Abstract
To study the efficacy of a treatment strategy for the management of hypertensive urgencies, the authors evaluated 549 patients admitted to the emergency department. They were first assigned to a 30‐minute rest period, then a follow‐up blood pressure measurement was carried out. Patients who did not respond to rest were randomly assigned to receive an oral dose of an antihypertensive drug with different mechanisms of action and pharmacodynamic properties (perindopril, amlodipine, or labetalol), and blood pressure was reassessed at 60‐ and 120‐minute intervals. A satisfactory blood pressure response to rest (defined as postintervention systolic blood pressure <180 mm Hg and diastolic blood pressure <110 mm Hg, with at least a 20 mm Hg reduction in basal systolic blood pressure and/or a 10‐mm Hg reduction in basal diastolic blood pressure) was observed in 31.9% of population. Among nonresponders, 79.1% had a satisfactory blood pressure response to the antihypertensive drug treatment in a 2‐hour average follow‐up period. No major adverse events were observed. This treatment strategy, based on standardized rest as an initial step and different antihypertensive drugs, can be effective and safe for the management of patients with hypertensive urgencies.
Patients with severe hypertension (SHT) with no acute target organ damage (ATOD), usually known as a hypertensive urgency, are frequently managed in the emergency department (ED). 1 In the past, an aggressive therapeutic approach was advised to lower blood pressure on the assumption that hypertensive urgency could be associated with an increased incidence of acute adverse events. Current recommendations from international scientific and regulatory associations advise a gradual and controlled decrease in blood pressure, avoiding fast‐action–onset antihypertensive drugs. 2 , 3 , 4
Hypertensive patients subjected to psychological stress demonstrate blood pressure reactivity that is highest in those with severe arterial hypertension. 5 Resting is a proven maneuver to reduce this alert reaction and could be associated with some blood pressure reduction. Treating these patients without considering this and the possibility of a spontaneous lowering of blood pressure may lead to overtreatment and tissue hypoperfusion. Several reports on cerebrovascular and myocardial autoregulation showed that an acute mean blood pressure reduction >20% has been associated with ischemic events. 6 , 7
The aim of this study was to determine the efficacy and safety of a stepped therapeutic strategy based on rest followed by the use of antihypertensive drugs with different profiles of action to manage patients with SHT without previous and ATOD in the ED.
Materials and Methods
Study Design and Setting
Between October 30, 2003, and April 30, 2004, we conducted a cohort study evaluating a stepped‐care management strategy with a nested, randomized, open‐label, parallel comparison of 3 intermediate‐acting antihypertensive drugs.
Patient Selection
We included male and female patients aged 18 years or older who presented in the ED with SHT, defined as a diastolic blood pressure (DBP) level ≥110 mm Hg and/or a systolic blood pressure (SBP) level ≥180 mm Hg. Patients were excluded if they presented with ATOD or any previous heart, renal, or brain disease (see Definitions); if they had recent surgery, acute trauma, infectious disease, body temperature >37°C, or acute psychiatric disease; or if they had received an antihypertensive drug within the previous 60 minutes. Pregnant women were also excluded.
Before any intervention, every patient signed an informed consent form that had been approved by the institutional ethics committee. The clinical research institutional board of the participant institutions also approved all protocols.
Methods
Blood pressure was measured following international guidelines. 3 , 4 An automated digital device (HEM 714IC‐IntelliSense, OMRON, Schaumburg, IL) with appropriate cuff sizes was used in all participating sites, and 3 separate consecutive readings were obtained. An average of the second and third readings was utilized.
Every eligible patient was evaluated with a complete physical examination, including fundoscopy and electrocardiography. Afterward, they were placed in a seated position to rest, in a comfortable and quiet room without talking or active listening during a 30‐minute period. Blood pressure response to rest was then assessed, and patients were identified as responders or nonresponders (see Definitions section). Responders were discharged and their blood pressure was controlled in the ambulatory care setting. Nonresponders to rest were randomly assigned to a single oral dose of one of the following drugs: amlodipine 5 mg, perindopril 4 mg, or labetalol 200 mg. Patients were maintained on rest, and blood pressure was reassessed at 60 and 120 minutes after the dose. Responders to drugs were discharged, adding the drug tested at the same daily dose to their therapeutic plan, while those not responding were considered to have treatment failures. They completed their study participation and were then managed by the physician as usual care (Figure 1). All patients were contacted by telephone 48 to 72 hours after discharge from the ED to verify any adverse events.
Figure 1.
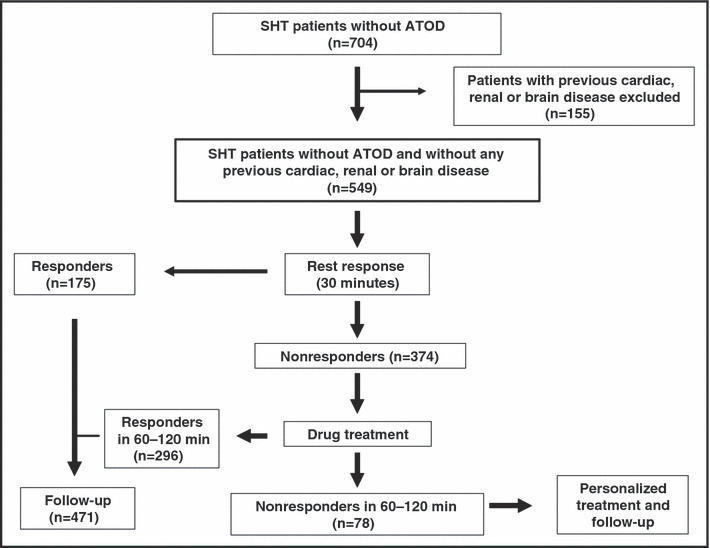
Study design. SHT indicates severe hypertension; ATOD, acute target organ damage.
Definitions
-
1
ATOD: Acute retinal changes (exudates, hemorrhage, or papillary edema), heart (acute myocardial ischemia, acute heart failure, acute aortic dissection), kidney (acute renal failure), or brain (stroke, hypertensive encephalopathy).
-
2
Blood pressure satisfactory response (responders): A patient with a postintervention SBP level <180 mm Hg and DBP level <110 mm Hg, with at least a 20‐mm Hg reduction in basal SBP and/or a 10‐mm Hg reduction in basal DBP.
-
3
Safety of the treatment strategy: We consider that the management of patients was safe if there were both (1) Lack or reduced number (<1%) of adverse events (see next point) in the ED setting and (2) a mean blood pressure reduction <20% from baseline during follow‐up in the ED.
-
4
Adverse events: Major adverse events were cardiovascular death, death due to any cause, myocardial ischemia, or cerebrovascular accident. Minor adverse events were hypotension, dizziness, or vertigo.
Statistical Analysis
The mean and standard deviations and, for categorical ones, proportions and their 95% confidence intervals (CIs) were utilized. Blood pressure changes within groups were compared using paired t‐test, and responses were expressed as percentages with the chi‐square test.
To obtain a precise estimate of the response to the overall strategy, assuming a response rate of 70% with a precision of 95%, it was estimated that the sample size would be at least 318 participants. We planned an effective sample size of 600 patients to get 3 groups of adequate size for comparing response to drugs.
Results
For the purposes of this study, 704 patients with SHT and without ATOD, recruited in 31 sites from Argentina, were identified. From those, 155 patients were excluded because of previous brain, cardiovascular, or renal disease; 549 were included from October 30, 2003, to April 30, 2004. The follow‐up period in the ED ranged from 30 to 60 minutes for those responding to rest and between 150 and 180 minutes for nonresponders.
Population characteristics of 704 SHT patients without ATOD are presented in Table.
Table.
Baseline Characteristics of Patients With Severe Hypertension Without Acute Target Organ Damage
| No. | 704 |
| Age, y | 58.9±14.4 |
| Male, % | 51% |
| Systolic blood pressure, mm Hg | 192.1±16 |
| Diastolic blood pressure, mm Hg | 106.4±13 |
| Heart rate, beats/min | 79.3±14 |
Blood Pressure Response to the Strategy
All patients showed a statistically significant blood pressure reduction in response to rest, and a satisfactory blood pressure response, as defined in the study, was achieved in 31.9% (175 of 549). Blood pressure changes are shown in Figure 2. Patients who did not respond to rest (n=374) were randomly assigned to one of the study drugs (amlodipine, 133; perindopril, 139; labetalol, 102). About half of these patients were classified as responders at 1 hour postdose (53.2%; 95% CI, 48.1%–51.2%). At the end of the second hour, 97 additional patients were classified as responders (25.9%; 95% CI, 21.7%–30.6%) (Figure 3). The overall cumulative response to the strategy (including the rest and drug treatment phases) was 85.8% (95% CI, 82.6%–88.5%).
Figure 2.
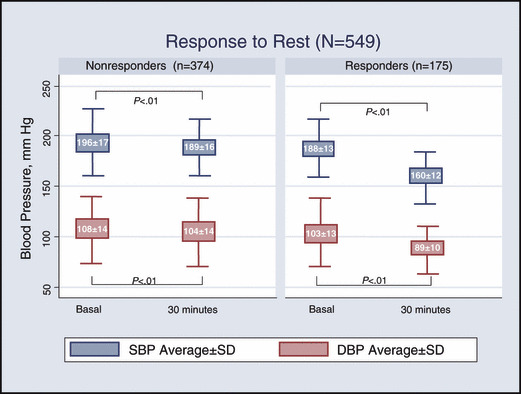
Blood pressure response to rest. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Figure 3.
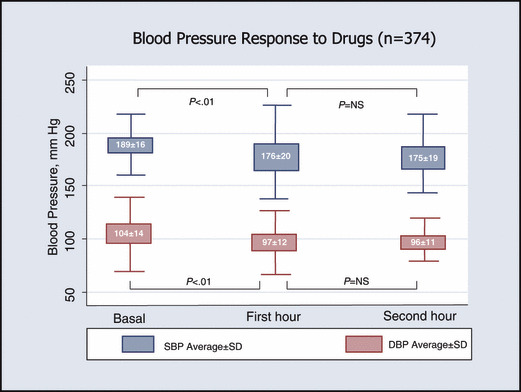
Blood pressure response to drugs. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Comparisons Among Drugs
Blood pressure favorable response (2 hours after drug administration) was observed in 70.7% (94 of 133), 75.5% (105 of 139), and 84.3% (86 of 102) of the amlodipine, perindopril, and labetalol groups, respectively (amlodipine vs labetalol vs perindopril, P=NS). At the end of the first hour, the following response rates were observed: amlodipine, 42.8% (57 of 133); perindopril, 51.8% (72 of 139); and labetalol, 68.6% (70 of 102) (labetalol vs perindopril, P<.01; labetalol vs amlodipine, P<.01; amlodipine vs perindopril, P=NS).
Safety of the Strategy and Adverse Events
In 5.7% (10 of 175) of responders to rest and 16.0% (60 of 374) of patients receiving drug treatment, the mean blood pressure decrease was >20% of the baseline value during observation in the ED. Statistically significant differences were observed among individual drugs in terms of the blood pressure decrease safety criteria. In the first hour, 22.5% (23 of 102) of patients receiving labetalol showed a mean blood pressure reduction >20 mm Hg vs 5.8% (8 of 139) of patients receiving perindopril and 4.5% (6 of 133) of patients receiving amlodipine (labetalol vs amlodipine, P<.01; labetalol vs perindopril, P<.01; amlodipine vs perindopril, P=NS). Overall, the proportion of patients who showed blood pressure decreases above the safety criteria was 31.4% (32 of 102) in the labetalol group, 9.8% (13 of 133) in the amlodipine group, and 10.0% in the perindopril group (14 of 139) (labetalol vs amlodipine, P<.001; labetalol vs perindopril, P<.01; amlodipine vs perindopril, P=NS). Only one patient, who received labetalol, had a mean blood pressure decrease >30%.
No SHT‐related or postintervention major or minor events were reported in any of the patients during their ED stay.
Discussion
The treatment strategy, based on the assumption that hypertensive urgency does not requires acute blood pressure reduction, was shown to be effective and safe. Notwithstanding that rest is recommended as initial treatment for some patients with hypertensive urgencies, 8 no previous reports could be found regarding its efficacy in a large population in the ED. However, Rodríguez Cerillo and associates 9 and Kotliar and colleagues, 10 respectively, found that 24% and 32% of 118 and 121 patients with SHT were satisfactorily managed without pharmacologic treatment. Although more patients could be classified as responders if the rest period had been more prolonged, practical reasons in the usually crowded ED may impose a limit to the time allocated to this strategy. However, Dieterle and coworkers, 11 evaluating the natural course of blood pressure in 45 medical ED patients presenting with moderate to severe hypertension but without target organ damage, showed that after 30 minutes’ rest only small additional changes in blood pressure could be expected.
Most of the patients who needed antihypertensive drugs in our study were classified as responders (79.1%). We employed 3 antihypertensive drugs with different mechanisms of action and pharmacodynamic properties, and despite the initial higher response with labetalol, all 3 drugs showed similar response rates at the end of the 2‐hour follow‐up period. Since the allocation of treatment groups was randomized but not blinded, there were more patients assigned to perindopril and amlodipine than to labetalol, suggesting that some bias may have been introduced; we suggest that the difference, if it exists, is probably small. As we expected, labetalol showed a higher proportion of a mean blood pressure reduction >20%, but only in the first hour postdose. No adverse events were reported in any of the patients in the ED; nevertheless, some minor adverse effects (orthostatic hypotension, dizziness, and vertigo) were observed during the follow‐up period outside the ED.
Drug selection could be an issue when deciding how to achieve a gradual and controlled reduction of blood pressure. We did not include diuretics as a therapeutic alternative, considering that in SHT patients with normal renal function, pressure/natriuresis reaction could be activated and a relative hypovolemic status might be present. The 3 drugs with different mechanisms of action used in our study showed similar response patterns at 2 hours postdose, suggesting that there are no reasons to select one of these drugs over the others for the treatment of SHT in patients without ATOD.
A nonaggressive stepped approach like the one evaluated could constitute an alternative to a more intensive intervention employing more rapid‐onset action antihypertensive drugs. Further research is needed to explore even less intense strategies to manage the hypertensive urgency in the ED and also the achievement of better long‐term blood pressure control during follow‐up to prevent cardiovascular events in the future.
Acknowledgments
Acknowledgments: The authors thank Biol, Pfizer, and Servier laboratories for supplying resources for this study (investigator’s travel expenses, blood pressure measurement digital devices, fax machines, logistic support, materials, and stationery). The authors also thank Sadie Campbell for her expert assistance in preparing the manuscript. Dr Forcada is a speaker for Servier. This program was organized by the Argentine Hypertension Council, from the Argentinian Society on Cardiology, Coordinated by the Austral University Hospital, and sponsored by the Argentine Ministry of Health and Environment. See the Appendix for a list of participant sites and principal investigators.
Executive Committee
Director: C. Kotliar.
Members: M. Bendersky, M. Díaz, D. Ferrante, R. Martín, M. Pellizzari, P. Rodríguez, D. Turri.
Scientific advisors: D. Grassi and K. Ferdinand.
Follow‐up and events committee: H. Fernández.
Executive secretary: P. Forcada.
Data entries and collaborators: M. P. Cobos, S. Riviera, A. Sáenz Valiente.
Principal investigators and participating sites: C. Bellido (Hospital de Clínicas, Bs. As.); E. Butori (Hospital Universitario Austral); C. Borrego (Sanatorio San Lucas, Bs. As.); M. Boscaro (Hospital de San Fernando, Bs. As.); G. Calderón (Sanatorio Franchin, Bs. As.); R. Celsi (Sanatorio Junín, Bs. As.); C. Cuneo (Hospital de San Bernardo, Salta); J. Chibaut (Hospital Zonal de Lobos, Bs. As.); J. O’Donell (Hospital Alemán, Bs. As.); M. Duarte (Hospital de Clínicas, Bs. As.); I. Elliff (Hospital Ramón Carrillo, Bs. As.); E. Farias (Instituto de Cardiología, Corrientes); A. Faurie (Hospital de Mercedes, Bs. As.); J. J. Fuselli (CEMIC, Bs. As.); R. Gambarte (Hospital Lagomaggiore, Mendoza); R. Ingaramo (CEHTA Cardiovascular, Trelew, Chubut); M. Marín (Policlínico Bancario, Bs. As.); R. Martingano (Sanatorio Trinidad, Bs. As.); R. Merbilhaá (ICYCC, Fundación Favaloro, Bs. As.); A. Mulassi (Hospital San Roque); L. Olmos Benegas (Hospital Militar de Córdoba, Córdoba); H. Peralta (Hospital Italiano, Bs. As.); J. C. Pereyra Redondo (CEMIC, Bs. As.); D. Piskorsz (Sanatorio Británico de Rosario, Santa Fe); R. Porcile (Hospital Universitario de la Universidad Abierta Interamericana, Bs. As.); J. .L. Rescio (Centro J. L. Rescio, Bs. As.); L. Romera (Hospital Quiroga, San Juan); P. Rumi (Hospital Santa Clara de Asís, Salta); A. Sáenz (Hospital Reina Fabiola, Córdoba); V. Tarasiuk (Hospital Central de San Isidro, Bs. As.); F. Villafaññe (Hospital Privado, Córdoba); N. Vita (Hospital Italiano de Rosario, Santa Fe); E. Ylarri (Hospital de Olavarría, Bs. As.).
References
- 1. Zampaglione B, Pascale C, Marchisio M, et al. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27:144–147. [DOI] [PubMed] [Google Scholar]
- 2. FDA (Food and Drug Administration, USA) reports . January 28,1996. Order No 711939.
- 3. The Seventh Report of the Joint National Committee on Prevention . Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. Consenso Latino Americano sobre Hipertensión Arterial. J Hypertens. 2001;6:83–110. [Google Scholar]
- 5. Grosse A, Bianchi JM, Díaz Puertas de Grosse CS, et al. Pressure responses of hypertensive patients treated with thiazides, beta blockers and clonidine during a psychological experimental stress situation. Medicina (B Aires). 1990;50(2):141–144. [PubMed] [Google Scholar]
- 6. Strandgaard S, Paulson OB. Cerebral blood flow and its pathophysiology in hypertension. Am J Hypertens. 1989;2:486–492. [DOI] [PubMed] [Google Scholar]
- 7. Kotliar C. Crisis Hipertensiva. In: Favaloro R, Mautner B, eds. Texto de Medicina Interna. Buenos Aires, Argentina: Centro Editor de la Fundación Favaloro. 1998. [Google Scholar]
- 8. Kaplan NM. Uncontrolled Severe Hypertension. In: Kaplan’s Clinical Hypertension. 9th ed. Philadelphia, PA: Lippincot Williams and Wilkins. 2006:321. [Google Scholar]
- 9. Rodríguez Cerillo M, Mateos Hernández P, Fernáández Pinilla C, et al. Hypertensive crises: prevalence and clinical aspects [in Spanish]. Rev Clin Esp. 2002;202:255–258. [DOI] [PubMed] [Google Scholar]
- 10. Kotliar C, Ramos F, Agranatti D, et al. Tratamiento de la hipertensión severa en guardia: ¿un cambio de rumbo es necesario? Rev Argent Cardiol. 1999;67:16(Resumen 30). [Google Scholar]
- 11. Dieterle T, Schuurmans M, Strubel W, et al. Moderate to severe blood pressure elevation at the emergency department entry. Hypertension or normotension? Am J Emerg Med. 2005;23:474–479. [DOI] [PubMed] [Google Scholar]


