Abstract
African Americans, particularly men, have the highest morbidity and mortality rates from hypertension in the United States. The authors studied 527 African Americans in a general clinical research center to determine whether there are sex differences in the relationships between hypertension with insulin resistance (IR) and aldosterone, which are risk factors for cardiovascular disease. Measurements included ambulatory blood pressure (BP), anthropometric measures, plasma renin activity, plasma aldosterone (PA) concentration, and fasting serum lipids, glucose, and insulin. IR was estimated using the Homeostasis Model Assessment (HOMA) model. BP correlated with aldosterone in both sexes. However, both BP and PA correlated with IR in men, but not in women. Compared with men in the lower tertile of HOMA‐IR, men in the upper tertile had higher mean systolic BP, a higher odds ratio of having hypertension, and higher levels of PA. The association of IR with both hypertension and PA in men, but not in women, may contribute to the high prevalence of cardiovascular disease in African American men.
Hypertension is frequently associated with additional cardiovascular disease risk factors, and clustering of risk factors increases the likelihood of subsequent morbidity and mortality. 1 , 2 African Americans, particularly men, have the highest morbidity and mortality from hypertension of any population group in the United States. 3 We have previously shown a strong association of aldosterone with blood pressure (BP) and hypertension among African Americans. 4 A similar but less robust association has been noted among Caucasians. 5 , 6 , 7 Insulin resistance (IR) is also associated with hypertension, although this association has been less consistent in African Americans than in Caucasians. 8 , 9 , 10 , 11 Moreover, plasma aldosterone (PA) is associated with IR and the metabolic syndrome in both African Americans and whites, independent of their association with hypertension. 12 , 13 , 14 In addition to being associated with hypertension, both PA and IR are independent cardiovascular risk factors. 15 , 16
Only limited information is available concerning the impact of sex on the associations between hypertension, IR, and aldosterone. For example, in a population‐based sample, Player and colleagues 17 have shown that prehypertension is associated with IR among men but not women. Moreover, in the Insulin Resistance Atherosclerosis Study (IRAS) family study, 18 unrelated to IR, visceral adipose tissue was found to be associated with hypertension in women but not in men. Some of these differences in risk factor associations may account for the difference in cardiovascular disease prevalence between men and women. The purpose of the present study was to evaluate the hypothesis that hypertension, IR, and PA are more tightly linked in African American men than in African American women.
Methods
African American participants were recruited from a variety of community resources and health providers within the Milwaukee, WI, area. Participants were defined as African American based on self‐identification, birth in the continental United States, both parents reported as being African American, and English as the native language. Informed consent was obtained from all participants, and the protocol was approved by the Froedtert Memorial Lutheran Hospital/Medical College of Wisconsin institutional review board.
Participants ranged in age from 18 to 55 years. Pregnant participants and participants with secondary hypertension, diabetes mellitus, serum creatinine concentrations >2.2 mg/dL (194.48 μmol/L), body mass index (BMI) >36 kg/m2, chronic debilitating illness, and substance abuse were excluded. Before further study, participants taking antihypertensive and lipid‐lowering medications discontinued these agents for at least 1 and 4 weeks, respectively. None of the participants were taking aldosterone antagonists. Participants were then admitted to an inpatient general clinical research center and placed on a weight‐maintaining diet containing 150 mEq sodium and 80 mEq potassium per day.
BPs were measured during a 24‐hour period with an Accutracker (Suntech Medical Instruments Inc, Morrisville, NC) every 30 minutes during the day (6 am–8 pm) and every 60 minutes during the night (8 pm–6 am). Participants were considered to have hypertension if their average overall BP was ≥130/85 mm Hg. For a variety of logistical reasons, some measurements were not obtained in a small percentage of participants. For participants in whom 24‐hour ambulatory BP measurements were not available (10% of total participants), hypertension was defined as screening clinic systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, or being on antihypertensive medications.
Standardized anthropometric measurements included height, weight, waist circumference, and skin‐fold thickness. Waist circumference was taken at the narrowest point between the umbilicus and superior iliac spine. Subscapular, suprailiac, bicep, and tricep skin‐fold thicknesses were measured with a standard protocol using a Lange skin‐fold caliper. All skin folds were measured 3 times and averaged. Based on these measurements, percent of total body fat was calculated with sex‐specific formulas using the Durnin and Womersley equation. 19 Peripheral venous blood was collected after an overnight fast for measurement of serum concentrations of total cholesterol, high‐density lipoprotein (HDL) cholesterol, triglycerides, glucose, and insulin. Plasma renin activity (PRA) and PA concentrations were measured in the midmorning after participants had been supine for 60 minutes followed by standing for 10 minutes.
Serum glucose was measured with an automated glucose oxidase enzymatic assay. Insulin was measured by using a commercially available double‐antibody equilibrium radioimmunoassay. Serum cholesterol was measured using a colorimetric enzymatic procedure, and HDL cholesterol was measured in a same‐day assay after selective precipitation of the low‐density lipoprotein (LDL) fraction. LDL cholesterol was calculated using Friedwald’s formula. Triglycerides were measured by an enzymatic procedure based on the conversion of triglycerides to glycerol and its subsequent conversion to dihydroxyacetone phosphate and hydrogen peroxide. All measurements of PRA and PA were carried out in the same core laboratory. PRA was measured by a modification of the method of Sealey and Laragh, with the use of angiotensin I antisera kindly provided by Dr Jean Sealey (Cornell University Medical Center). 20 PA concentrations were measured by radioimmunoassay with a commercially available assay kit. In our laboratory, the interassay coefficients of variation for the commercially available low and high aldosterone pools are 10.2% and 9.2%, respectively. The respective intraassay coefficients of variation are 5.1% and 4.4%. Twenty‐four‐hour urine sodium concentration was analyzed by flame photometry.
Based on Adult Treatment Panel (ATP III) guidelines, the metabolic syndrome was defined as the presence of ≥3 of the following criteria 21 : (1) abdominal obesity (waist circumference >102 cm in men and >88 cm in women; (2) hypertriglyceridemia (≥150 mg/dL or 1.695 mmol/L); (3) low HDL cholesterol (<40 mg/dL or 1.036 mmol/L in men and <50 mg/dL or 1.295 mmol/L in women); (4) elevated ambulatory BP (≥130/85 mm Hg); and (5) elevated fasting glucose (≥100 mg/dL or 5.55 mmol/L). These criteria were also used for a binary classification of these variables. IR was calculated with the Homeostasis Model Assessment of IR (HOMA‐IR) index, a Web‐based program made available by Oxford University. 22 The degree of IR is related to the height of the index. HOMA‐IR has been shown to correlate well with the euglycemic clamp technique in both normotensive and hypertensive individuals. 23 Participants were considered to be insulin resistant if they were in the upper tertile of HOMA‐IR. 24
Reported BPs were mean values obtained from 24‐hour ambulatory monitoring. Continuous variables were reported as mean ± standard error of the mean (SEM). BPs, PRA, PA, 24‐hour urine sodium, serum insulin, and IR (HOMA‐IR) were not normally distributed and were log transformed to achieve normal distribution for statistical analyses. Triglycerides remained skewed even after log transformation; hence, nonparametric tests were used to compare the significance of group differences of triglycerides. The statistical significance of differences in continuous variables of participant characteristics between men and women were examined either by t test or Wilcoxon rank sum test depending on the distribution of variables. To simultaneously assess the effects of hypertension and sex on the selected phenotypes, a 2‐way analysis of variance with a Bonferroni correction was conducted. Spearman rank correlation was used to test the relationship of BP, aldosterone, and HOMA‐IR with each other and other variables. Differences in the proportions of metabolic risk factors among different groups were tested by chi‐square test. P value <.05 was considered statistically significant. Statistical analyses were performed using Stata SE 9.0 (Stata Corporation, College Station, TX).
Results
A total of 527 African American participants (52% female, 47% hypertensive) were evaluated. There were no statistically significant sex differences in age, average ambulatory systolic and diastolic BPs, or waist circumference (Table I). Women had higher mean BMIs and percent body fat (P<.001) but lower serum glucose (<.05), LDL cholesterol (P<.05), and triglycerides (P<.001). No sex differences were noted in the serum levels of HDL cholesterol, insulin, or IR. There were also no sex differences in PA or 24‐hour sodium excretion, although PRA tended to be lower in women (P<.01).
Table I.
The Mean (±SEM) Values of the Variables Measured in Women and Men
| Variable | Women (n=276) | Men (n=251) |
|---|---|---|
| Age, y | 44±0.4 | 43±0.4 |
| Systolic blood pressure/diastolic blood pressure, mm Hg | 128±1/76±1 | 131±1/79±1 |
| Waist circumference, cm | 90±1 | 92±1 |
| BMI, kg/m2 | 29.5±0.3 | 27.7±0.3c |
| Percent body fat, % | 39.1±0.3 | 24.2±0.4c |
| Fasting glucose, mg/dL | 88±1 | 90±1a |
| Fasting insulin, μU/mL | 12.35±0.36 | 11.71±0.42 |
| Insulin resistance (HOMA‐IR) | 1.57±0.04 | 1.51±0.05 |
| HDL cholesterol, mg/dL | 47±1 | 45±1 |
| LDL cholesterol, mg/dL | 110±2 | 117±2a |
| Triglycerides, mg/dL | 88±4 | 105±4c |
| Plasma aldosterone, ng/dL | 6.9±0.4 | 7.4±0.4 |
| Plasma renin activity, ng/mL/h | 1.19±0.12 | 1.64±.15b |
| 24‐h urine sodium, meq/24 h | 186±6 | 196±7 |
Abbreviations: BMI, body mass index; HOMA‐IR, Homeostasis Model Assessment of Insulin Resistance; SEM, standard error of the mean. To convert high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; and plasma aldosterone to nmol/L, multiply by 0.0277. a P<.05. b P<.01. c P<.001.
Overall, and within each sex, hypertensive participants were older (P<.01) and had a higher waist circumference (P<.001), BMI (P<.05), LDL cholesterol (P<.05), PA (P<.01), and lower PRA (P<.05) compared with normotensive participants (Table II). Overall, hypertensives were also more insulin resistant than normotensives. Among men, but not women, hypertensives had higher percent body fat (P<.05), serum insulin (P<.01), serum glucose (P<.05), and were more insulin resistant (P<.01), but had lower serum HDL cholesterol (P<.05) than normotensives (Table II). In contrast, among women, but not men, serum triglyceride concentrations were higher among hypertensive than normotensive participants (P<.001). Among hypertensives, men had lower BMIs (P<.05), percent body fat (P<.001), and HDL cholesterol (P<.05) than women.
Table II.
The Mean (±SEM) Values of the Variables Measured in Men and Women Based on BP Status
| Variable | Normotensive Women (n=123–154) | Hypertensive Women (n=95–122) | Normotensive Men (n=114–126) | Hypertensive Men (n=100–125) |
|---|---|---|---|---|
| Age, y | 42±1 | 45±1b | 42±1 | 45±1b |
| Systolic BP/diastolic BP, mm Hg | 115±1/69±1 | 146±1/87±1 | 116±1/69±1 | 146±1/88±1 |
| Waist circumference, cm | 87±1 | 93±1c | 88±1 | 95±1c |
| BMI, kg/m2 | 28.9±0.4 | 30.3±0.4a | 26.6±0.3e | 28.8±0.4b,d |
| Percent body fat, % | 38.59±0.46 | 39.78±0.51 | 23.32±0.51e | 25.14±0.63a,e |
| Fasting glucose, mg/dL | 87±1 | 88±1 | 88±1 | 91±1a |
| Fasting insulin, μU/mL | 11.86±0.44 | 12.98±0.59 | 10.73±0.56d | 12.71±0.61b |
| Insulin resistance (HOMA‐IR) | 1.51±0.06 | 1.65±0.07 | 1.38±0.07 | 1.64±0.08b |
| HDL cholesterol, mg/dL | 47±1 | 48±2 | 47±1 | 43±1a,d |
| LDL cholesterol, mg/dL | 106±3 | 116±4a | 112±3 | 122±4a |
| Triglycerides, mg/dL | 79±3 | 100±7c | 98±4e | 112±7 |
| Plasma aldosterone, ng/dL | 5.9±0.5 | 8.2±0.5c | 6.3±0.4 | 8.6±0.6b |
| Plasma renin activity, ng/mL/h | 1.45±0.19 | 0.86±0.11a | 2.18±0.25e | 1.04±0.14c |
| 24‐h urine sodium, meq/24 h | 193±8 | 177±9 | 188±8 | 204±10 |
Abbreviations: BMI, body mass index; HOMA‐IR, Homeostasis Model Assessment of Insulin Resistance; SEM, standard error of the mean. To convert high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; and plasma aldosterone to nmol/L, multiply by 0.0277. Significance between normotensives and hypertensives of the same sex: a P<.05, b P<.01, c P<.001. Significance between men and women in same blood pressure (BP) category: d P<.05, e P<.001.
Based on ATP III criteria, the overall prevalence of the metabolic syndrome was 39% among women compared with 23% among men (P<.0001). The prevalence of IR was similar among hypertensive and normotensive women (30% vs 32%). Hypertensive men had a significantly higher prevalence of IR compared with normotensive men (38% vs 20%; P<.005) (Table III). In addition, the prevalence of the metabolic syndrome was higher among normotensive women than normotensive men (53% vs 19%; P<.0005).
Table III.
Prevalence of Metabolic Syndrome Components Among Men and Women Based on Hypertension Status
| Variable | Normotensive Women, % | Hypertensive Women, % | Normotensive Men, % | Hypertensive Men, % |
|---|---|---|---|---|
| High waist circumference (>88 cm in women and >102 cm in men) | 65/138 (47) | 78/111 (70)a | 8/117 (7)b | 28/110 (25)a,b |
| High triglycerides (>150 mg/dL) | 6/148 (4) | 14/116 (12)a | 14/121 (12)b | 22/118 (19) |
| Low HDL cholesterol (<50 mg/dL in women and <40 mg/dL in men) | 96/149 (64) | 74/116 (64) | 43/121 (36)b | 60/118 (51)a,b |
| Insulin resistance (top 3rd tertile of HOMA‐IR) | 43/143 (30) | 35/110 (32) | 23/113 (20) | 42/111 (38)a |
| Metabolic syndrome (>3 criteria) | 72/135 (53) | 72/107 (67)a | 22/114 (19)b | 47/109 (43)a,b |
Abbreviation: HOMA‐IR, Homeostasis Model Assessment of Insulin Resistance. To convert high‐density lipoprotein (HDL) cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113. a P<.05: significance between normotensives and hypertensives of the same gender. b P<.05: significance between men and women in same blood pressure category.
Among both men and women, mean systolic BP was positively correlated with waist circumference, BMI, percent body fat, and PA concentration and negatively correlated with PRA (Table IV). In addition, in men, but not in women, systolic BP was correlated with HOMA‐IR and inversely correlated with HDL cholesterol. In contrast, systolic BP was significantly correlated with serum triglycerides only in women. Among both men and women, IR was positively correlated with waist circumference (r=.48, P<.001; r=.38, P<.001) and triglycerides (r=.36, P<.001; r=.13, P=.04) and inversely correlated with HDL cholesterol (r=−.22; P<.001; r=−.41; P<.001). PA was positively correlated with waist circumference, BMI, triglycerides, and IR and inversely correlated with HDL cholesterol among men, but, in women, these relationships failed to reach statistical significance (Table IV).
Table IV.
Spearman Correlations of Systolic Blood Pressure and Plasma Aldosterone Concentration
| Variable | Systolic Blood Pressure | Plasma Aldosterone Concentration | ||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Plasma aldosterone | 0.31 (<.001) | 0.20 (0.003) | – | – |
| Insulin | 0.1 (0.15) | 0.21 (0.003) | 0.00 (0.99) | 0.29 (<0.001) |
| HOMA‐IR | 0.1 (0.15) | 0.21 (0.003) | −0.00 (0.99) | 0.30 (<.001) |
| Waist circumference | 0.33 (<.001) | 0.41 (<.001) | 0.11 (0.1) | 0.17 (0.02) |
| Body mass index | 0.19 (0.003) | 0.30 (<.001) | −0.05 (0.49) | 0.21 (0.002) |
| Percent body fat | 0.16 (0.02) | 0.22 (0.002) | −0.05 (0.5) | 0.11 (0.14) |
| Plasma renin activity | −0.16 (0.02) | −0.32 (<.001) | 0.18 (0.007) | 0.14 (0.04) |
| Triglycerides | 0.23 (<.001) | 0.1 (0.15) | 0.06 (0.38) | 0.14 (0.04) |
| HDL cholesterol | −0.009 (0.91) | −0.19 (0.006) | −0.003 (0.97) | −0.15 (0.03) |
Abbreviation: HDL, high‐density lipoprotein cholesterol; HOMA‐IR, Homeostasis Model Assessment of Insulin Resistance. Values in parentheses are significance levels.
To further evaluate the association of IR with hypertension and aldosterone, all participants were grouped by tertiles of HOMA‐IR in each sex group. Compared with men in the lowest tertile of HOMA‐IR, men in the uppermost tertile (ie, most insulin resistant) had higher mean systolic BP (P=.002), an increased prevalence of hypertension (P=.018), and an increased odds ratio (P=.002) of having hypertension (Figure 1). In contrast, among women, average BP, hypertension prevalence, and odds for having hypertension did not differ among the tertiles of IR. In addition, among men, but not among women, there was a progressive increase in PA concentration from the lowest to the highest tertile of IR (Figure 2).
Figure 1.
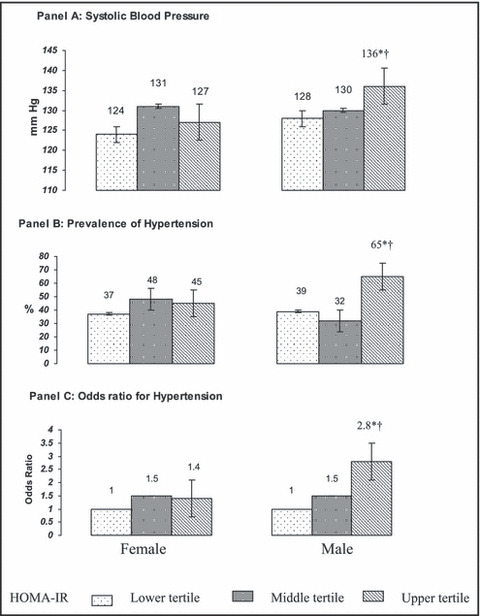
Mean systolic blood pressure (Panel A), prevalence (Panel B) and odds ratio (Panel C) for hypertension according to tertiles of insulin resistance (HOMA‐IR) in African American women and men. Corresponding HOMA‐IR values to the tertiles of insulin resistance include: lower tertile – 0.4–1.1, middle tertile – 1.2–1.7, and top tertile – 1.8–6.3. *Statistically significant difference from first tertile of insulin resistance within each sex (P<.05). †Statistically significant difference within each tertile of insulin resistance across the sex (P<.05).
Figure 2.
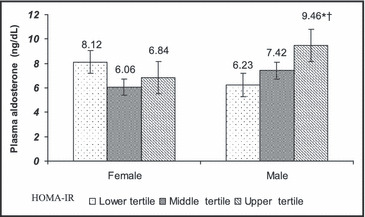
Plasma aldosterone concentration by tertiles of insulin resistance (HOMA‐IR) in African American women and men. Corresponding HOMA‐IR values to the tertiles of insulin resistance include: lower tertile – 0.4–1.1, middle tertile – 1.2–1.7, and top tertile – 1.8–6.3. *Statistically significant difference from the first tertile of insulin resistance within each sex (P<.05). †Statistically significant difference within each tertile of insulin resistance across the sex (P<.05). To convert aldosterone ng/dL to nmol/L, multiply by 0.0277.
Discussion
We have previously reported a significant association of IR with BP and aldosterone in African Americans in sex‐adjusted models. 4 , 6 We now report that these associations of IR are sex specific. In more than 500 African‐American participants studied under controlled standardized conditions, BP levels, the odds ratio for hypertension, the prevalence of hypertension, and PA concentrations were related to IR in men but did not reach statistical significance in women. Nevertheless, BP was associated with PA levels in both men and women.
These results are consistent with earlier observations suggesting that the relationship between IR and BP may be sex specific. Player and colleagues17 have previously described an association of fasting insulin concentration with prehypertension in a nationally representative sample of men, but not women. The IRAS Family Study reported an association between the odds of having hypertension and visceral adipose tissue, independent of IR among African American and Hispanic women, but not men. 25 In the current study, although the degree of IR and serum insulin concentrations did not differ between men and women, BP was not related to IR in women. In addition, normotensive women had an equivalent level of IR and prevalence of metabolic syndrome compared with hypertensive women, further suggesting that IR does not play a significant role in the development of hypertension in African American women.
We and others have previously described an association of PA with the metabolic syndrome, IR and BMI in both Caucasians and African Americans in sex‐adjusted models. 12 , 14 , 26 , 27 , 28 , 29 The results of the present study indicate that aldosterone is associated with IR, BMI, and components of the metabolic syndrome only in men. In apparent contrast, Goodfriend and colleagues 30 reported an association between IR and PA in women but not in men. However, that study had a sample size of fewer than 30 women and was restricted to normotensive participants. In addition, the participants in that study were Caucasian in contrast to African American participants in our study. It has been suggested that the association of centripetal obesity and IR with PA concentration may be secondary to a putative aldosterone secretagogue from visceral adipose tissue. 12 , 28 , 31 However, this would not account for the currently observed sex differences in the association of aldosterone with IR.
This is an observational study and, consequently, we can only speculate about the sex‐specific mechanisms for an effect of IR on BP and aldosterone. One potential explanation may be deficiency of nitric oxide at the tissue level, induced by IR, which has been shown to be associated with development of hypertension as well as an increase in aldosterone production. 32 , 33 , 34 , 35 , 36 , 37 In women, this effect may be offset by estrogen‐induced nitric oxide synthesis. 38 , 39 Additional studies in premenopausal and postmenopausal women will be required to test this hypothesis. Although only 8% of women were taking exogenous estrogens (either oral contraceptives or hormone replacement therapy), we do not have reliable information about menopausal status or phase of menstrual cycle.
Additional limitations of this study should be noted. For safety reasons, antihypertensive agents were withdrawn for only 1 week among hypertensive participants on drug therapy. Sixty‐five percent of the hypertensive participants had been on prior antihypertensive therapy, and average 24‐hour systolic BPs were slightly higher among these individuals compared with untreated hypertensive participants (P<.05). Similar studies will also be required in other racial and ethnic groups to determine whether the reported sex‐specific correlates are unique to African Americans.
Conclusions
IR is a notable cardiovascular disease risk factor and a harbinger for the development of hypertension. 8 In these African American participants, IR was associated with BP and PA concentration in men but not in women. We speculate that the sex specificity of the association of IR with BP and aldosterone in men may contribute to the high rates of hypertension‐related cardiovascular disease in African American men. The sexual dimorphism of many physiologic traits, as well as susceptibility to disease, including hypertension, has long been recognized. Further, it is becoming increasingly apparent that there are sex‐specific genetic determinants to physiologic traits associated with BP and hypertension. 40 , 41 Recognizing sex differences in the relationships among physiologic measures may provide clues about pathogenic mechanisms of hypertension. In addition, these findings highlight the importance of sex‐specific studies for designing targeted strategies for risk factor modification and therapeutic interventions.
Disclosures:
This study was supported by National Institutes of Health grants HL07011 and 5‐M01‐RR‐00058 (General Clinical Research Center). Dr Kidambi was supported by National Institutes of Health T32HL07792 hypertension training grant.
References
- 1. Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. [DOI] [PubMed] [Google Scholar]
- 2. McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11‐year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. [DOI] [PubMed] [Google Scholar]
- 3. Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. [DOI] [PubMed] [Google Scholar]
- 4. El‐Gharbawy AH, Nadig VS, Kotchen JM, et al. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension. 2001;37:845–850. [DOI] [PubMed] [Google Scholar]
- 5. Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. [DOI] [PubMed] [Google Scholar]
- 6. Grim CE, Cowley AW Jr, Hamet P, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005;45:766–772. [DOI] [PubMed] [Google Scholar]
- 7. Walker WG, Whelton PK, Saito H, et al. Relation between blood pressure and renin, renin substrate, angiotensin II, aldosterone and urinary sodium and potassium in 574 ambulatory subjects. Hypertension. 1979;1:287–291. [DOI] [PubMed] [Google Scholar]
- 8. Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab. 2003;88:2399–2403. [DOI] [PubMed] [Google Scholar]
- 9. Manolio TA, Savage PJ, Burke GL, et al. Association of fasting insulin with blood pressure and lipids in young adults. The CARDIA study. Arteriosclerosis. 1990;10:430–436. [DOI] [PubMed] [Google Scholar]
- 10. Donahue RP, Prineas RJ, Bean JA, et al. The relation of fasting insulin to blood pressure in a multiethnic population: the Miami Community Health Study. Ann Epidemiol. 1998;8:236–244. [DOI] [PubMed] [Google Scholar]
- 11. Saad MF, Lillioja S, Nyomba BL, et al. Racial differences in the relation between blood pressure and insulin resistance. N Engl J Med. 1991;324:733–739. [DOI] [PubMed] [Google Scholar]
- 12. Kidambi S, Kotchen JM, Grim CE, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007;49:704–711. [DOI] [PubMed] [Google Scholar]
- 13. Krug AW, Ehrhart‐Bornstein M. Aldosterone and metabolic syndrome: is increased aldosterone in metabolic syndrome patients an additional risk factor? Hypertension. 2008;51:1252–1258. [DOI] [PubMed] [Google Scholar]
- 14. Ingelsson E, Pencina MJ, Tofler GH, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–992. [DOI] [PubMed] [Google Scholar]
- 15. Connell JMC, MacKenzie SM, Freel EM, et al. A lifetime of aldosterone excess: long‐term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr Rev. 2008;29:133–154. [DOI] [PubMed] [Google Scholar]
- 16. Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res. 2005;2:105–112. [DOI] [PubMed] [Google Scholar]
- 17. Player MS, Mainous AG 3rd, Diaz VA, et al. Prehypertension and insulin resistance in a nationally representative adult population. J Clin Hypertens (Greenwich). 2007; 9:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foy CG, Hsu FC, Haffner SM, et al. Visceral fat and prevalence of hypertension among African Americans and Hispanic Americans: findings from the IRAS family study. Am J Hypertens. 2008;21:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. [DOI] [PubMed] [Google Scholar]
- 20. Sealey JE, Laragh JH. Radioimmunoassay of plasma renin activity. Semin Nucl Med. 1975;5:189–202. [DOI] [PubMed] [Google Scholar]
- 21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 22. Homa Calculator v 2.2.2. http://www.dtu.ox.ac.uk. Accessed December 26, 2007. [Google Scholar]
- 23. Lansang MC, Williams GH, Carroll JS. Correlation between the glucose clamp technique and the homeostasis model assessment in hypertension. Am J Hypertens. 2001; 14:51–53. [DOI] [PubMed] [Google Scholar]
- 24. Tenenbaum A, Adler Y, Boyko V, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. 2007;153:559–565. [DOI] [PubMed] [Google Scholar]
- 25. Nelson TL, Bessesen DH, Marshall JA. Relationship of abdominal obesity measured by DXA and waist circumference with insulin sensitivity in Hispanic and non‐Hispanic white individuals: the San Luis Valley Diabetes Study. Diabetes Metab Res Rev. 2008;24:33–40. [DOI] [PubMed] [Google Scholar]
- 26. Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. [DOI] [PubMed] [Google Scholar]
- 27. Colussi G, Catena C, Lapenna R, et al. Insulin resistance and hyperinsulinemia are related to plasma aldosterone levels in hypertensive patients. Diabetes Care. 2007; 30:2349–2354. [DOI] [PubMed] [Google Scholar]
- 28. Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids. 1999;60:401–405. [DOI] [PubMed] [Google Scholar]
- 29. Rossi GP, Belfiore A, Bernini G, et al. Body mass index predicts plasma aldosterone concentrations in overweight‐obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–2571. [DOI] [PubMed] [Google Scholar]
- 30. Goodfriend TL, Kelley DE, Goodpaster BH, et al. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–362. [DOI] [PubMed] [Google Scholar]
- 31. Goodfriend TL, Ball DL, Egan BM, et al. Epoxy‐keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. [DOI] [PubMed] [Google Scholar]
- 32. Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91:1063–1077,viii. [DOI] [PubMed] [Google Scholar]
- 33. Scherrer U, Sartori C. Defective nitric oxide synthesis: a link between metabolic insulin resistance, sympathetic overactivity and cardiovascular morbidity. Eur J Endocrinol. 2000;142:315–323. [DOI] [PubMed] [Google Scholar]
- 34. Williams IL, Wheatcroft SB, Shah AM, et al. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26:754–764. [DOI] [PubMed] [Google Scholar]
- 35. Muldowney JA 3rd, Davis SN, Vaughan DE, et al. NO synthase inhibition increases aldosterone in humans. Hypertension. 2004;44:739–745. [DOI] [PubMed] [Google Scholar]
- 36. Natarajan R, Lanting L, Bai W, et al. The role of nitric oxide in the regulation of aldosterone synthesis by adrenal glomerulosa cells. J Steroid Biochem Mol Biol. 1997;61:47–53. [DOI] [PubMed] [Google Scholar]
- 37. Hanke CJ, Drewett JG, Myers CR, et al. Nitric oxide inhibits aldosterone synthesis by a guanylyl cyclase‐independent effect. Endocrinology. 1998;139:4053–4060. [DOI] [PubMed] [Google Scholar]
- 38. Weiner CP, Lizasoain I, Baylis SA, et al. Induction of calcium‐dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994;91:5212–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goetz RM, Morano I, Calovini T, et al. Increased expression of endothelial constitutive nitric oxide synthase in rat aorta during pregnancy. Biochem Biophys Res Commun. 1994;205:905–910. [DOI] [PubMed] [Google Scholar]
- 40. Rana BK, Insel PA, Payne SH, et al. Population‐based sample reveals gene‐gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. [DOI] [PubMed] [Google Scholar]
- 41. Seda O, Tremblay J, Gaudet D, et al. Systematic, genome‐wide, sex‐specific linkage of cardiovascular traits in French Canadians. Hypertension. 2008;51:1156–1162. [DOI] [PubMed] [Google Scholar]


