercise and the cardiovascular system. Mod Concepts Cardiovasc Dis. 1972;41:11–15. [PubMed] [Google Scholar]
- 14. Donald KW, Lind AR, McNicol GW, et al. Cardiovascular responses to sustained (static) contractions. Circ Res. 1967;20(suppl 1):15–18. [Google Scholar]
- 15. MacDougall JD, Tuxen D, Sale DG, et al. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58:785–790. [DOI] [PubMed] [Google Scholar]
- 16. Bristow JD, Honour AJ, Pickerin GW, et al. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;39:48–54. [DOI] [PubMed] [Google Scholar]
- 17. Gribbin B, Pickering TG, Sleight P, et al. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–431. [DOI] [PubMed] [Google Scholar]
- 18. Hoel BL, Lorentsen E, Lund‐Larsen PG. Hemodynamic responses to sustained handgrip in patients with hypertension. Acta Med Scand. 1970;188:413–421. [DOI] [PubMed] [Google Scholar]
- 19. Abinader EG, Sharif D, Sagiv M, et al. The effects of isometric stress on left ventricular filling in athletes with isometric or isotonic training compared to hypertensive and normal controls. Eur Heart J. 1996;17:457–461. [DOI] [PubMed] [Google Scholar]
- 20. Fisher ML, Nutter DO, Jacobs W, et al. Hemodynamic responses to isometric exercise (handgrip) in patients with heart disease. Br Heart J. 1973;35:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris KA, Holly RG. Physiological response to circuit weight training in borderline hypertensive subjects. Med Sci Sports Exerc. 1987;19:246–252. [PubMed] [Google Scholar]
- 22. Cononie CC, Graves JE, Pollock ML, et al. Effects of exercise training on blood pressure in 70‐to‐79‐yr‐old men and women. Med Sci Sports Exerc. 1991;23:505–511. [PubMed] [Google Scholar]
- 23. Taylor AC, McCartney N, Kamath MV, et al. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc. 2003;35:251–256. [DOI] [PubMed] [Google Scholar]
- 24. McGowan CL, Visocchi A, Faulkner M, et al. Isometric handgrip training improves local flow‐mediated dilation in medicated hypertensives. Eur J Appl Physiol. 2006;98:355–362. [DOI] [PubMed] [Google Scholar]
- 25. Millar PJ, Bray SF, McGowan CL, et al. Effects of isometric handgrip training among people medicated for hypertension: a multilevel analysis. Blood Press Monit. 2007;12:307–314. [DOI] [PubMed] [Google Scholar]
- 26. Kelley GA, Kelley KS. Isometric handgrip exercise and resting blood pressure: a meta‐analysis of randomized control trials. J Hypertens. 2010;28:411–418. [DOI] [PubMed] [Google Scholar]
- 27. Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease. 2007 update. Circulation. 2007;116:572–584. [DOI] [PubMed] [Google Scholar]
- 28. Cornelissen VA, Fagaro RH. Effect of resistance training on resting blood pressure, a meta‐analysis of randomized controlled trials. J Hypertens. 2005;23:251–259. [DOI] [PubMed] [Google Scholar]
- 29. Kelemen MH, Effron MB, Valenti SA, et al. Exercise training combined with antihypertensive drug therapy: effects on lipids, blood pressure and left ventricular mass. JAMA. 1990;263:2766–2771. [PubMed] [Google Scholar]
- 30. Phillips SM. Resistance exercise: good for more than just Grandmama and Grandpapa’s muscles. Appl Physiol Nutr Metab. 2007;32:1198–1205. [DOI] [PubMed] [Google Scholar]
- 31. Harvard Medical School . Squeezing your way to lower blood pressure. Harv Heart Lett. 2006;17:5. [PubMed] [Google Scholar]


