Abstract
J Clin Hypertens (Greenwich). 2010;12:776‐783. © 2010 Wiley Periodicals, Inc.
This study was performed to test whether morning hypertension defined by the morning‐evening difference in home blood pressure (BP) (MEdif) and the average of morning and evening BP (MEave) is a determinant of concentric left ventricular hypertrophy (LVH). The authors enrolled patients with untreated hypertension and performed echocardiography and home BP monitoring for 14 consecutive days. All patients were classified into 4 groups by the MEave and MEdif and morning hypertension was defined by MEave ≥135 mm Hg and MEdif ≥15 mm Hg. Left ventricular (LV) geometry was classified as normal, concentric remodeling, eccentric LVH, or concentric LVH. The morning hypertensive patients had a higher LV mass index and relative wall thickness than the other groups. According to multivariable logistic regression analysis, morning hypertensive patients had a significantly increased risk of the concentric LVH (odds ratio, 6.5; 95% confidence interval, 2.5–17.2; P<.001) compared with home normotensive patients with MEdif <15 mm Hg, after adjusting for confounders. Moreover, even among the home normotensives (white‐coat hypertensives), patients with MEdif ≥15 mm Hg had a higher percentage of concentric remodeling than those with MEdif <15 mm Hg (32.5% vs 14.7%, P=.017). Morning hypertension defined by the MEdif and MEave is a strong determinant of concentric LVH, suggesting that this definition could be used to determine the cardiovascular risk of morning hypertension.
Morning hypertension (MHT) defined by home blood pressure (BP) measurement has been reported to be a significant predictor of cardiovascular events 1 and target organ damage. 2 , 3 Although there is no consensus on the definition of MHT, 4 a condition in which BP is specifically higher in the morning than at other times of day, may be regarded as MHT. As the criterion of hypertension based on home BP is 135/85 mm Hg, 5 an average BP in the morning of ≥135/85 mm Hg and average BP in the evening of <135/85 mm Hg could be one of the definitions of MHT. 1
We have recently demonstrated that the morning‐evening difference in self‐measured BP at home (MEdif) in systolic BP (SBP) was associated with left ventricular hypertrophy (LVH) and remodeling in untreated hypertensive patients, independent of the average of morning and evening BP (MEave). 6 The recent guidelines of home BP have shown that home hypertension should be diagnosed by MEave. 7 , 8 Thus, we hypothesized that home hypertension with an increased MEdif, a condition that we termed morning hypertension, could more precisely reflect the cardiac risk of hypertensive patients. The assessment of left ventricular (LV) geometry, in addition to increased LV mass (LVM), is important in terms of cardiovascular risk stratification. Hypertensive patients with concentric LVH have a higher risk of cardiovascular events than those with any other LV geometric patterns. 9 This study was designed to assess whether MHT as defined by both MEdif and MEave can be a determinant of concentric LVH in never‐treated hypertensive patients.
Methods
Study Participants
Patients with untreated hypertension from the Department of Internal Medicine for Outpatients in Miwa Municipal Hospital were enrolled from June 2004 to December 2007. Office hypertension was defined as an average office SBP of at least 140 mm Hg or diastolic BP (DBP) of at least 90 mm Hg or both on 2 different occasions (with at least a 2‐week interval) during the run‐in period (4 weeks) in advance of entry. Patients who had secondary hypertension, arrhythmias, a history of heart failure, a history of stroke or coronary artery disease, renal insufficiency (serum creatinine>2 mg/dL), mental disorders, severe noncardiovascular disease, or chronic inflammatory disease were excluded. Asymptomatic patients with a low LV ejection fraction (<50%), regional wall motion abnormalities, or significant valvular heart disease were excluded from this study. This study was approved by the institutional review board of Miwa Municipal Hospital and written informed consent was obtained from all patients.
At baseline, these patients underwent a medical interview, anthropometric measurements, blood examinations, echocardiography, and pulse wave analysis. Diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dL or use of antidiabetic medication. Hyperlipidemia was defined as a total cholesterol level ≥240 mg/dL, a triglyceride level ≥150 mg/dL, or the use of lipid‐lowering drugs. Habitual drinking was defined as drinking alcohol ≥5 days per week regardless of the amount. Smoking was defined as having a current smoking habit.
BP Measurements
Home BP was measured 3 times each in the morning and evening for 14 consecutive days after the enrollment for this study. The patients were instructed to place the cuff on the nondominant arm, take a 5‐minute rest before the first reading, and take a 15‐second interval between the readings. Morning BP was measured within 1 hour after waking, after urination, and before breakfast. 5 Evening BP was measured just before going to bed and at least 60 minutes after taking a bath. 5 , 10 The device used for home BP was a validated oscillometric device, which incorporates an integrated circuit memory and clock to store the BP readings and time of measurement. 11 Patients who took home BP for <5 consecutive days were excluded from the analysis due to insufficient home BP measurement. The BP data for the first day were discarded, 7 and the mean of all available measurements recorded for the remaining 13 days was calculated for each patient and used for further analysis.
Office BP was measured during the screening period (twice) and at the end of the home BP measurement term, using the same device as used for home BP. At each office visit, 3 consecutive readings were taken on the left arm with a 15‐second interval after a 5‐minute rest in a sitting position. The average of the office BPs on 3 occasions was used for the analyses. Office BP was taken by attending physicians in the morning.
Definitions of MHT
Although there is no available evidence concerning the criteria for MEdif, the cutoff of MEdif was set at 15 mm Hg because the border of the highest quartile of the MEdif was around 15 mm Hg in this study 6 and in treated hypertensives. 3 The patients were classified into 4 groups according to the MEave and MEdif: home normotension without morning BP rise (NT) (MEave <135 mm Hg and MEdif <15 mm Hg); home normotension with morning BP rise (MNT) (MEave <135 mm Hg and MEdif ≥15 mm Hg); hypertension without morning BP rise (HT) (MEave ≥135 mm Hg and MEdif <15 mm Hg); and MHT (MEave ≥135 mm Hg and MEdif ≥15 mm Hg).
Next, the patients were classified into 4 groups according to the morning and evening SBP: home normotension (both morning SBP and evening SBP <135 mm Hg); evening hypertension (morning SBP <135 mm Hg and evening SBP ≥135 mm Hg); sustained hypertension (both morning SBP and evening SBP ≥135 mm Hg); and MHT (morning SBP ≥135 mm Hg and evening SBP <135 mm Hg).
Echocardiographic Measurements
M‐mode echocardiography, guided by 2‐dimensional echocardiography, was performed as previously described. 6 The LVM index (LVMI) was calculated by dividing LVM by body surface area. The presence of LVH was defined by sex‐specific criteria (LVMI ≥125 g/m2 in men and ≥110 g/m2 in women). 12 The LV relative wall thickness (RWT) was calculated as twice the posterior wall thickness divided by the end‐diastolic LV dimension. 9 Concentric geometry was defined as an RWT ≥0.42. 12 Patterns of LV geometry were defined as follows: 9 normal geometry, no LVH, and RWT <0.42; concentric remodeling, no LVH, and RWT ≥0.42; eccentric LVH, LVH, and RWT <0.42; and concentric LVH, LVH, and RWT ≥0.42. The LV diastolic filling pattern was recorded from the apical transducer position with the sample volume situated between the mitral leaflet tips. The reproducibility of the echocardiographic measurements was described in a previous report. 6
Arterial Stiffness Measurements
Arterial stiffness was assessed by brachial‐ankle pulse wave velocity (baPWV) and the carotid augmentation index (AIx). 13 These parameters were measured with a volume‐plethysmographic device with a carotid tonometry sensor. The method and reproducibility of this device have been previously reported. 13
Blood Examinations
Blood samples were collected in the morning in a fasting state. Plasma B‐type natriuretic peptide (BNP) was measured using high‐sensitivity, noncompetitive radioimmunoassays. The estimated glomerular filtration rate (eGFR) was calculated using a validated equation based on the Modification of Diet in Renal Disease study in Japan: eGFR (mL/min/1.73m2)=194 × serum creatinine −1.094 × age −0.287 (if female × 0.739). 14 All assays were performed at Mitsubishi Biochemical Laboratory (Tokyo, Japan).
Statistical Analyses
The data were expressed as the mean ± standard deviation or percentage. Since the distribution of BNP was highly skewed, this parameter was log‐transformed before statistical analyses. One‐way analysis of variance was performed to detect differences among groups, and Tukey’s honestly significant differences test was performed for multiple pairwise comparisons of the means among groups. The chi‐square test was used to compare proportions. Analysis of covariance was performed to detect significant differences among groups after adjustment for potential confounding factors, and the Bonferroni test was used for multiple pairwise comparisons. The likelihood of each LV remodeling pattern in each of the 4 groups based on home BP was analyzed with multivariable logistic regression analysis after adjusting for confounding factors. The null hypothesis was rejected when the 2‐tailed P value was <.05. All statistical analyses were performed with SPSS version 16.0 (SPSS Inc, Chicago, IL).
Results
Characteristics of the Patients
A total of 356 patients completed the study protocol. 6 The characteristics of the 4 groups are shown in Table I. There was no significant difference in the MEave in SBP between the NT patients and the MNT patients (P=.71) or between the HT patients and the MHT patients (P=.12). The MHT patients and the MNT patients had lower MEdif in pulse rate than the NT patients and the HT patients. The MHT patients were significantly older and had a higher percentage of habitual drinkers and a higher BNP than the NT patients and the HT patients. The MHT patients had a higher office SBP and baPWV than the other groups, and a higher carotid AIx than the NT patients (Table I).
Table I.
Characteristics of the Patients According to the MEave in SBP and MEdif in SBP
| Normotension Without Morning BP Rise (n=102) | Normotension With Morning BP Rise (n=40) | Hypertension Without Morning BP Rise (n=140) | Morning Hypertension (n=74) | P Value | |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age, y | 63.7±9.7 | 69.4±10.1a | 65.8±11.6 | 70.3±11.0b,d | <.001 |
| Male sex, % | 31 | 55a | 48a | 64b | <.001 |
| Body height, cm | 154.2±8.1 | 154.8±10.7 | 155.1±10.1 | 155.8±9.2 | .74 |
| Body mass index, kg/m2 | 23.3±3.1 | 22.7±3.2 | 23.7±3.5 | 22.8±2.7 | .13 |
| Waist circumference, cm | 81.0±9.5 | 80.9±9.1 | 83.7±9.1 | 82.3±8.8 | .12 |
| Duration of hypertension, y | 1.6±4.0 | 2.8±7.4 | 3.5±5.5 | 4.9±7.6a | .003 |
| Current smoking, % | 14 | 23 | 18 | 14 | .52 |
| Habitual drinking, % | 17 | 32 | 23 | 49b,e | <.001 |
| Diabetes mellitus, % | 9 | 13 | 11 | 4 | .30 |
| Hyperlipidemia, % | 36 | 27 | 31 | 24 | .38 |
| Blood pressure | |||||
| MEave in SBP, mm Hg | 124±8 | 126±7 | 151±13b,c | 154±14b,c | <.001 |
| MEave in DBP, mm Hg | 72±8 | 71±7 | 83±9b,c | 82±10b,c | <.001 |
| MEave in pulse rate, beats per min | 67±8 | 67±9 | 69±9 | 68±9 | .36 |
| MEdif in SBP, mm Hg | 4±6 | 21±6b | 4±8c | 26±9b,c,e | <.001 |
| MEdif in pulse rate, beats per min | −4±5 | −7±6a | −4±6c | −8±7b,e | <.001 |
| Office SBP, mm Hg | 142±11 | 145±16 | 158±16b,c | 165±17b,c,e | <.001 |
| Office DBP, mm Hg | 82±9 | 81±9 | 87±10b | 89±12b,c | <.001 |
| Office pulse rate, beats per min | 71±10 | 70±11 | 73±11 | 70±12 | .33 |
| Arterial stiffness | |||||
| baPWV, m/s | 15.8±2.8 | 17.4±3.9 | 18.9±3.8b | 20.7±4.9b,c,e | <.001 |
| Carotid AIx, % | 30.1±8.7 | 32.7±10.9 | 31.6±10.4 | 34.4±8.3a | .02 |
| Cardiorenal functions | |||||
| BNP (geometric mean), pg/mL | 17.6 | 23.9 | 18.7 | 31.2b,e | <.001 |
| eGFR, mL/min/1.73m2 | 78.5±16.7 | 78.2±14.8 | 79.9±17.9 | 74.0±15.2 | .10 |
| Echocardiographic parameters | |||||
| LV end‐diastolic dimension, mm | 44.8±4.5 | 45.3±4.2 | 45.8±4.9 | 46.2±5.2 | .19 |
| LV end‐systolic dimension, mm | 27.6±4.7 | 27.3±3.6 | 28.5±4.6 | 29.0±5.1 | .11 |
| Interventricular septal wall thickness, mm | 8.9±1.2 | 9.8±1.4a | 10.1±1.7b | 10.6±1.6b | <.001 |
| LV posterior wall thickness, mm | 8.7±1.2 | 9.4±1.2a | 9.9±1.6b | 10.6±1.2b,c,e | <.001 |
| LV mass, g | 147.8±41.3 | 169.4±37.1 | 186.9±60.7b | 204.1±47.7b,c | <.001 |
| LV mass index, g/m2 | 96.6±24.3 | 112.0±24.1a | 120.4±36.0b | 133.4±30.7b,c,d | <.001 |
| Relative wall thickness | 0.39±0.06 | 0.42±0.08 | 0.44±0.08b | 0.47±0.08b,c,d | <.001 |
| Left atrial dimension, mm | 32.8±4.4 | 32.8±3.9 | 33.1±4.7 | 34.6±4.5 | .10 |
| LV ejection fraction, % | 68.4±8.3 | 69.8±7.3 | 67.6±7.6 | 67.2±7.7 | .31 |
| E/A ratio | 0.89±0.23 | 0.82±0.14 | 0.79±0.18a | 0.73±0.21b | <.001 |
| Deceleration time of E wave, msec | 209.6±35.4 | 219.9±24.0 | 232.1±44.7a | 245.1±47.8b | <.001 |
| Heart rate at echocardiography, beats per min | 67.7±11.6 | 67.4±11.2 | 68.4±11.4 | 67.0±12.1 | .85 |
Abbreviations: AIx, augmentation index; baPWV, brachial‐ankle pulse wave velocity; BNP, B‐type natriuretic peptide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LV, left ventricular; MEave, the average of morning and evening blood pressure; MEdif, morning minus evening blood pressure; SBP, systolic blood pressure. Data are shown as the mean ± SD or percentage. P values were calculated by analysis of variance and Tukey’s honestly significant differences or chi‐square test. a P<.05. b P<.001 vs normotension without morning BP rise. c P<.01 vs normotension with morning BP rise. d P<.05. e P<.01 vs hypertension without morning BP rise. [Correction added after online publication 16‐Jul‐2010: The footnotes and citations have been updated.]
The MHT patients had a higher LVMI and RWT than the other groups. The LV diastolic function (lower E/A ratio and longer deceleration time) in the MHT patients was impaired to a greater extent than in the NT patients (Table I). Even after adjustment for significant confounding factors such as age, sex, duration of hypertension, habitual drinking, diabetes mellitus, office SBP, and heart rate at echocardiography, the MHT patients had significantly higher LVMI, RWT, and deceleration time than the NT patients (P=.003; P=.001; P=.029). However, the significant difference in the E/A ratio among the 4 groups disappeared after adjustment for the aforementioned confounders (P=.09).
MHT and Concentric LVH
Figure 1 shows a comparison of the mean values of the LVMI and RWT adjusted for age and sex among the 4 groups. This figure shows that the stratification by the MEave and MEdif clearly reflected an increase in the LVMI and RWT. When the distribution of the LV geometric patterns was compared among the 4 groups, concentric LVH was more prevalent in the MHT patients (48.6%) than in the NT patients (7.8%, P<.001), the MNT patients (20.0%, P=.007), or the HT patients (30.0%, P=.003) (Figure 2). On the other hand, there were no significant differences in the percentage of eccentric LVH among the 4 groups. The MNT group had a higher percentage of concentric remodeling than the NT group (32.5% vs 14.7%, P=.017). The likelihood of having concentric LVH was estimated for each of the 4 groups with multivariable logistic regression analysis. As shown in Figure 3, the MHT patients had the highest risk for concentric LVH (odds ratio [OR], 6.5; 95% confidence interval [CI], 2.5–17.2; P<.001) after adjusting for age, sex, duration of hypertension, current smoking, habitual drinking, diabetes mellitus, office SBP, and heart rate at echocardiography.
Figure 1.
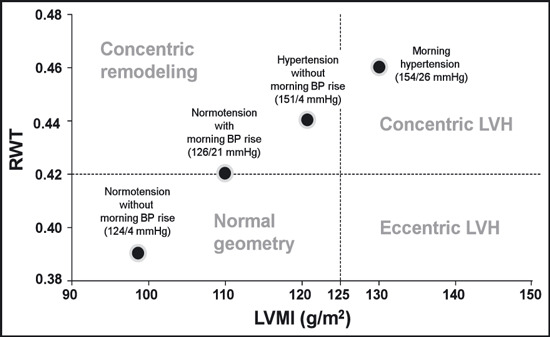
Comparison of the left ventricular geometric pattern to the left ventricular mass index (LVMI) (abscissa) and relative wall thickness (RWT) (ordinate) coordinates after adjusting for age and sex in each of the 4 hypertension groups. The average of the morning and evening systolic blood pressure (BP) and the difference between the morning and the evening systolic BP are indicated in parentheses for each group. LVH indicates left ventricular hypertrophy.
Figure 2.
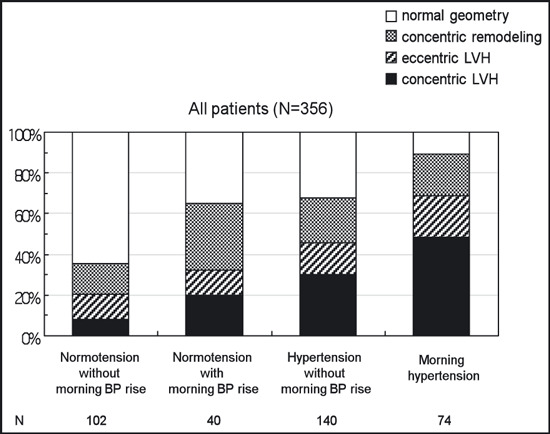
Distribution of left ventricular geometric patterns in normotensives without morning blood pressure (BP) rise, normotensives with morning BP rise, hypertensives without morning BP rise, and morning hypertensives. LVH indicates left ventricular hypertrophy.
Figure 3.
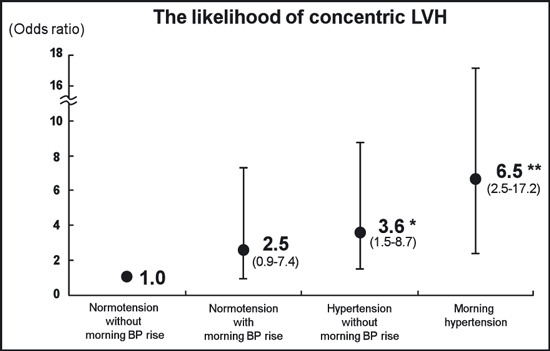
Odds ratio for concentric left ventricular hypertrophy (LVH) in normotensives without morning blood pressure (BP) rise, normotensives with morning BP rise, hypertensives without morning BP rise, and morning hypertensives after adjusting for age, sex, duration of hypertension, current smoking, habitual drinking, diabetes mellitus, office systolic BP, and heart rate at echocardiography. *P=.005. **P<.001 vs normotensives without morning BP rise.
Another Definition of MHT
The characteristics of the 4 groups established according to the MHT definition based on morning and evening SBP are shown in Table II. The sustained hypertensives had a higher MEave, office BP, and RWT than the morning hypertensives, while there was no significant difference in LVMI and BNP between the 2 groups (Table II). The percentage of concentric LVH in the morning hypertensives (30.0%) was significantly higher than in the normotensives (2.2%, P<.001) and the evening hypertensives (8.3%, P=.04), but was not significantly different from that in the sustained hypertensives (38.9%, P=.14). After adjusting for the aforementioned confounders, there was a significantly higher risk for concentric LVH both in the morning hypertensives (OR, 14.6; 95% CI, 3.3–65.1; P<.001) and the sustained hypertensives (OR, 18.5; 95% CI, 4.3–83.5; P<.001) compared with the normotensives, but not in the evening hypertensives (OR, 4.0; 95% CI, 0.3–50.1; P=.27).
Table II.
Characteristics of the Patients According to the Morning and Evening SBP
| Normotension (n=90) | Evening Hypertension (n=12) | Sustained Hypertension (n=154) | Morning Hypertension (n=100) | P Value | |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age, y | 64.2±9.8 | 59.1±16.4 | 67.8±11.5c | 67.7±9.9c | .005 |
| Male sex, % | 33 | 50 | 49 | 56a | .01 |
| Body height, cm | 153.5±8.6 | 156.8±9.6 | 154.9±9.6 | 156.0±9.7 | .31 |
| Body mass index, kg/m2 | 23.2±3.1 | 25.3±4.2 | 23.3±3.1 | 23.1±3.2 | .15 |
| Waist circumference, cm | 80.5±9.3 | 85.4±11.6 | 83.1±9.1 | 82.4±8.7 | .12 |
| Duration of hypertension, y | 1.2±3.3 | 1.5±4.8 | 3.8±6.4a | 4.1±6.8a | .002 |
| Current smoking, % | 15 | 8 | 18 | 17 | .74 |
| Habitual drinking, % | 17 | 17 | 28 | 38a | .01 |
| Diabetes mellitus, % | 10 | 25 | 8 | 7 | .22 |
| Hyperlipidemia, % | 36 | 50 | 25 | 33 | .14 |
| Blood pressure | |||||
| MEave in SBP, mm Hg | 121±7 | 134±4b | 156±13b,d | 137±7b,f | <.001 |
| MEave in DBP, mm Hg | 70±7 | 79±10b | 84±10b | 77±8b,f | <.001 |
| MEave in pulse rate, beats per min | 67±8 | 67±11 | 69±9 | 68±9 | .74 |
| MEdif in SBP, mm Hg | 5±8 | −6±7a | 9±12d | 19±11b,d,f | <.001 |
| MEdif in pulse rate, beats per min | −4±6 | −4±4 | −5±6 | −6±7a | .007 |
| Office SBP, mm Hg | 140±11 | 147±15 | 162±17b,d | 153±16b,f | <.001 |
| Office DBP, mm Hg | 81±9 | 85±14 | 87±10b | 85±11a | <.001 |
| Office pulse rate, beats per min | 72±10 | 71±13 | 72±11 | 71±11 | .86 |
| Arterial stiffness | |||||
| baPWV, m/s | 15.6±2.8 | 15.8±4.9 | 19.9±4.4b,d | 18.0±3.6b,f | <.001 |
| Carotid AIx, % | 30.8±8.8 | 24.6±13.0 | 32.9±9.0c | 32.1±10.4c | .02 |
| Cardio‐renal functions | |||||
| BNP (geometric mean), pg/mL | 17.1 | 13.4 | 22.9 | 23.5 | .02 |
| eGFR, mL/min/1.73m2 | 78.6±16.0 | 85.0±23.0 | 78.1±17.1 | 76.8±16.2 | .34 |
| Echocardiographic parameters | |||||
| LV end‐diastolic dimension, mm | 44.4±4.5 | 47.5±4.2 | 45.6±5.2 | 46.1±4.2 | .05 |
| LV end‐systolic dimension, mm | 27.4±4.7 | 30.2±5.1 | 28.5±4.8 | 28.3±4.1 | .14 |
| Interventricular septal wall thickness, mm | 8.8±1.1 | 9.0±1.6 | 10.4±1.7b,c | 9.9±1.4b,e | <.001 |
| LV posterior wall thickness, mm | 8.6±1.1 | 8.6±1.3 | 10.2±1.6b,d | 9.8±1.3b,c | <.001 |
| LV mass, g | 142.9±36.9 | 166.8±59.1 | 194.8±60.4b | 182.1±43.6b | <.001 |
| LV mass index, g/m2 | 94.2±21.2 | 101.9±26.6 | 126.6±36.7b,c | 118.3±28.2b | <.001 |
| Relative wall thickness | 0.39±0.06 | 0.36±0.05 | 0.45±0.08b,d | 0.43±0.07a,c,e | <.001 |
| Left atrial dimension, mm | 32.7±4.6 | 34.0±3.0 | 33.4±4.7 | 33.5±4.3 | .47 |
| LV ejection fraction, % | 68.3±8.7 | 66.0±8.1 | 67.5±7.9 | 68.7±6.8 | .51 |
| E/A ratio | 0.88±0.24 | 0.87±0.14 | 0.77±0.18b | 0.80±0.20a | <.001 |
| Deceleration time of E wave, msec | 208.0±34.4 | 212.4±38.4 | 237.3±47.0b | 229.3±38.0a | <.001 |
| Heart rate at echo, beats per min | 67.6±11.9 | 68.2±11.1 | 68.1±11.5 | 67.4±11.6 | .96 |
Abbreviations: AIx, augmentation index; baPWV, brachial‐ankle pulse wave velocity; BNP, B‐type natriuretic peptide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LV, left ventricular; MEave, the average of morning and evening blood pressure; MEdif, morning minus evening blood pressure; SBP, systolic blood pressure. P values were calculated by analysis of variance and Tukey’s honestly significant differences or chi‐square test. Data are shown as the mean ± SD or percentage. a P<.05. b P<.001 vs normotension. c P<.05. d P<.01 vs evening hypertension. e P<.05. f P<.01 vs sustained hypertension. [Correction added after online publication 16‐Jul‐2010: The footnotes and citations have been updated.]
Discussion
The main finding of this study was that MHT defined by MEdif and MEave is a stronger predictor of concentric LVH than the other BP groups. On the other hand, when MHT was defined by high morning BP and normal evening BP, there was no significant difference in the risk for concentric LVH between the MHT and sustained hypertension groups.
MHT and Concentric LVH
The present study showed that the morning hypertensives with an increased MEdif had a higher prevalence of concentric LVH than the other groups. This result is reinforced by our previous finding that morning hypertensives have a higher BNP level than other hypertensives 3 and by a report that BNP secretion is augmented to a greater extent in concentric LVH. 15 Four possible mechanisms may explain the relationship between MHT and concentric LVH. First, because the MEdif was reported to be a marker of the BP variability of home BP, 16 hypertensives with an increased MEdif may have a larger daytime BP variability, which has been reported to be associated with LVH. 17 Morning hypertensives may have advanced LVH due to high BP variability, including morning BP surge. Second, an increased MEdif was reported to be associated with the severity of sleep apnea, 18 which is closely associated with LVH. Thus, sleep apnea may have partly contributed to the close association between MHT and LVH, although the absence of relationship between the MEdif and body mass index in the present study does not completely support the involvement of obstructive sleep apnea.
Third, patients with an increased MEdif had a higher value of carotid AIx, a measure of wave reflection, than the other groups, and the carotid AIx was significantly correlated with the MEdif (data not shown). Rizzioni and colleagues 19 demonstrated that small artery remodeling is significantly correlated with a morning BP surge diagnosed by ambulatory BP monitoring in hypertension. Small‐artery remodeling is a cause of increased peripheral artery resistance, which partly determines the magnitude of the reflected wave. It has been reported that an increased wave reflection is associated with increased LVM 20 and, therefore, small‐artery remodeling may contribute to the LVH in patients with an increased MEdif. Fourth, in the present study, the morning hypertensives had a higher baPWV than the other groups. An increased arterial stiffness may amplify the morning BP surge through an impaired baroreceptor sensitivity, or vice versa, an exaggerated morning BP surge may further increase arterial stiffness. 21 Considering the previous reports in which both increased PWV 22 and impaired baroreceptor sensitivity 23 were associated with LV concentric geometry in hypertension, these hemodynamic factors may contribute to the LV concentric change in the LVH of the morning hypertensives in the present study.
Morning BP Rise in White‐Coat Hypertension
The present study conducted in office hypertensives showed that patients with home normotension, ie, white‐coat hypertension, plus an increased MEdif had a significantly higher prevalence of concentric remodeling than those with white‐coat hypertension without an increased MEdif, despite the fact that the MEave values were similar between the 2 groups. Because patients with concentric remodeling have a worse cardiovascular outcome than those with normal geometry, 24 our results suggest that white‐coat hypertension patients with an increased MEdif are at increased cardiovascular risk. Both patients with morning BP rise and those with white‐coat hypertension may have similarly large BP reactivity to physical and psychological stress, and these conditions could be regarded as indicating a pre–sustained hypertension status. 21 , 25 Thus, patients with white‐coat hypertension and morning BP rise should be carefully assessed for possible early target organ damage.
Optimal Definition of MHT
MHT defined by the MEdif and MEave may better differentiate the presence of concentric LVH than MHT defined using morning and evening BP. The latter definition 1 could underestimate the cardiac damage of MHT and is inconsistent with the gold‐standard diagnosis of home hypertension using MEave. 7 , 8 The MEdif is higher both in normotensives 26 and hypertensives 27 in Japan than in America and the European countries, because evening BP was measured in the early evening in the American and European studies but in late evening in Japan. Of note, evening BP tends to decrease by bathing and drinking alcohol in the evening. 10 , 26 , 27 Thus, because there are many patients with high morning BP relative to evening BP in Japan, it may be important to stratify the risk of these patients. The definition of MHT including the MEdif may reflect not only the risk of morning BP surge but also the risk of BP variability assessed by home monitoring.
Study Limitations
There were several limitations in this study. First, the directional nature of cause‐and‐effect relations between MHT and concentric LVH remains to be clarified because the present study was cross‐sectional. Second, because there may be a racial difference in the prevalence of MHT, the study findings might not be evenly applicable to other populations. Third, the numbers of patients in the 4 groups were not balanced because, in Japan, there are few hypertensives whose late evening BP is specifically higher than at the other times of day. Finally, because the definition of sustained hypertension (Table II) captures most patients with MEdif ≥15 mm Hg, patients with an increased MEdif may simply describe patients who might have had an overall higher daytime BP.
Conclusions
The present study demonstrated that MHT defined by both increased MEdif and MEave is a strong predictor of concentric LVH in patients with untreated hypertension. The more optimal definition of MHT remains unknown in terms of prognostic ability, therefore future research is needed to compare the different definitions of MHT in terms of their ability to predict future cardiovascular events.
References
- 1. Asayama K, Ohkubo T, Kikuya M, et al. Prediction of stroke by home “morning” versus “evening” blood pressure values: the Ohasama study. Hypertension. 2006;48:737–743. [DOI] [PubMed] [Google Scholar]
- 2. Shibuya Y, Ikeda T, Gomi T. Morning rise of blood pressure assessed by home blood pressure monitoring is associated with left ventricular hypertrophy in hypertensive patients receiving long‐term antihypertensive medication. Hypertens Res. 2007;30:903–911. [DOI] [PubMed] [Google Scholar]
- 3. Ishikawa J, Hoshide S, Shibasaki S, et al. Relationship between morning hypertension identified by home blood pressure monitoring and brain natriuretic peptide and estimated glomerular filtration rate: the Japan Morning Surge 1 (JMS‐1) Study. J Clin Hypertens (Greenwich). 2008;10:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stergiou G, Parati G. Further insights into the 24‐h blood pressure profile by home blood pressure monitoring: the issue of morning hypertension. J Hypertens. 2009;27:696–699. [DOI] [PubMed] [Google Scholar]
- 5. Imai Y, Otsuka K, Kawano Y, et al. Japanese society of hypertension (JSH) guidelines for self‐monitoring of blood pressure at home. Hypertens Res. 2003;26:771–782. [DOI] [PubMed] [Google Scholar]
- 6. Matsui Y, Eguchi K, Shibasaki S, et al. Association between the morning‐evening difference in home blood pressure and cardiac damage in untreated hypertensive patients. J Hypertens. 2009;27:712–720. [DOI] [PubMed] [Google Scholar]
- 7. Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526. [DOI] [PubMed] [Google Scholar]
- 8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1–9. [DOI] [PubMed] [Google Scholar]
- 9. Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. [DOI] [PubMed] [Google Scholar]
- 10. Kawabe H, Saito I. Influence of nighttime bathing on evening home blood pressure measurements: how long should the interval be after bathing? Hypertens Res. 2006;29:129–133. [DOI] [PubMed] [Google Scholar]
- 11. Anwar YA, Giacco S, McCabe EJ, et al. Evaluation of the efficacy of the Omron HEM‐737 IntelliSense device for use on adults according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1998;3:261–265. [PubMed] [Google Scholar]
- 12. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 13. Matsui Y, Kario K, Ishikawa J, et al. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res. 2004;27:851–857. [DOI] [PubMed] [Google Scholar]
- 14. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 15. Nishikimi T, Yoshihara F, Morimoto A, et al. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension. 1996;28:22–30. [DOI] [PubMed] [Google Scholar]
- 16. Imai Y, Nishiyama A, Sekino M, et al. Characteristics of blood pressure measured at home in the morning and in the evening: the Ohasama study. J Hypertens. 1999;17:889–898. [DOI] [PubMed] [Google Scholar]
- 17. Zakopoulos NA, Tsivgoulis G, Barlas G, et al. Impact of the time rate of blood pressure variation on left ventricular mass. J Hypertens. 2006;24:2071–2077. [DOI] [PubMed] [Google Scholar]
- 18. Lavie‐Nevo K, Pillar G. Evening‐morning differences in blood pressure in sleep apnea syndrome: effect of gender. Am J Hypertens. 2006;19:1064–1069. [DOI] [PubMed] [Google Scholar]
- 19. Rizzoni D, Porteri E, Platto C, et al. Morning rise of blood pressure and subcutaneous small resistance artery structure. J Hypertens. 2007;25:1698–1703. [DOI] [PubMed] [Google Scholar]
- 20. Hashimoto J, Nichols WW, O’Rourke MF, et al. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. Am J Hypertens. 2008;21:329–333. [DOI] [PubMed] [Google Scholar]
- 21. Kario K. Preceding linkage between a morning surge in blood pressure and small artery remodeling: an indicator of prehypertension? J Hypertens. 2007;25:1573–1575. [DOI] [PubMed] [Google Scholar]
- 22. Schillaci G, Mannarino MR, Pucci G, et al. Age‐specific relationship of aortic pulse wave velocity with left ventricular geometry and function in hypertension. Hypertension. 2007;49:317–321. [DOI] [PubMed] [Google Scholar]
- 23. Milan A, Caserta MA, Del Colle S, et al. Baroreflex sensitivity correlates with left ventricular morphology and diastolic function in essential hypertension. J Hypertens. 2007;25:1655–1664. [DOI] [PubMed] [Google Scholar]
- 24. Pierdomenico SD, Lapenna D, Bucci A, et al. Prognostic value of left ventricular concentric remodeling in uncomplicated mild hypertension. Am J Hypertens. 2004;17:1035–1039. [DOI] [PubMed] [Google Scholar]
- 25. Ugajin T, Hozawa A, Ohkubo T, et al. White‐coat hypertension as a risk factor for the development of home hypertension: the Ohasama study. Arch Intern Med. 2005;165:1541–1546. [DOI] [PubMed] [Google Scholar]
- 26. Kawabe H, Saito I. Determinants of exaggerated difference in morning and evening home blood pressure in Japanese normotensives. Hypertens Res. 2009;32:1028–1031. [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa J, Kario K, Hoshide S, et al. Determinants of exaggerated difference in morning and evening blood pressure measured by self‐measured blood pressure monitoring in medicated hypertensive patients: Jichi Morning Hypertension Research (J‐MORE) Study. Am J Hypertens. 2005;18:958–965. [DOI] [PubMed] [Google Scholar]


