Abstract
J Clin Hypertens (Greenwich).
This study was performed to determine the effectiveness of a cardiac rehabilitation and exercise training program on metabolic parameters and coronary risk factors in patients with the metabolic syndrome and coronary heart disease. The study involved 642 patients with coronary heart disease. Of them, 171 (26.7%) fulfilled criteria for the metabolic syndrome. Clinical data, laboratory tests, and exercise testing were performed before and after the program, which lasted 2 to 3 months. Except for waist circumference, there were no significant differences between groups; blood pressure, high‐density lipoprotein cholesterol, triglycerides, and fasting glucose improvements during the follow‐up were higher in patients with the metabolic syndrome (all P<.001). At study end, in patients with the metabolic syndrome, functional capacity increased by 26.45% ( P<.001), as measured by metabolic equivalents, with a slight increase of 1.25% ( P=not significant) in the double product. Patients with the metabolic syndrome who took part in this secondary prevention program reported improvements in cardiovascular risk profile and functional capacity.
The metabolic syndrome (MS) is characterized by a cluster of cardiovascular risk factors including hypertension, dyslipidemia, impaired glycemic control, and abdominal obesity. 1 , 2 It has been estimated that the prevalence of MS is about 25% in the adult US population. 3 Taking into account the progressive advance of diabetes and obesity in Western countries, however, it is not surprising that this prevalence will increase in the coming years. 4 The MS is associated with increased cardiovascular morbidity and mortality. Thus, persons with MS are 3 times more likely to die of coronary heart disease. 5 Without intervention this will almost certainly result in an increase in cardiovascular morbidity. 6 , 7
The application of cardiac rehabilitation programs (CRPs) following acute myocardial infarction (MI) has demonstrated an increase functional capacity and control of cardiovascular risk factors. 8 , 9 There is scarce information about the effects of CRPs in patients with a recent acute coronary syndrome and MS, however. 10 , 11 , 12 Considering that it is well established that exercise training at least partly reverses the MS, it would be useful to determine the efficacy of CRPs in this population.
This study was performed with the aim of assessing the effect of a CRP, developed in a university hospital in Spain, in patients with MS after a MI. The main results of the study population included in the CRP have been recently published. 13
Patients and Methods
The methods and objectives of the CRP have been previously described. 13 Briefly, the CRP was developed by a group of physicians, including a cardiologist who led the program, a rehabilitation physician, a psychologist, a physiotherapist, a nurse, and a nursing auxiliary.
Patient Selection
Although all patients with heart disease could be incorporated into the CRP, only patients with a history of coronary heart disease were included for the present analysis. Patients were included by consecutive sampling and prospectively followed‐up. Indications for the program were assessed by their respective cardiologists based on clinical criteria. No conditions were set for population selection, except for being able to engage in physical exercise and to visit the hospital to undergo the program. The mean time from the acute coronary event to program entry was 3 to 4 months; the total program duration (from inclusion, 3–4 months after the acute event; to program end, after patient completed the exercise training program and was discharged from the CRP) was around 2 to 3 months.
Program Protocol
Medical Visit. The cardiologists performed the medical history and the physical examination and developed the training program for each patient. Blood pressure (BP) measurement was performed by the nurse. The medical history was recorded during the first visit. During this visit, a complete general examination including BP measurements and electrocardiography were also performed. Seated BP was measured after 5 minutes of rest with a validated Omron device. Two measurements were taken and the mean was recorded. BP was considered to be well controlled using the guidelines of the European Society of Hypertension (<140/90 mm Hg for the general population and <130/80 mm Hg for diabetic patients). 14 In the second visit, patients brought with them the results of the baseline exercise testing and laboratory tests. A training program was provided if there were no contraindications. Initial heart rate was established as around 70% of the maximum reached in exercise testing, without symptoms or signs. Treatment was prescribed, or medication adjusted if necessary, for clinical and cardiovascular risk factor control. Oral and written advice was given on diet and avoiding stress. Smoking habits, alcohol consumption, and other factors were determined during the interview.
To quit smoking, patients received advice and simple instructions by the cardiologist, nurse, and psychologist at group meetings. Dietary counseling was provided by cardiologists and nurses according to the current recommendations. 14 , 15 Treatment compliance was also recorded. At the end of the program, the cardiologist made a complete clinical report with the results obtained, dietary recommendations, pharmacologic treatment, and reinsertion into the workplace.
Group Meetings. All members of the medical team, patients, and family attended the group meetings. These were held once a week and dealt with, for example, healthy lifestyles, exercise, substance abuse, and stress.
Cardiovascular Training. Physical training consisted of three 1‐hour sessions per week in the hospital gym. At each session, patients followed a table of physiotherapy and aerobic training on mats. During training, heart rate was calculated for each patient following stress exercise treadmill tests (Bruce protocol) at the beginning and at the end of the program. When ischemia or low exercise levels were detected in the initial test, patients were excluded from the program and indicated for coronary angiography. Patients with exercise tests without ischemia had their objective heart rate set at 75% of the maximum achieved for the first 6 weeks of training and 85% for the last 6 weeks. This was done in the unit’s gymnasium, conducted by a physiotherapist, under medical supervision. The intensity and duration of training increased during the course of the program. The physiotherapist measured the BP of the patients and heart rate at the beginning and end of each session and the data were recorded in the program registry. Supervised training in the gym was complemented by progressively increasing daily walks of ≤1 hour, with the goal of maintaining the heart rate achieved during training. Walks were individually undertaken by patients and were unsupervised.
Test Methodology
Electrocardiography was performed using a Cardioline Delta 3 plus system. Symptom‐limited exercise testing was performed on a Spacelab Quest 600 treadmill, following the Bruce protocol.
Blood tests were performed in a central laboratory after at least 12‐hour fasting. Analytic parameters in plasma were automatically obtained using an EDTA 1‐mg/mL tube. Samples underwent ultracentrifugation following the Lipid Research Clinic criteria. Total cholesterol and triglycerides were determined by enzymatic methods and high‐density lipoprotein cholesterol (HDL‐C) by direct methods.
MS was diagnosed using Third Report of the Adult Treatment Panel National Cholesterol Education Program (NCEP‐ATP III) criteria, requiring the presence of ≥3 of the following: abdominal obesity (waist circumference >102/88 cm or >40/35 in men/women); triglycerides ≥150 mg/dL; HDL‐C <40/50 mg/dL (men/women); fasting glucose ≥110 mg/dL; or BP ≥130/85 mm Hg. 15 Waist circumference was measured at the midway point between the iliac crest and the costal margin. Diabetes was defined as a fasting plasma glucose ≥126 mg/dL on repeat measurement, or postload plasma glucose >198 mg/dL, or a history of diabetes and taking oral antidiabetics or insulin. The presence of diabetes was not an exclusion criterion.
Statistical Analysis
Qualitative data are presented as percentages and quantitative data as mean (standard deviation). Various statistical tests were performed depending on the nature of variables being compared. Preprogram and postprogram quantitative data were compared using the Student t test for paired data. Significant changes in qualitative data were analyzed using the McNemar symmetry test. Database design was subjected to internal consistency rules and ranges to control inconsistencies/inaccuracies in the collection and tabulation of data. The statistical analysis was performed using SPSS statistical software 9.0 (SPSS Inc, Chicago, IL). All the statistical tests were two‐tailed, and P values <.05 were considered significant.
Results
Overall, a total of 642 patients were included in the program. Of them, 171 patients (26.7%) fulfilled criteria for MS; 27.3% of men and 23.7% of women (P=not significant [NS] between sexes). The mean age of the patients with MS was 55.9±8.2 years. At study end, the prevalence of MS decreased to 19.3% (P<.001). Baseline treatments according to the presence of MS are shown in Table I.
Table I.
Baseline Treatments According to the Presence of the Metabolic Syndrome (MS)
| Treatments | Overall (n=642; 100%) | MS (n=171; 26.7%) | No MS (n=471; 73.3%) | P Value |
|---|---|---|---|---|
| Antiplatelets, % | 97.2 | 96.4 | 97.5 | Not significant |
| Anticoagulants, % | 9.0 | 11.2 | 8.3 | <.05 |
| β‐Blockers, % | 74.5 | 72.6 | 75.1 | Not significant |
| Angiotensin‐renin system inhibitor, % | 51.7 | 45.3 | 54.1 | <.05 |
| Lipid‐lowering drugs, % | 99.7 | 100 | 99.6 | Not significant |
| Antidiabetics, % | 11.7 | 21.7 | 7.4 | <.05 |
Table II shows the quantitative changes during the study of the criteria for MS and different biochemical parameters according to the presence of MS. As expected, patients with MS reported baseline higher waist circumference, BP, triglycerides, and glucose values and lower HDL cholesterol levels than patients without MS. Except for waist circumference, there were no significant differences between groups; BP, HDL cholesterol, triglycerides and fasting glucose improvements during the follow‐up were higher in patients with MS (all P<.001) (Table III).
Table II.
Quantitative Changes of MS Criteria and Different Biochemical Parameters According to the Presence of MS
| N=642 | MS (n=171; 26.7%) | No MS (n=471; 73.3%) | P MS | P No MS | P MS vs No MS | |
|---|---|---|---|---|---|---|
| Body mass index, kg/m2 | Baseline | 28.9±3.9 | 26.2±2.9 | NS | NS | <.001 |
| Study end | 28.8±3.8 | 26.3±3.0 | ||||
| Waist circumference, cm | Baseline | 100.8±9.3 | 95.3±8.7 | NS | NS | .006 |
| Study end | 99.4±9.3 | 95.4±9.1 | ||||
| Systolic blood pressure, mm Hg | Baseline | 138.6±17.6 | 124.4±18.4 | <.001 | NS | <.001 |
| Study end | 129.9±17.6 | 124.7±18.9 | ||||
| Diastolic blood pressure, mm Hg | Baseline | 86.4±9.5 | 80.3±11.5 | NS | NS | <.001 |
| Study end | 82.8±10.1 | 79.5±11.0 | ||||
| Pulse pressure, mm Hg | Baseline | 52.2±6.3 | 44.1±5.1 | .01 | NS | <.001 |
| Study end | 47.1±5.2 | 45.2±5.3 | ||||
| Total cholesterol, mg/dL | Baseline | 187.4±40.1 | 182.8±42.0 | <.001 | <.001 | NS |
| Study end | 167.7±34.6 | 166.7±30.8 | ||||
| LDL cholesterol, mg/dL | Baseline | 119.4±35.5 | 116.0±35.6 | <.001 | <.001 | NS |
| Study end | 99.5±25.1 | 99.1±25.5 | ||||
| NEFA, mg/dL | Baseline | 213.8±69.3 | 148.1±50.4 | NS | NS | <.001 |
| Study end | 201.6±93.3 | 152.5±57.0 | ||||
| HDL cholesterol, mg/dL | Baseline | 35.6±8.7 | 43.7±12 | <.001 | <.001 | <.001 |
| Study end | 38.5±9.4 | 45.3±12.2 | ||||
| VLDL cholesterol, mg/dL | Baseline | 32.7±26.8 | 22.6±19.9 | <.001 | <.001 | <.001 |
| Study end | 27.7±18.7 | 21.4±11.4 | ||||
| Triglycerides, mg/dL | Baseline | 176.8±90.9 | 111.9±46.1 | <.001 | NS | <.001 |
| Study end | 149.9±73.2 | 107.7±44.6 | ||||
| Apolipoprotein B, mg/dL | Baseline | 107.4±26.6 | 93.7±26.2 | <.001 | <.001 | <.001 |
| Study end | 97.0±27.1 | 87.0±29.9 | ||||
| TG/HDL cholesterol | Baseline | 5.3±3.5 | 2.8±1.6 | <.001 | NS | <.001 |
| Study end | 4.3±3.2 | 2.6±1.6 | ||||
| Fasting glucose, mg/dL | Baseline | 125.6±37.0 | 102.9±22.6 | <.001 | NS | <.001 |
| Study end | 120.1±42.2 | 103.2±19.5 | ||||
| HbA1c, % | Baseline | 7.7±2.4 | 5.6±0.3 | NS | NS | .025 |
| Study end | 7.7±2.7 | 5.6±0.4 | ||||
| HOMA index | Baseline | 4.9±2.9 | 2.9±1.9 | .05 | NS | .059 |
| Study end | 3.9±3.1 | 3.1±2.8 | ||||
Abbreviations: HbA1c, glycated hemoglobin A1c; HDL, high‐density lipoprotein; HOMA, homeostasis model assessment; LDL, low‐density lipoprotein; MS, metabolic syndrome; NEFA, nonesterified fatty acid; NS, not significant; P MS, P value between baseline and study end in patients with MS; P MS vs No MS, P value between baseline values in patients with and without MS; P No MS, P value between baseline and study end in patients without MS; TG, triglycerides; VLDL, very low‐density lipoprotein.
Table III.
Qualitative Changes of the Metabolic Syndrome (MS) Criteria According to the Presence of MS
| MS (Δ%) | No MS (Δ%) | P Value | |
|---|---|---|---|
| Waist circumference | −1.38 | +0.10 | NS |
| Systolic blood pressure | −6.27 | +0.24 | <.001 |
| Diastolic blood pressure | −4.16 | −0.99 | <.001 |
| HDL cholesterol | +8.14 | +3.66 | <.001 |
| Triglycerides | −15.21 | −3.75 | <.001 |
| Fasting glucose | −4.37 | +0.29 | <.001 |
Abbreviations: Δ%, percentage change; HDL, high‐density lipoprotein; NS, not significant.
Achievement of goals in patients with MS during the study is represented in the Figure. At study end, a higher proportion of patients with MS achieved HDL‐C, triglycerides, fasting glucose, and systolic and diastolic BP control rates, with a trend to a lower body mass index.
Figure.
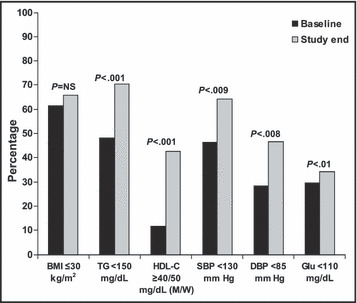
Cardiovascular risk factor control rates during the study in patients with the metabolic syndrome. BMI indicates body mass index; TG, triglyerides; HDL‐C, HDL‐cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; Glu, fasting glucose; M, men; W, women; NS, not significant.
Table IV reports the results of exercise testing. There was a 26.45% increase (P<.001) in functional capacity assessed in metabolic equivalents (METs) and a slight increase of 1.25% (P=NS) in the double product (systolic BP × heart rate).
Table IV.
Results of Exercise Testing at the Beginning and End of the Program in Patients With the Metabolic Syndrome
| Baseline | Study End | P Value | |
|---|---|---|---|
| Metabolic equivalents | 7.07±2.43 | 8.94±2.29 | <.001 |
| Double product | 20.853±5.895 | 21.115±5.999 | Not significant |
Discussion
The presence of MS is associated with a worse prognosis and lower functional recovery in patients with previous MI. 16 There is general agreement that it is necessary to improve prevention and treatment strategies after MI to reduce outcomes. If this is true in the general population after an acute coronary syndrome, this is critical in higher‐risk patients such as those with MS. 17 As a result, this study aimed to assess the efficacy of a CRP in patients with recent MI and MS.
In our study, the prevalence of MS was 26.7%. This is in accordance with other studies performed in Mediterranean countries. Ambrosetti and Mariani 11 reported a prevalence of 26% in patients with coronary artery disease or peripheral arterial obstructive disease attending a CRP, with the highest prevalence (31%) among those admitted after acute coronary syndromes. In studies developed in countries with a higher prevalence of coronary disease, however, such as the United States, the proportion of patients with MS entering CRPs reached 50%. 12 This is not surprising, taking into account that in the general US population, prevalence has been calculated to be 24%. 18 These differences can be explained by the healthier lifestyle found in the Mediterranean area compared with other Western countries and may also be due to the fact that patients referred to these programs depend on the cardiologists’ decision. It is expected, however, that this proportion will rise worldwide in the following years due to the increase of diabetes, obesity, and sedentary lifestyle. 19 In this context, the exercise therapy developed in the CRP remains the better approach for this population. 20
After the CRP, the prevalence of MS decreased from 26.7% to 19.3% (P<.001). This is in accordance with the significant reductions of BP, fasting glucose, triglycerides, and higher HDL‐C values found in our study. Moreover, these reductions were more significant in patients with MS than in the population without MS. These improvements extended not only to the variables that define MS, but also to low‐density lipoprotein cholesterol and, more significantly, the small and dense particles of low‐density lipoprotein cholesterol that have been shown to be more atherogenic. 21 There was also a trend toward decrease in nonesterified fatty acid values. This is relevant since increased nonesterified fatty acid levels reduce insulin‐mediated glucose uptake, thereby causing insulin resistance, 22 which has been proposed as the underlying feature of the MS. 1 , 2 Importantly, our data show that after the CRP, patients with MS reported an improvement in homeostasis model assessment index values.
On the other hand, available data show that high pulse pressure increases cardiovascular mortality in patients with MS. 23 , 24 In this study, only patients with MS reported a significant decrease in pulse pressure. This supports an added value of CRP in this population. All these data show that CRPs reduce MS prevalence and result in an improvement in risk factor control for MS patients after a recent MI. 10 The efficacy of CRP concerning weight loss has been disappointing. Several studies have reported that the weight loss associated with CRPs is quite modest. 25 , 26 Our data did not show significant differences in body mass index or waist circumference during the study. This could be related to the fact that a significant proportion of patients that are included in CRPs are treated with surgical revascularization. As it is known, weeks or even months after surgery, patients tend to gain weight. Longer follow‐up after CRPs has shown significant weight loss. 10
Our data showed that cardiovascular training and improvements in clinical and cardiovascular risk factors led to an increase in efficiency in cardiac work capacity. Thus, functional capacity increased by 26.45%, with only a 1.25% increase in the double product. That is, the heart is capable of more work with only slightly higher oxygen needs (BP and heart rate are the main determinants of myocardial oxygen consumption). These results are in agreement with those found by others. 17
Limitations
The main limitation of the study lies in the lack of a control group (patients with MS after MI that do not follow a CRP) and, thus, it is not known which part of the improvement in the risk profile is attributable to being included in the CRP. Nevertheless, when compared with other studies performed in Spain of patients with similar clinical characteristics and treatments but without an exercise training program, our data suggest that patients included in a CRP have a better prognosis. 27 Moreover, the high number of the patients included and the meticulousness of the data recorded diminish this potential bias. Mean time from the acute coronary event to program entry was 3 to 4 months. Taking into account that the moment of the inclusion may have some influence in the metabolic parameters, the results of our study can be applied only to those CRPs with a similar delay. Since the program duration was only around 2 to 3 months in our study, we cannot determine the effects of CRP in this population if follow‐up were longer. Nevertheless, some studies have shown that longer follow‐up reports similar results. 10 Taking into account that the aim of this study was to assess the effects of CRPs in patients after an acute coronary event in conditions of clinical practice, intensification of drug therapy was permitted during the program. Although this could have some influence in the results, the intensification of the therapy was similar in patients with and without MS, which reduces potential bias.
Conclusions
CRPs reduce MS prevalence and increase the cardiovascular risk factor control rates and functional capacity of patients with MS and coronary heart disease.
Acknowledgments
Acknowledgments: The authors wish to express their sincere gratitude to all nurses and physiotherapists that actively work in the cardiac rehabilitation program of hospital La Paz. Without their dedication and quality of work, the present publication would not have been possible.
References
- 1. Sundstrom J, Riserus U, Byberg L, et al. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haffner SM. Risk constellations in patients with the metabolic syndrome: epidemiology, diagnosis, and treatment patterns. Am J Med. 2006;119:S3–S9. [DOI] [PubMed] [Google Scholar]
- 3. James PT, Rigby N, Leach R. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004;11:3–8. [DOI] [PubMed] [Google Scholar]
- 4. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. [DOI] [PubMed] [Google Scholar]
- 5. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002;288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 7. Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–376. [DOI] [PubMed] [Google Scholar]
- 8. Brubaker PH, Rejeski WJ, Smith MJ, et al. A home‐based maintenance exercise program after center‐based cardiac rehabilitation: effects on blood lipids, body composition, and functional capacity. J Cardiopulm Rehabil. 2000;20: 50–56. [DOI] [PubMed] [Google Scholar]
- 9. Franklin B, Bonsheim K, Warren J, et al. Effects of a contemporary, exercise‐based rehabilitation and cardiovascular risk‐reduction program on coronary patients with abnormal baseline risk factors. Chest. 2002;122:338–343. [DOI] [PubMed] [Google Scholar]
- 10. Gayda M, Brun C, Juneau M, et al. Long‐term cardiac rehabilitation and exercise training programs improve metabolic parameters in metabolic syndrome patients with and without coronary heart disease. Nutr Metab Cardiovasc Dis. 2008;18:142–151. [DOI] [PubMed] [Google Scholar]
- 11. Ambrosetti M, Mariani P. Metabolic syndrome and related dietary intervention among patients with coronary and peripheral arterial disease attending cardiovascular rehabilitation programs. Monaldi Arch Chest Dis. 2007;68:227–230. [DOI] [PubMed] [Google Scholar]
- 12. Savage PD, Banzer JA, Balady GJ, et al. Prevalence of metabolic syndrome in cardiac rehabilitation/secondary prevention programs. Am Heart J. 2005;149:627–631. [DOI] [PubMed] [Google Scholar]
- 13. Plaza I, García S, Madero R, et al. Secondary prevention program: impact on cardiovascular risk. Rev Esp Cardiol. 2007;60:205–208. [PubMed] [Google Scholar]
- 14. 2003 European Society of Hypertension – European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 15. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel) III . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 16. Caminiti G, Volterrani M, Marazzi G, et al. Metabolic syndrome predicts lower functional recovery in female but not in male patients after an acute cardiac event. Int J Cardiol. 2009;135:296–301. [DOI] [PubMed] [Google Scholar]
- 17. Tjønna AE, Lee SJ, Rognmo Ø, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Survey. JAMA. 2002; 287:356–359. [DOI] [PubMed] [Google Scholar]
- 19. Audelin MC, Savage PD, Ades PA. Changing clinical profile of patients entering cardiac rehabilitation/secondary prevention programs: 1996 to 2006. J Cardiopulm Rehabil Prev. 2008;28:299–306. [DOI] [PubMed] [Google Scholar]
- 20. Blaha MJ, Bansal S, Rouf R, et al. A practical “ABCDE” approach to the metabolic syndrome. Mayo Clin Proc. 2008;83:932–941. [DOI] [PubMed] [Google Scholar]
- 21. Mauger JF, Lichtenstein AH, Ausman LM, et al. Effect of different forms of dietary hydrogenated fats on LDL particle size. Am J Clin Nutr. 2003;78:370–375. [DOI] [PubMed] [Google Scholar]
- 22. Maison P, Byrne CD, Hales CN, et al. Hypertension and its treatment influence changes in fasting nonesterified fatty acid concentrations: a link between the sympathetic nervous system and the metabolic syndrome? Metabolism. 2000;49:81–87. [DOI] [PubMed] [Google Scholar]
- 23. Barrios V, Escobar C, Echarri R, et al. Pulse pressure and the metabolic syndrome in patients with hypertension J Cardiometab Syndr. 2009;4:72–75. [DOI] [PubMed] [Google Scholar]
- 24. Mazza A, Zamboni S, Tikhonoff V, et al. Pulse hypertension: a new component of the metabolic syndrome in elderly women? J Hum Hypertens. 2007;21:934–941. [DOI] [PubMed] [Google Scholar]
- 25. Bader D, Maquire T, Spahn C, et al. Clinical profile and outcome of obese patients in cardiac rehabilitation as stratified according to NHLBI criteria. J Cardiopulm Rehabil. 2001;21:210–217. [DOI] [PubMed] [Google Scholar]
- 26. Milani RV, Lavie CJ. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92:50–54. [DOI] [PubMed] [Google Scholar]
- 27. De Velasco JA, Cosín J, López‐Sendón JL, et al. New data on secondary prevention of myocardial infarction in Spain. Results of the PREVESE II study. Rev Esp Cardiol. 2002;55:801–809. [DOI] [PubMed] [Google Scholar]


